Stabilization and Crystal Characterization of Electric Arc Furnace Oxidizing Slag Modified with Ladle Furnace Slag and Alumina
Abstract
1. Introduction
2. Materials and Methods
2.1. Slag Sampling and Characterization
2.2. Dephosphorization of Steels
2.3. XRD and SEM Analysis
2.4. Image-Pro-Plus Processing and Analysis
2.5. f-CaO Content Determination
2.6. Laboratory-Scale Volume Expansion Testing
3. Results and Discussion
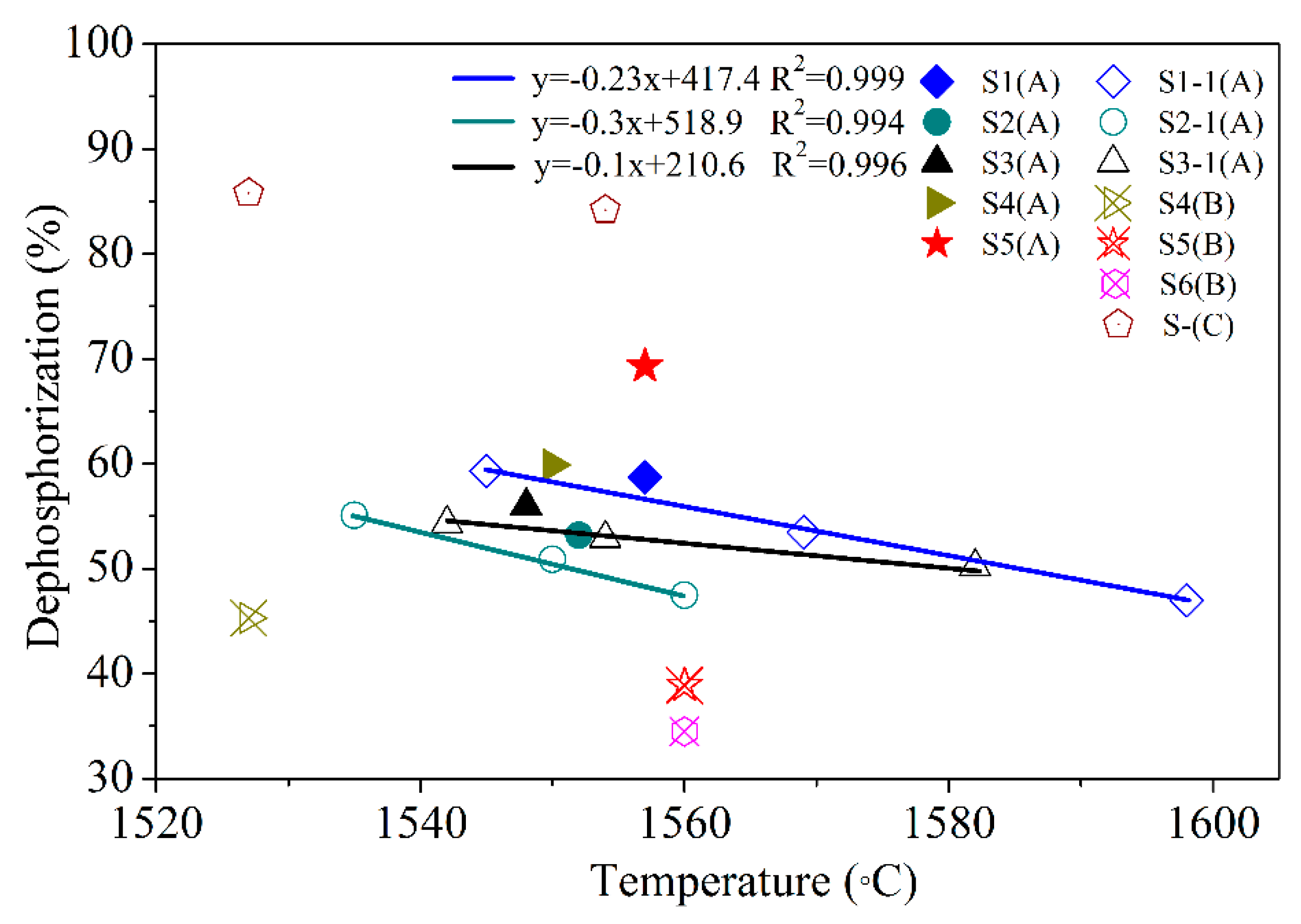
3.1. Dephosphorization of Modified Oxidizing Slag
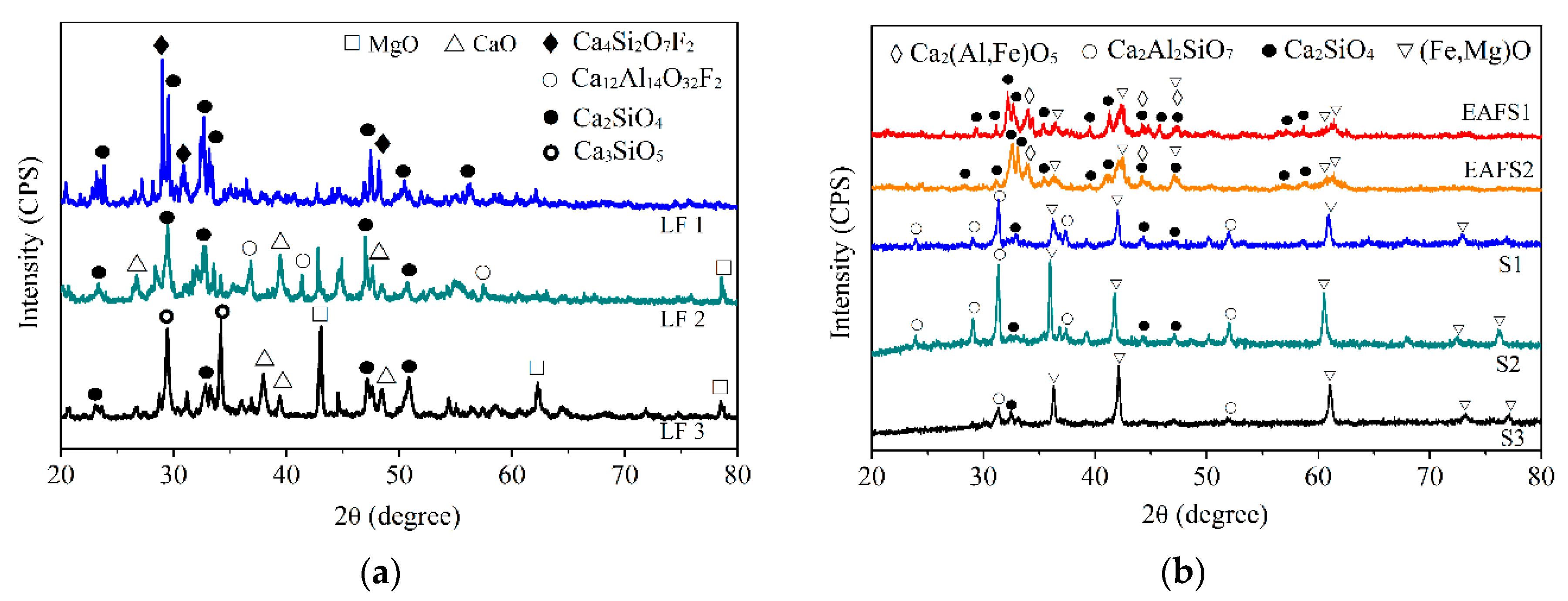
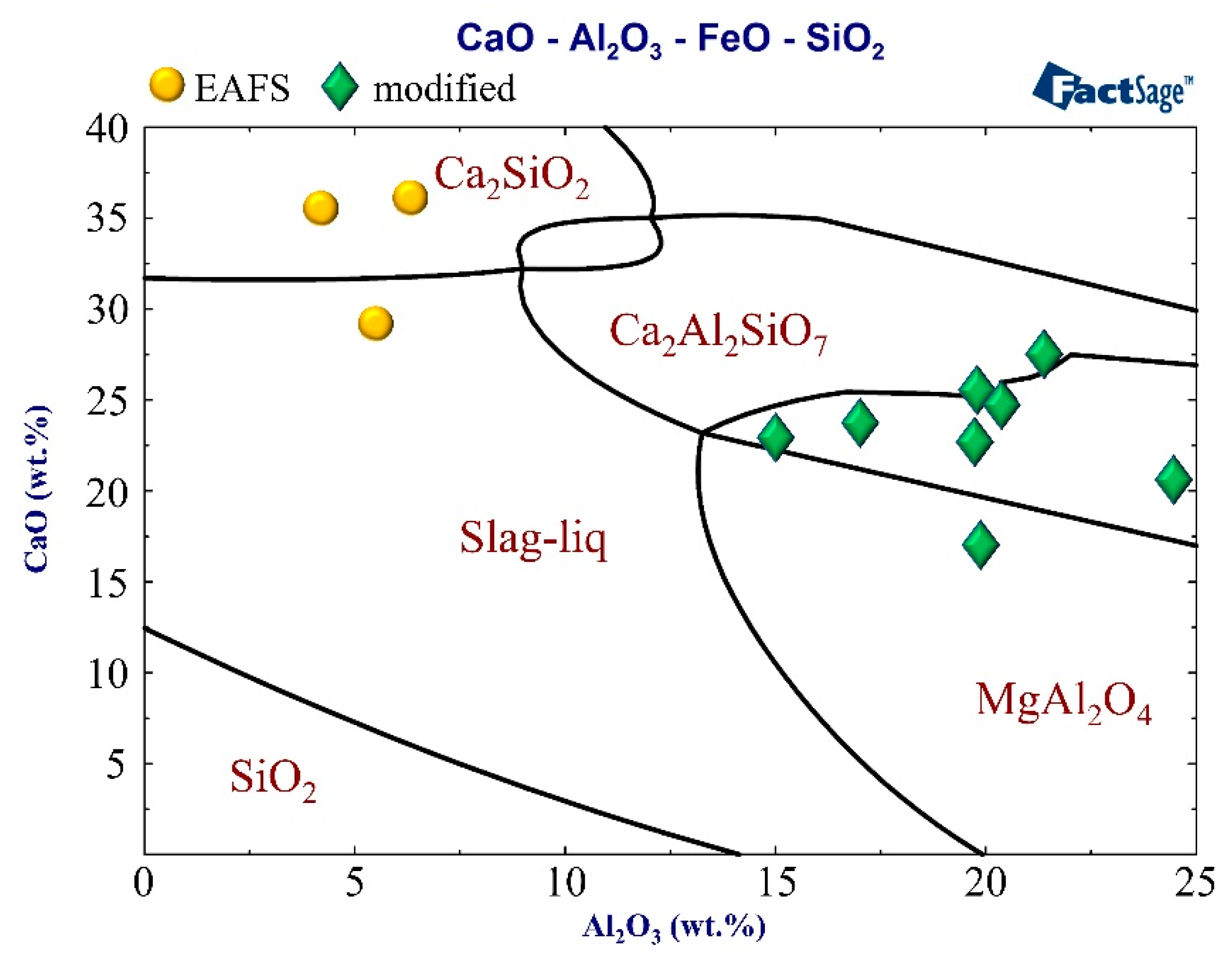
3.2. XRD Analysis
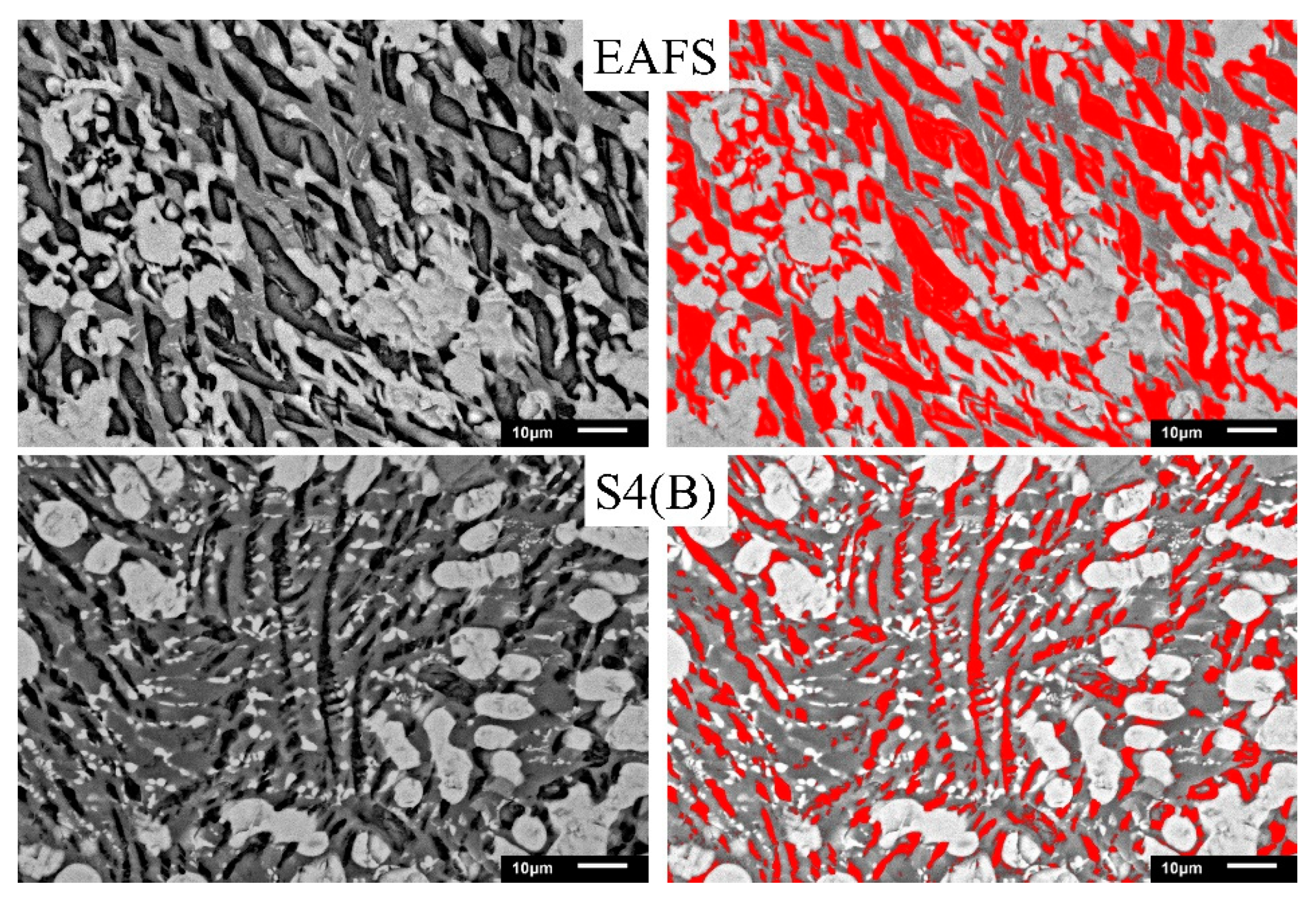
3.3. SEM Analysis
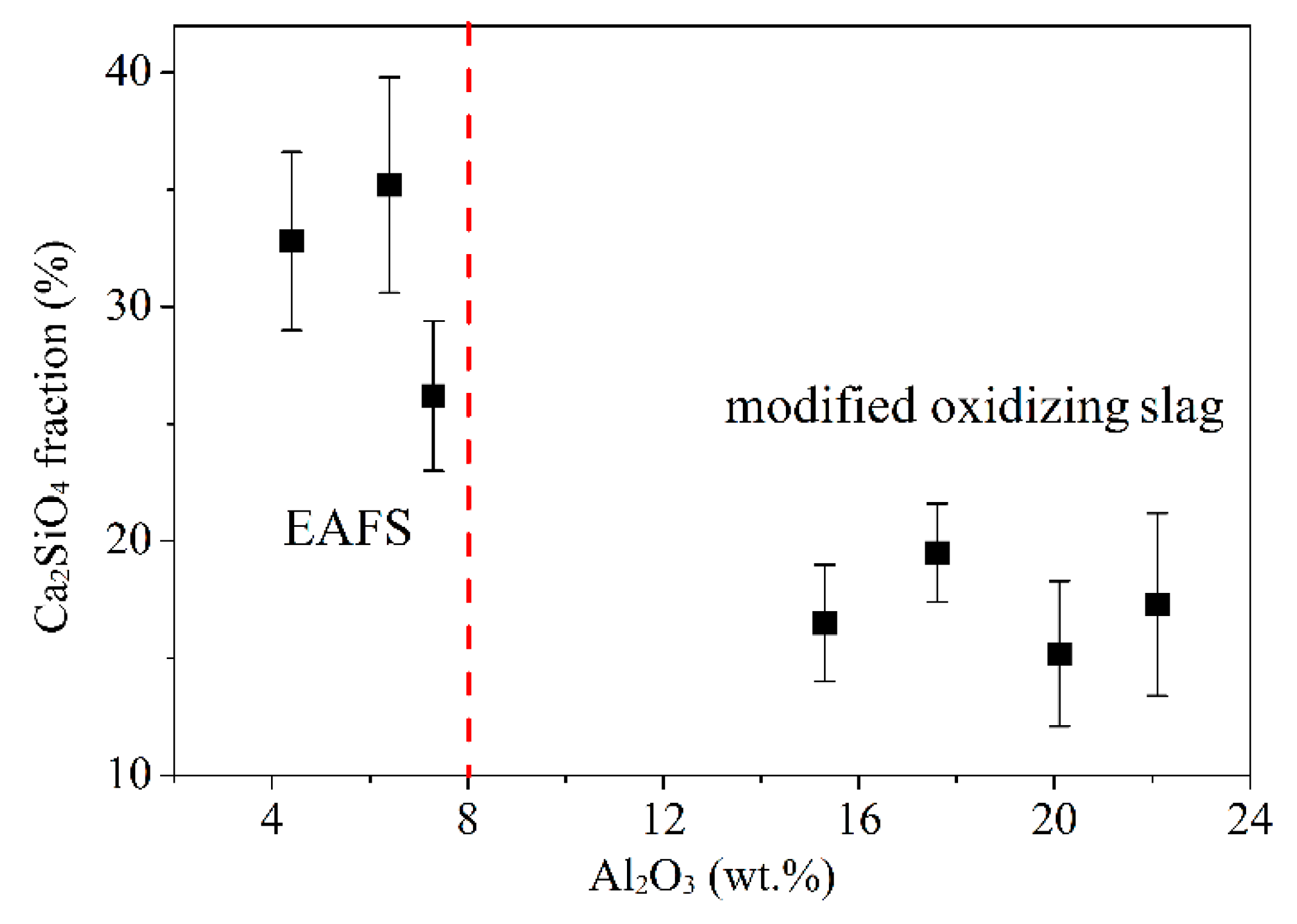
3.4. Image-Pro-Plus Analysis
3.5. Comparison of Expansion and f-CaO Results
4. Conclusions
- (1)
- The dephosphorization rate of different LFSs using supplement A (LF/CaO = 1/1, Al2O3 content = 15–25%) was more than 45% between 1520 °C and 1600 °C. The phosphorus content of steel is less than 0.03% to meet the set standards of general engineering steel.
- (2)
- XRD results showed that different LFSs had C2S, C3S, CaO, and MgO as the major phases. The characteristics of LFS were altered after high-temperature slag modification, and the primary crystalline phases were FeO and gehlenite (Ca2Al2SiO7).
- (3)
- SEM analysis confirmed that phosphorus was concentrated in the C2S phase during dephosphorization. As the Al2O3 content exceeds 15%, about 2–8% of Al atoms replaced Si atoms, and the C2S phase fraction decreased from 31.4% to 17.1%.
- (4)
- The f-CaO content of the modified oxidizing slag was less than 0.41% (while those of LFS and EAFS were 0.9–3.4% and 1.2–1.4%, respectively). The modified oxidizing slag showed almost no expansion and conformed to the ASTM D2940 standards.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Setien, J.; Hernandez, D.; Gonzalez, J.J. Characterization of ladle furnace basic slag for use as a construction material. Constr. Build. Mater. 2009, 23, 1788–1794. [Google Scholar] [CrossRef]
- Durinck, D.; Arnout, S.; Mertens, G.; Boydens, E.; Jones, P.T.; Elsen, J.; Blanpain, B.; Wollants, P. Borate distribution in stabilized stainless-steel slag. J. Am. Ceram. Soc. 2008, 91, 548–554. [Google Scholar] [CrossRef]
- Shi, C.J. Characteristics and cementitious properties of ladle slag fines from steel production. Cem. Concr. Res. 2002, 32, 459–462. [Google Scholar] [CrossRef]
- Shi, C.J.; Qian, J.S. High performance cementing materials from industrial slags—A review. Resour. Conserv. Recycl. 2000, 29, 195–207. [Google Scholar] [CrossRef]
- Lee, H.-S.; Lim, H.-S.; Ismail, M.A. Quantitative evaluation of free cao in electric furnace slag using the ethylene glycol method. Constr. Build. Mater. 2017, 131, 676–681. [Google Scholar] [CrossRef]
- Loncnar, M.; van der Sloot, H.A.; Mladenovic, A.; Zupancic, M.; Kobal, L.; Bukovec, P. Study of the leaching behaviour of ladle slags by means of leaching tests combined with geochemical modelling and mineralogical investigations. J. Hazard. Mater. 2016, 317, 147–157. [Google Scholar] [CrossRef]
- Bocci, E. Use of ladle furnace slag as filler in hot asphalt mixtures. Constr. Build. Mater. 2018, 161, 156–164. [Google Scholar] [CrossRef]
- Worldsteel. Steel Statistical Yearbook. Available online: https://www.worldsteel.org/steel-by-topic/statistics/steel-statistical-yearbook.html (accessed on 27 March 2020).
- Serjun, V.Z.; Mirtic, B.; Mladenovic, A. Evaluation of ladle slag as a potential material for building and civil engineering. Mater. Tehnol. 2013, 47, 543–550. [Google Scholar]
- Branca, T.A.; Colla, V.; Valentini, R. A way to reduce environmental impact of ladle furnace slag. Ironmak. Steelmak. 2009, 36, 597–602. [Google Scholar] [CrossRef]
- Okoye, P.U.; Abdullah, A.Z.; Hameed, B.H. Stabilized ladle furnace steel slag for glycerol carbonate synthesis via glycerol transesterification reaction with dimethyl carbonate. Energy Conv. Manag. 2017, 133, 477–485. [Google Scholar] [CrossRef]
- Gu, X.; Yu, B.; Dong, Q.; Deng, Y. Application of secondary steel slag in subgrade: Performance evaluation and enhancement. J. Clean. Prod. 2018, 181, 102–108. [Google Scholar] [CrossRef]
- Papayianni, I.; Anastasiou, E. Effect of granulometry on cementitious properties of ladle furnace slag. Cem. Concr. Compos. 2012, 34, 400–407. [Google Scholar] [CrossRef]
- Guzzon, M.; Mapelli, C.; Sahajwalla, V.; Saha-Chaudhury, N.; Memoli, F.; Pustorino, M. The behaviour of the secondary metallurgy slag into the eaf. How to create a good foamy slag with the appropriate basicity using a mix of lime and recycled ladle slag as eaf slag former. In Proceedings of the 38th Seminário de Aciaria, Belo Horizonte, Brazil, 14 November 2019. [Google Scholar]
- Li, J.X.; Yu, Q.J.; Wei, J.X.; Zhang, T.S. Structural characteristics and hydration kinetics of modified steel slag. Cem. Concr. Res. 2011, 41, 324–329. [Google Scholar] [CrossRef]
- Wang, Q.A.; Yan, P.Y.; Feng, J.W. A discussion on improving hydration activity of steel slag by altering its mineral compositions. J. Hazard. Mater. 2011, 186, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, D.Q.; Zhuang, S.Y. The soundness of steel slag with different free cao and mgo contents. Constr. Build. Mater. 2017, 151, 138–146. [Google Scholar] [CrossRef]
- Rashad, A.M. A synopsis manual about recycling steel slag as a cementitious material. J. Mater. Res. Technol. 2019, 8, 4940–4955. [Google Scholar] [CrossRef]
- Guzzon, M.; Mapelli, C.; Memoli, F.; Marcozzi, M. Recycling of ladle slag in the eaf: Improvement of the foaming behaviour and decrease of the environmental impact. Rev. de Métall. 2007, 104, 171–178. [Google Scholar] [CrossRef]
- Varanasi, S.S.; More, V.M.R.; Rao, M.B.V.; Alli, S.R.; Tangudu, A.K.; Santanu, D. Recycling ladle furnace slag as flux in steelmaking: A review. J. Sustain. Metall. 2019, 5, 449–462. [Google Scholar] [CrossRef]
- Skaf, M.; Manso, J.M.; Aragón, Á.; Fuente-Alonso, J.A.; Ortega-López, V. Eaf slag in asphalt mixes: A brief review of its possible re-use. Resour. Conserv. Recycl. 2017, 120, 176–185. [Google Scholar] [CrossRef]
- Yildirim, I.Z.; Prezzi, M. Experimental evaluation of eaf ladle steel slag as a geo-fill material: Mineralogical, physical & mechanical properties. Constr. Build. Mater. 2017, 154, 23–33. [Google Scholar] [CrossRef]
- Brand, A.S.; Roesler, J.R. Steel furnace slag aggregate expansion and hardened concrete properties. Cem. Concr. Compos. 2015, 60, 1–9. [Google Scholar] [CrossRef]
- González-Ortega, M.A.; Cavalaro, S.H.P.; de Rodríguez Sensale, G.; Aguado, A. Durability of concrete with electric arc furnace slag aggregate. Constr. Build. Mater. 2019, 217, 543–556. [Google Scholar] [CrossRef]
- Mombelli, D.; Mapelli, C.; Barella, S.; Gruttadauria, A.; Le Saout, G.; Garcia-Diaz, E. The efficiency of quartz addition on electric arc furnace (eaf) carbon steel slag stability. J. Hazard. Mater. 2014, 279, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, D.; Mapelli, C.; Barella, S.; Di Cecca, C.; Le Saout, G.; Garcia-Diaz, E. The effect of microstructure on the leaching behaviour of electric arc furnace (eaf) carbon steel slag. Proc. Saf. Environ. Protect. 2016, 102, 810–821. [Google Scholar] [CrossRef]
- Suito, H.; Inoue, R. Behavior of phosphorous transfer from cao-feto-p2o5(-sio2) slag to cao particles. ISIJ Int. 2006, 46, 180–187. [Google Scholar] [CrossRef]
- Deniz, D.; Tutumluer, E.; Popovics, J.S. Evaluation of expansive characteristics of reclaimed asphalt pavement and virgin aggregate used as base materials. Transp. Res. Rec. 2010, 10–17. [Google Scholar] [CrossRef]
- Li, C.C.; Lin, C.M.; Hung, C.Y.; Chang, K.L.; Wu, W.T. Effect of al2o3/sio2 ratio on viscosity and structure of cao-al2o3-sio2-caf2mgo slag. Int. J. Mater. Res. 2019, 110, 247–252. [Google Scholar] [CrossRef]
- Diao, J.; Xie, B.; Wang, Y.; Guo, X. Recovery of phosphorus from dephosphorization slag produced by duplex high phosphorus hot metal refining. ISIJ Int. 2012, 52, 955–959. [Google Scholar] [CrossRef]
- Kondratiev, A.; Jak, E. Predicting coal ash slag flow characteristics (viscosity model for the al2o3-cao-’feo’-sio2 system). Fuel 2001, 80, 1989–2000. [Google Scholar] [CrossRef]
- Wu, W.; Lin, C.M.; Chen, J.H.; Li, C.C.; Lin, K.H. Detection of Calcium in the Active Oxide. I600903; 1 October 2017. Available online: https://twpat1.tipo.gov.tw/tipotwoc/tipotwkm?!!FR_I600903 (accessed on 11 April 2020).
- Wang, G.; Wang, Y.H.; Gao, Z.L. Use of steel slag as a granular material: Volume expansion prediction and usability criteria. J. Hazard. Mater. 2010, 184, 555–560. [Google Scholar] [CrossRef]
- Waligora, J.; Bulteel, D.; Degrugilliers, P.; Damidot, D.; Potdevin, J.L.; Measson, M. Chemical and mineralogical characterizations of ld converter steel slags: A multi-analytical techniques approach. Mater. Charact. 2010, 61, 39–48. [Google Scholar] [CrossRef]
- Ortega-Lopez, V.; Manso, J.M.; Cuesta, I.I.; Gonzalez, J.J. The long-term accelerated expansion of various ladle-furnace basic slags and their soil-stabilization applications. Constr. Build. Mater. 2014, 68, 455–464. [Google Scholar] [CrossRef]
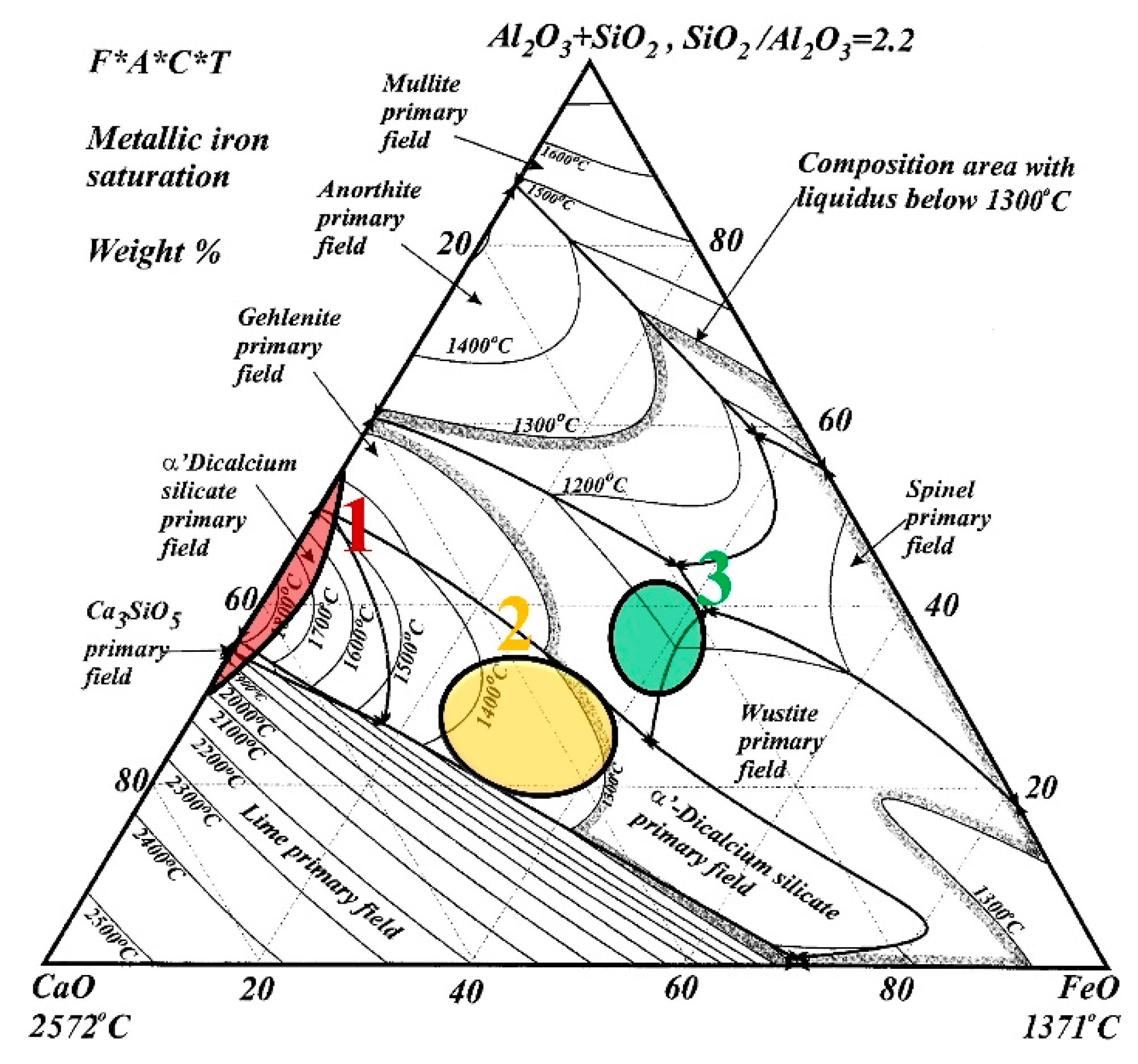



| Specimen | Types | Chemical Composition (wt.%) | ||||||
|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | MgO | T-Fe | MnO | S | ||
| LFS 1 | (1) | 45.9 | 21.6 | 4.2 | 8.6 | 1.8 | 0.3 | 0.867 |
| LFS 2 | (2) | 50.2 | 11.7 | 19.9 | 4.9 | 2.1 | 0.3 | 0.727 |
| LFS 3 | (3) | 44.1 | 17.5 | 3.4 | 17.9 | 2.3 | 0.6 | 0.567 |
| LFS 4 | (1) | 55.4 | 27.2 | 2.7 | 5.8 | 1.5 | 0.4 | 0.547 |
| LFS 5 | (1) | 52.1 | 19.3 | 14.8 | 4.9 | 0.7 | 0.1 | 0.994 |
| LFS 6 | (2) | 52.3 | 5.1 | 28.9 | 5.4 | 0.5 | 0.1 | 0.911 |
| LFS 7 | (1) | 46.4 | 21.1 | 3.9 | 7.0 | 1.6 | 0.5 | 0.772 |
| LFS 8 | (1) | 52.6 | 23.6 | 4.2 | 8.8 | 0.3 | 0.2 | 0.828 |
| LFS 9 | (3) | 40.3 | 15.9 | 4.8 | 13.1 | 4.5 | 0.6 | 0.542 |
| LFS 10 | (1) | 47.7 | 21.8 | 4.3 | 10.4 | 2.2 | 0.7 | 0.690 |
| EAFS 1 | 37.8 | 11.2 | 7.4 | 6.2 | 25.9 | 3.4 | 0.102 | |
| EAFS 2 | 28.8 | 11.9 | 6.4 | 3.7 | 28.6 | 4.9 | 0.241 | |
| EAFS 3 | 35.6 | 10.7 | 4.5 | 6.9 | 28.4 | 4.5 | 0.097 | |
| Supplements | LFS (g) | CaO (g) | Al2O3 (%) |
|---|---|---|---|
| A (for 200 g metal) | 3 | 3 | 15–25 |
| A | 525 | 525 | 15–25 |
| B | 1050 | 0 | 15–25 |
| C | 0 | 1050 | 15–25 |
| Sample | Chemical Composition (wt.%) | Initial [P] | Final [P] | De-P 1 | ||||
|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | FexO | P2O5 | (%) | |||
| S1 (A) | 24.4 | 8.4 | 21.7 | 41.3 | 0.551 | 0.0477 | 0.0197 | 58.7 |
| S2 (A) | 25.8 | 7.9 | 20.1 | 40.2 | 0.455 | 0.0451 | 0.0211 | 53.2 |
| S3 (A) | 24.5 | 8.6 | 17.6 | 44.5 | 0.546 | 0.0432 | 0.0191 | 55.8 |
| S4 (A) | 27.2 | 7.3 | 22.3 | 27.2 | 0.643 | 0.0389 | 0.0156 | 59.9 |
| S5 (A) | 22.3 | 8.1 | 20.4 | 33.6 | 0.757 | 0.0496 | 0.0152 | 69.4 |
| S4 (B) | 23.4 | 9.9 | 15.3 | 35.6 | 0.445 | 0.0403 | 0.0221 | 45.2 |
| S5 (B) | 16.6 | 13.9 | 20.3 | 33.8 | 0.375 | 0.0314 | 0.0192 | 38.9 |
| S6 (B) | 20.8 | 8.8 | 24.8 | 25.9 | 0.397 | 0.0495 | 0.0324 | 34.5 |
| S-(C) | 32.1 | 4.3 | 28.7 | 26.2 | 0.844 | 0.0514 | 0.0081 | 84.2 |
| Sample | Phase | Chemical Composition, at.% | ||||||
|---|---|---|---|---|---|---|---|---|
| Ca | Si | Al | Fe | Mg | P | O | ||
| S1 | I-FeO | 1.9 | 0.0 | 0.6 | 41.1 | 6.6 | 0.00 | 49.8 |
| II-matrix | 14.8 | 5.8 | 14.0 | 7.1 | 0.8 | 0.00 | 57.4 | |
| III-C2S | 25.7 | 10.4 | 2.6 | 1.1 | 0.3 | 0.32 | 59.5 | |
| S2 | I-FeO | 2.5 | 0.2 | 0.9 | 40.1 | 7.6 | 0.00 | 48.7 |
| II-matrix | 14.5 | 5.3 | 14.5 | 8.7 | 1.2 | 0.00 | 55.9 | |
| S3 | I-FeO | 0.9 | 0.0 | 0.4 | 36.7 | 13.1 | 0.00 | 48.9 |
| II-matrix | 15.1 | 6.1 | 13.2 | 5.8 | 1.0 | 0.00 | 58.9 | |
| III-C2S | 28.6 | 8.2 | 7.3 | 1.1 | 0.4 | 0.28 | 54.1 | |
| EAFS | I-FeO | 1.1 | 0.2 | 0.7 | 35.8 | 8.3 | 0.00 | 53.9 |
| II-matrix | 13.3 | 4.6 | 14.8 | 4.5 | 0.1 | 0.00 | 61.9 | |
| III-C2S | 33.1 | 17.5 | 0.4 | 1.0 | 0.1 | 0.47 | 47.4 | |
| Sample | S1A | S2A | S3A | S4A | S5A | S4B | S5B | S6B | S-C | EAF1 | EAF2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| f-CaO (%) | 0.31 | 0.31 | 0.26 | 0.41 | 0.37 | 0.18 | 0.13 | 0.21 | 0.7 | 1.2 | 1.4 |
| Expansion (%) | 0.027 | NA 1 | NA 1 | 0.037 | NA 1 | 0.023 | 0.029 | NA 1 | 0.11 | 0.17 | 0.15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.-C.; Lin, C.-M.; Chang, Y.-E.; Chang, W.-T.; Wu, W. Stabilization and Crystal Characterization of Electric Arc Furnace Oxidizing Slag Modified with Ladle Furnace Slag and Alumina. Metals 2020, 10, 501. https://doi.org/10.3390/met10040501
Li C-C, Lin C-M, Chang Y-E, Chang W-T, Wu W. Stabilization and Crystal Characterization of Electric Arc Furnace Oxidizing Slag Modified with Ladle Furnace Slag and Alumina. Metals. 2020; 10(4):501. https://doi.org/10.3390/met10040501
Chicago/Turabian StyleLi, Chia-Chun, Chi-Ming Lin, Yu-En Chang, Wei-Ti Chang, and Weite Wu. 2020. "Stabilization and Crystal Characterization of Electric Arc Furnace Oxidizing Slag Modified with Ladle Furnace Slag and Alumina" Metals 10, no. 4: 501. https://doi.org/10.3390/met10040501
APA StyleLi, C.-C., Lin, C.-M., Chang, Y.-E., Chang, W.-T., & Wu, W. (2020). Stabilization and Crystal Characterization of Electric Arc Furnace Oxidizing Slag Modified with Ladle Furnace Slag and Alumina. Metals, 10(4), 501. https://doi.org/10.3390/met10040501






