Morphology, Structure and Mechanical Properties of Copper Coatings Electrodeposited by Pulsating Current (PC) Regime on Si(111)
Abstract
1. Introduction
2. Materials and Methods
Characterization of the Produced Cu Coatings
3. Results
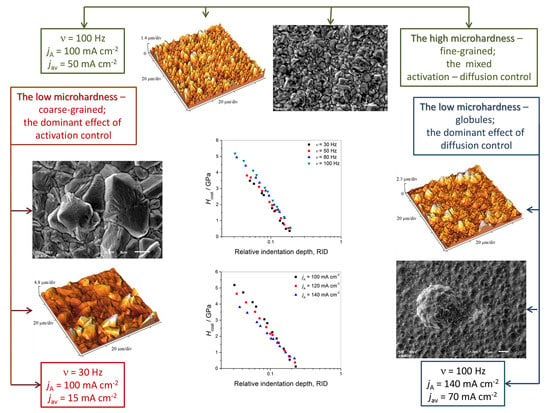
3.1. Basic Facts Necessary for Understanding of the Presented Results
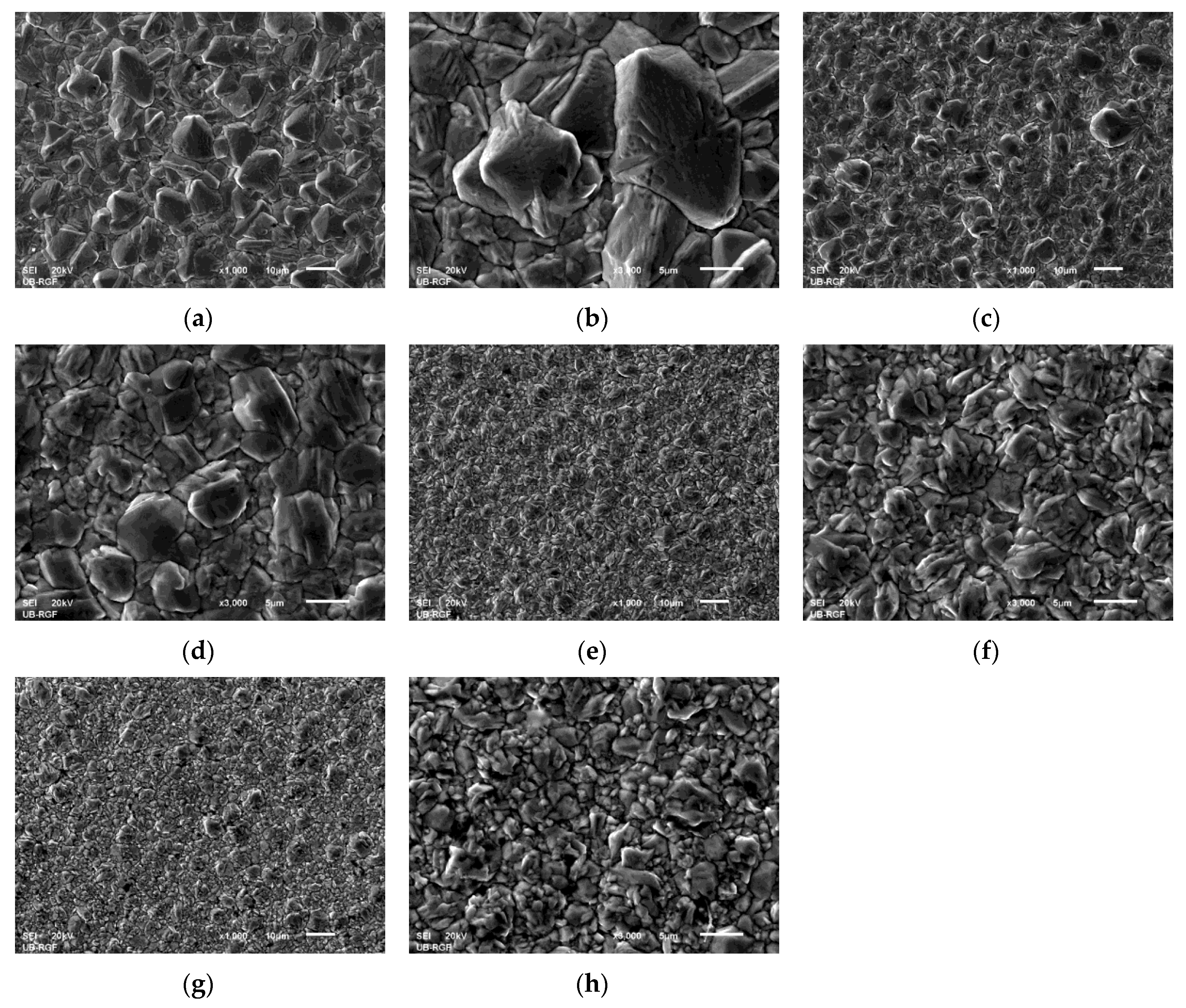
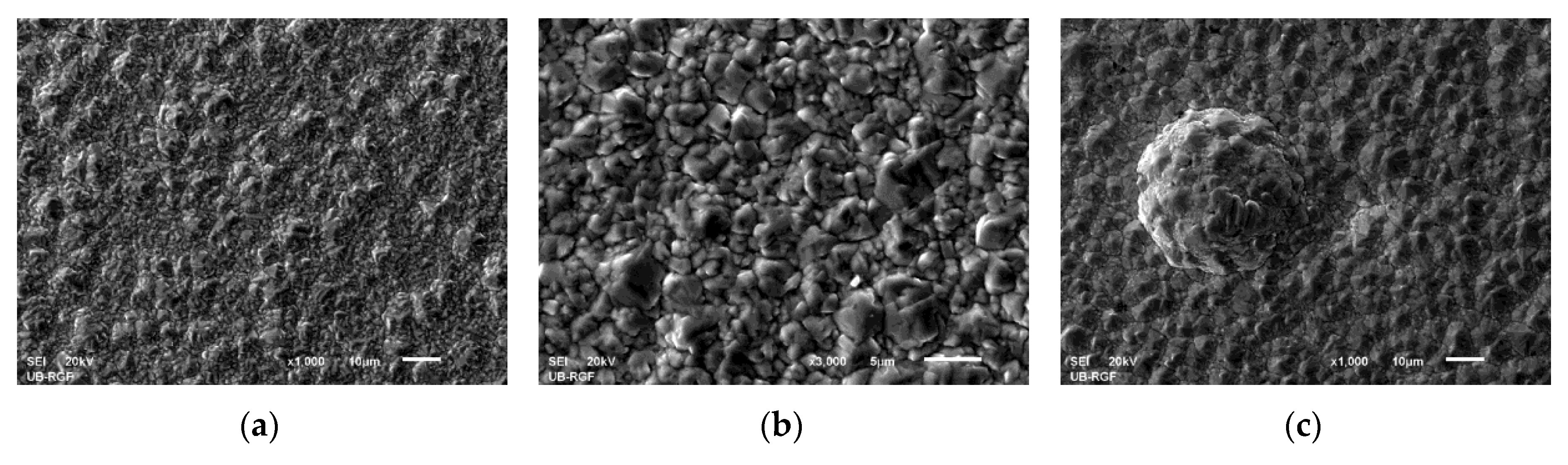
3.2. Morphology of the Cu Coatings Obtained by Application of the PC Regime
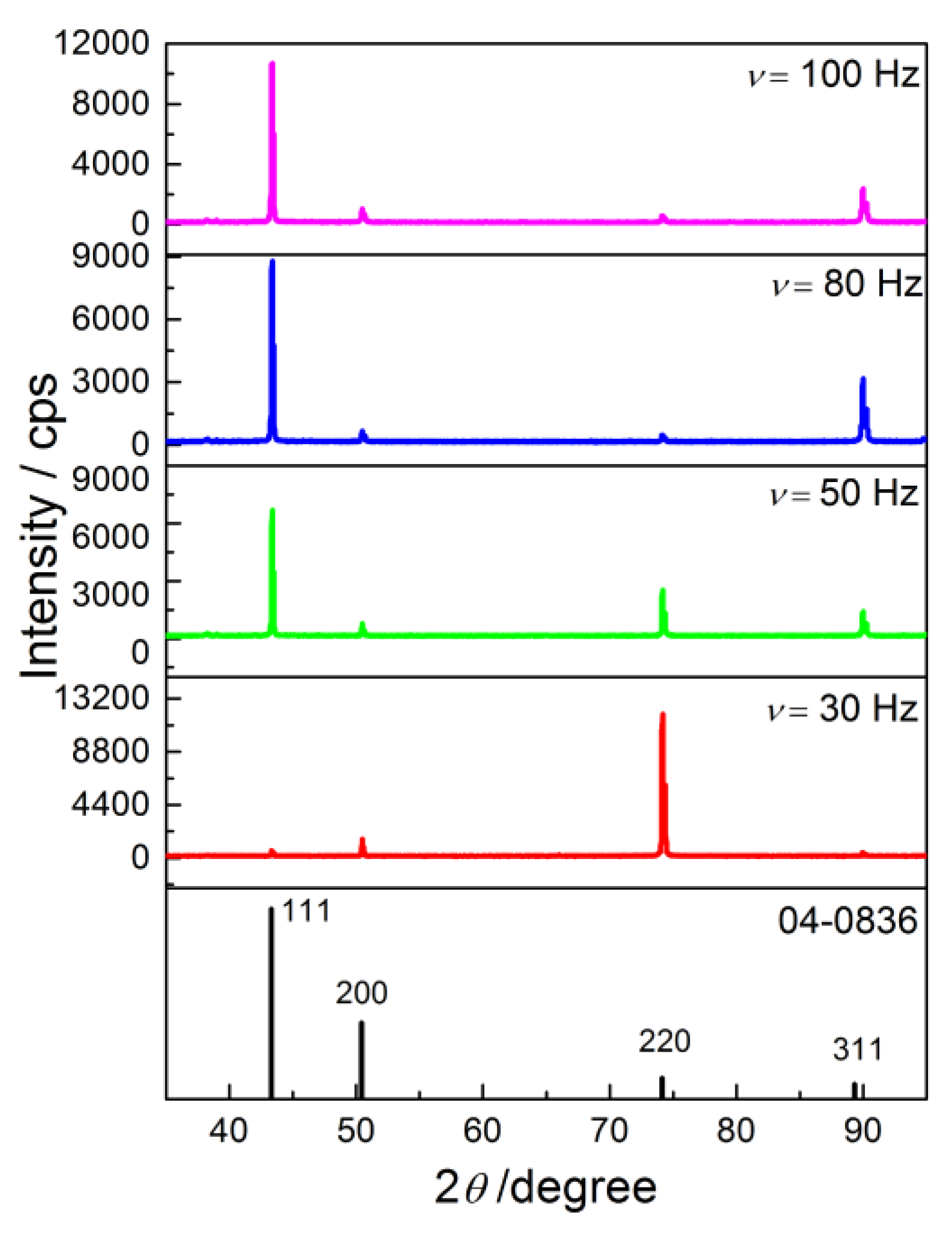
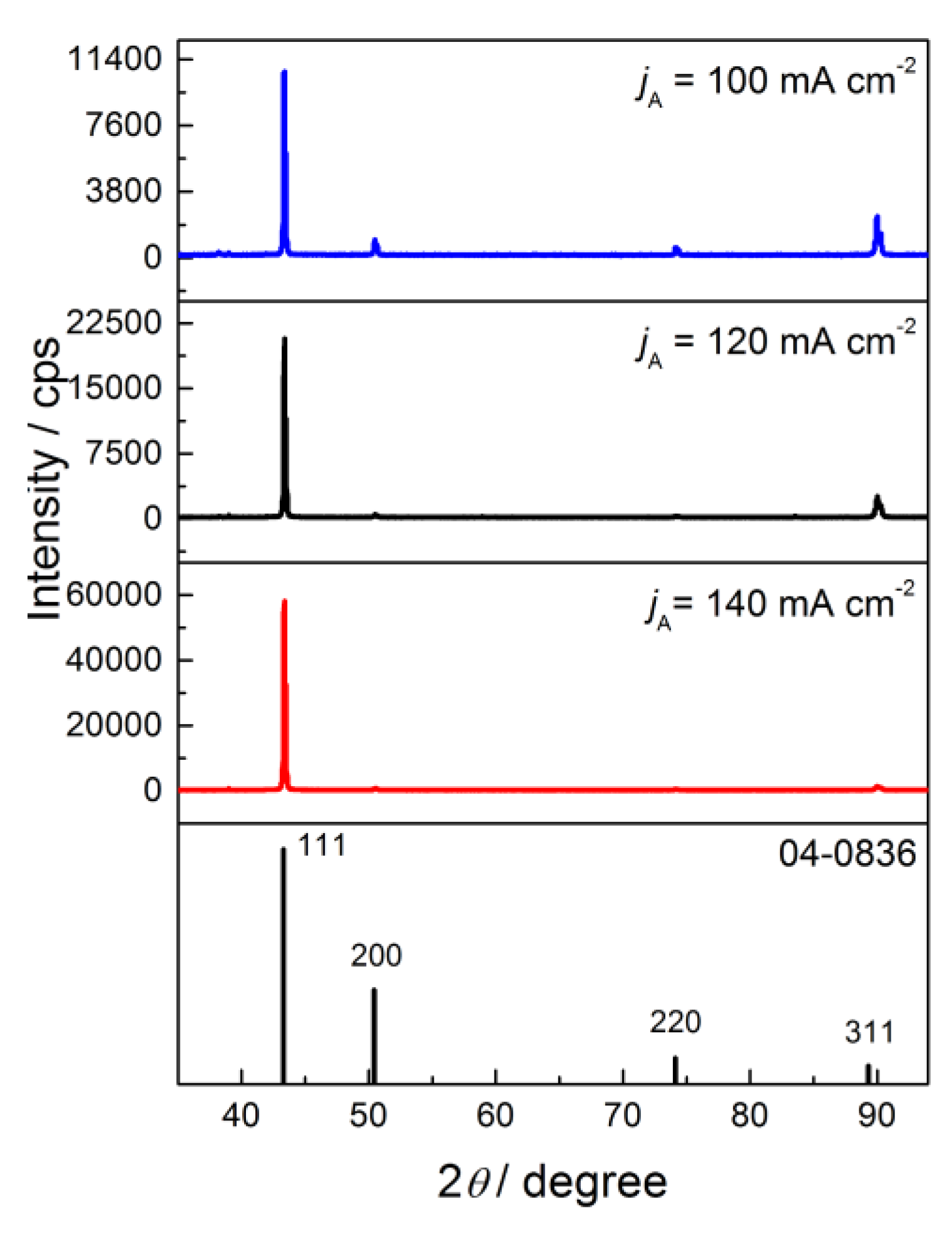
3.3. X-ray Diffraction (XRD) Analysis of the Produced Cu Coatings
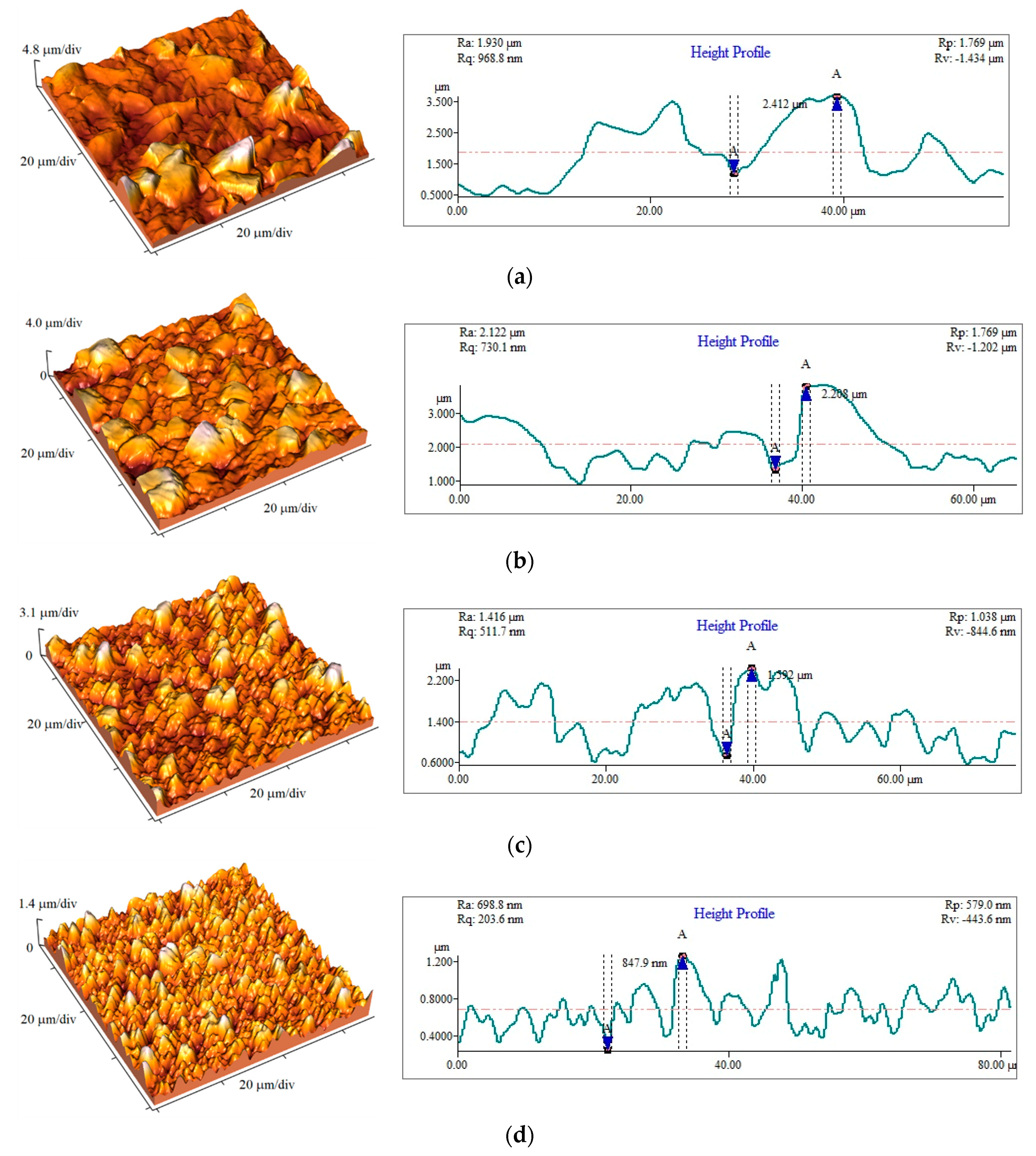
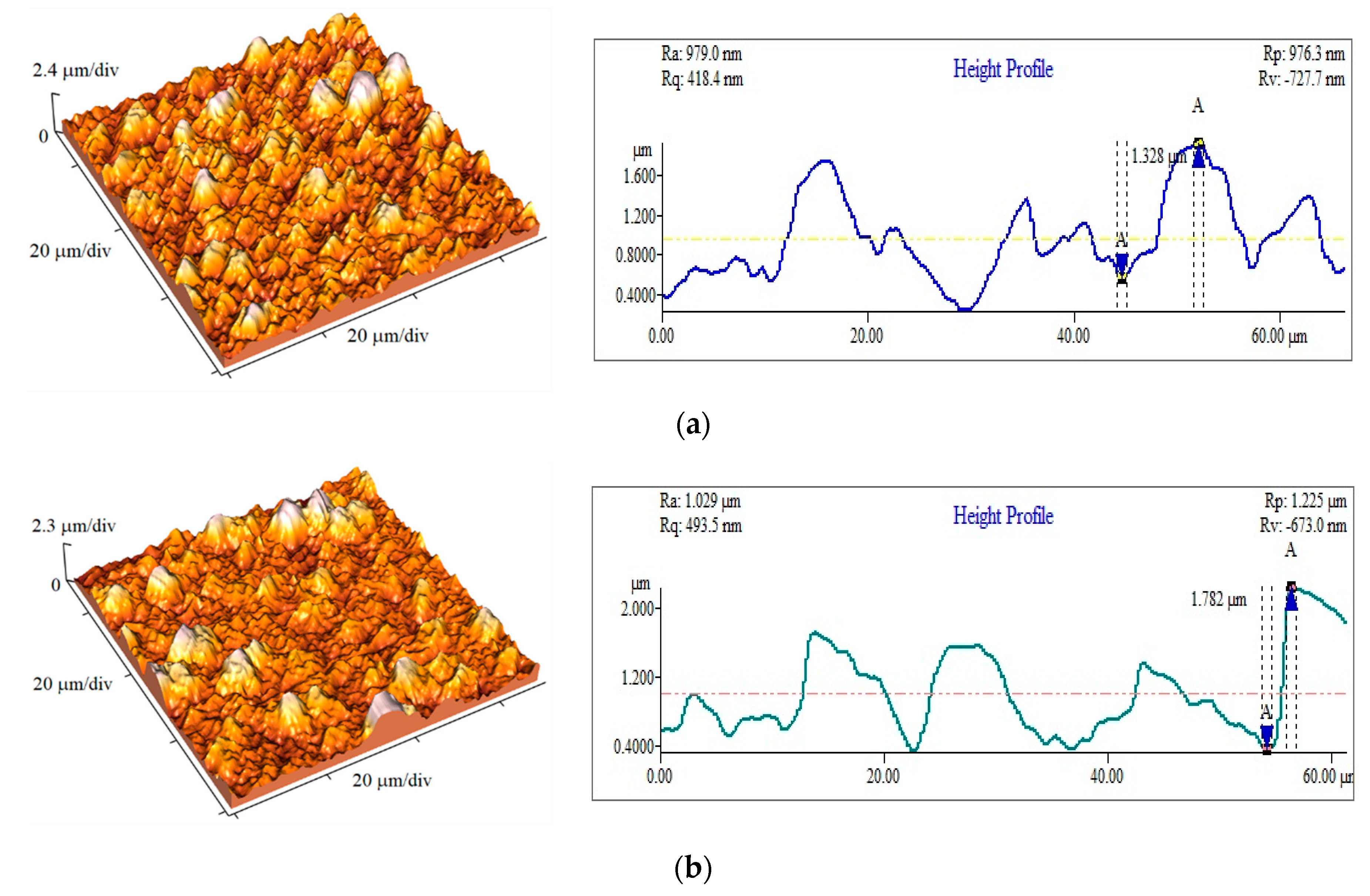
3.4. The Roughness Analysis of the Cu Coatings
3.5. Microhardness
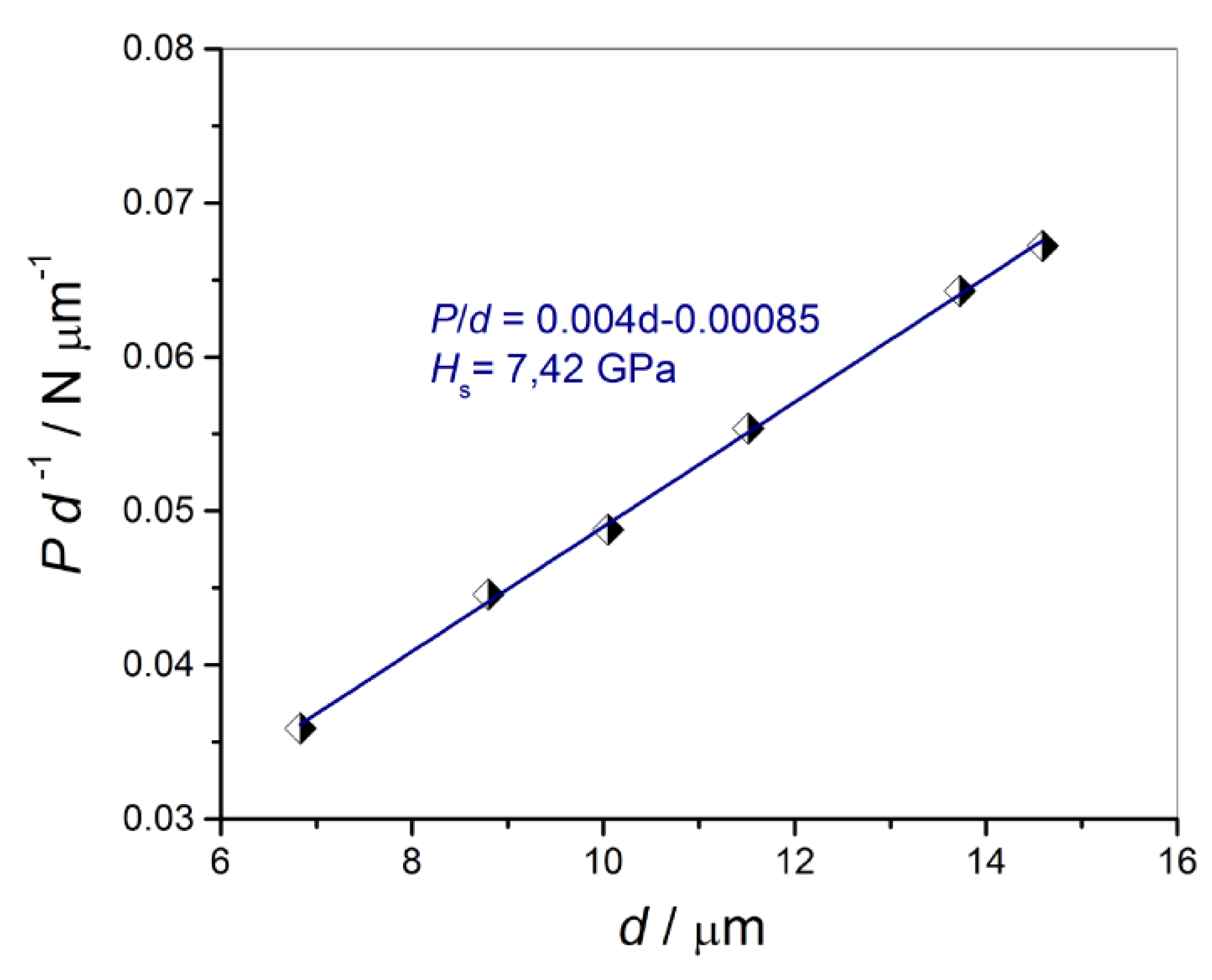
3.5.1. Absolute Hardness of the Substrate
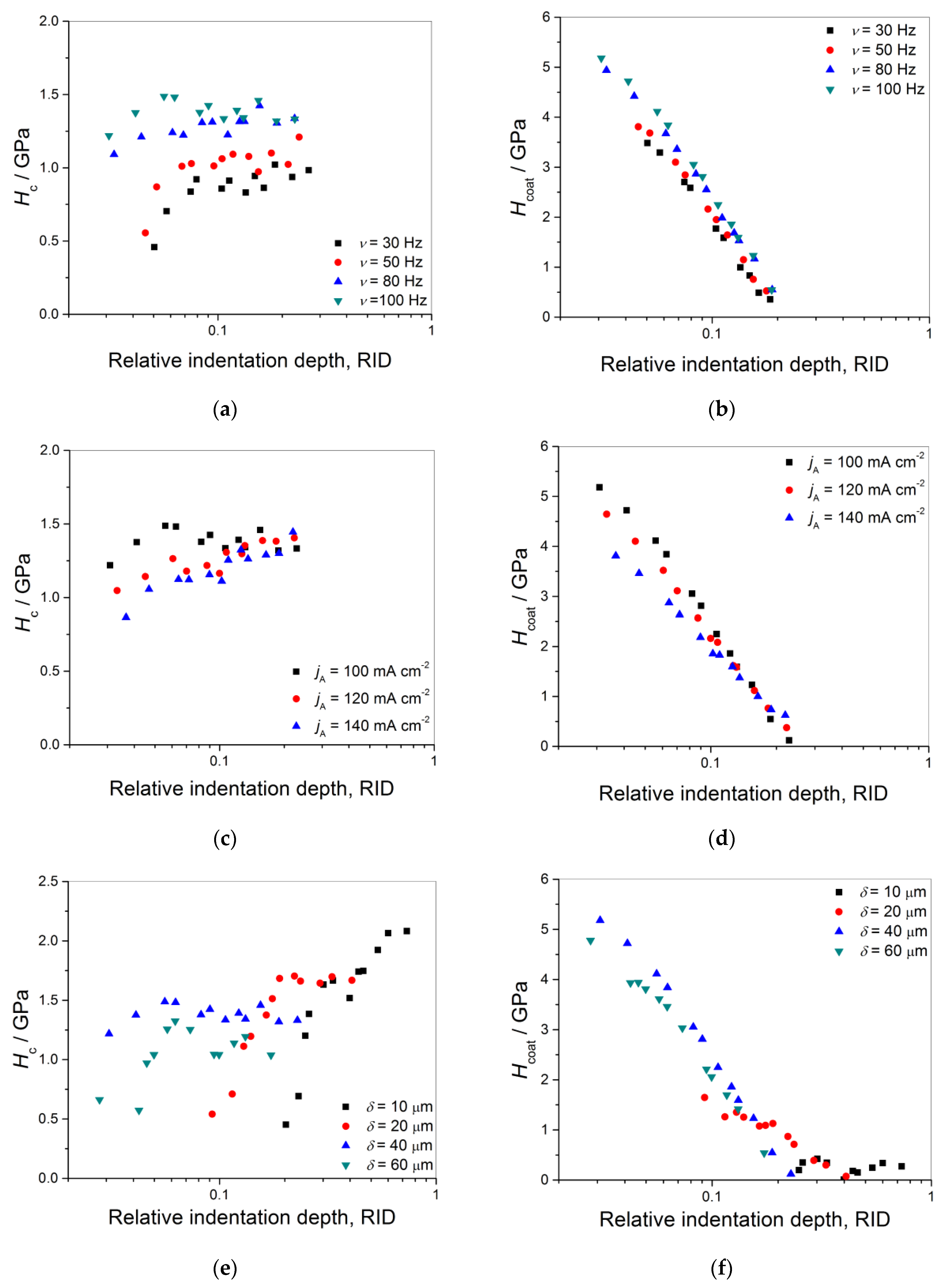
3.5.2. The Composite and the Coating Hardness
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Theory of the Composite Hardness Model
Appendix B
Appendix C
References
- Rohan, J.F.; Thompson, D. Frontiers of Cu Electrodeposition and Electroless plating for on-chip Interconnects. In Copper Electrodeposition for nanofabrication of Electronics Devices; Kondo, K., Alkolkar, R.N., Barkey, D.P., Yokoi, M., Eds.; Springer: New York, NY, USA, 2014; Volume 171, pp. 99–101. [Google Scholar] [CrossRef]
- Pellicer, E.; Varea, A.; Pané, S.; Sivaraman, K.M.; Nelson, B.J; Suriñach, S.; Baró, M.D.; Sort, J. A comparison between fine-grained and nanocrystalline electrodeposited Cu–Ni films Insights on mechanical and corrosion performance. Surf. Coat. Technol. 2011, 205, 5285–5293. [Google Scholar] [CrossRef]
- Banthia, S.; Sengupta, S.; Das, S.; Das, K. Cu, Cu-SiC functionally graded coating for protection against corrosion and wear. Surf. Coat. Technol. 2019, 374, 833–844. [Google Scholar] [CrossRef]
- Banthia, S.; Sengupta, S.; Das, S.; Das, K. Synthesis and characterization of novel Cu, Cu-Si functionally graded coating by pulse reverse electrodeposition. Appl. Surf. Sci. 2019, 467–468, 567–579. [Google Scholar] [CrossRef]
- Gupta, S.K.; Misra, R.D. An experimental investigation on flow boiling heat transfer enhancement using Cu-TiO2 nanocomposite coating on copper substrate. Exp. Therm. Fluid Sci. 2018, 98, 406–419. [Google Scholar] [CrossRef]
- Gupta, S.K; Misra, R.D. Effect of two-step electrodeposited Cu–TiO2 nanocomposite coating on pool boiling heat transfer performance. J. Therm. Anal. Calorim. 2019, 136, 1781–1793. [Google Scholar] [CrossRef]
- Gupta, S.K.; Misra, R.D. Experimental study of pool boiling heat transfer on copper surfaces with Cu-Al2O3 nanocomposite coatings. Int. Commun. Heat Mass Transf. 2018, 97, 47–55. [Google Scholar] [CrossRef]
- Cortés, M.; Martínez, S.; Serre, C.; Gómez, E.; Pérez-Rodríguez, A.; Vallés, E. Design and electrochemical preparation of inductive copper coil for magnetic particles detection. Sens. Actuators B 2012, 173, 737–744. [Google Scholar] [CrossRef]
- Ming, P.M; Zhu, D.; Zeng, Y.B; Hu, Y.Y. Wear resistance of copper EDM tool electrode electroformed from copper sulfate baths and pyrophosphate baths. Int. J. Adv. Manuf. Technol. 2010, 50, 635–641. [Google Scholar] [CrossRef]
- Delbos, E.; Omnès, L.; Etcheberry, A. Bottom-up filling optimization for efficient TSV metallization. Microelectron. Eng. 2010, 87, 514–516. [Google Scholar] [CrossRef]
- Arai, S.; Hasegawa, T.; Kaneko, N. Fabrication of three-dimensional Cu/Ni multilayered microstructure by wet process. Electrochim. Acta 2004, 49, 945–950. [Google Scholar] [CrossRef]
- Ladani, L.; Awad, I.; She, Y.; Dardona, S.; Schmidt, W. Fabrication of Carbon Nanotube/Copper and Carbon Nanofiber/Copper Composites for Microelectronics. Mater. Today Commun. 2017, 11, 123–131. [Google Scholar] [CrossRef]
- Frost, G.; Ladani, L. Development of High-Temperature-Resistant Seed Layer for Electrodeposition of Copper for Microelectronic Applications. J. Electron. Mater. 2020, 49, 1387–1395. [Google Scholar] [CrossRef]
- Marro, J.B.; Darroudi, T.; Okorod, C.A.; Obeng, Y.S.; Richardson, K.C. The influence of pulse plating frequency and duty cycle on the microstructure and stress state of electroplated copper films. Thin Solid Films 2017, 621, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Pasa, A.A.; Schwarzacher, W. Electrodeposition of Thin Films and Multilayers on Silicon. Phys. Status Solid. A 1999, 173, 73–84. [Google Scholar] [CrossRef]
- Kristof, P.; Pritzker, M. Improved Copper Plating Through the Use of Current Pulsing & Ultrasonic Agitation. Plat. Surf. Finish. 1999, 85, 237–240. [Google Scholar]
- Tantavichet, N.; Pritzker, M.D. Effect of plating mode, thiourea and chloride on the morphology of copper deposits produced in acidic sulphate solutions. Electrochim. Acta 2005, 50, 1849–1861. [Google Scholar] [CrossRef]
- Tantavichet, N.; Damronglerda, S.; Chailapakulb, O. Influence of the interaction between chloride and thiourea on copper electrodeposition. Electrochim. Acta 2009, 55, 240–249. [Google Scholar] [CrossRef]
- Baral, A.; Sarangi, C.K.; Tripathy, B.C.; Bhattacharya, I.N.; Subbaiah, T. Copper electrodeposition from sulfate solutions—Effects of selenium. Hydrometallurgy 2014, 146, 8–14. [Google Scholar] [CrossRef]
- Mallik, A.; Ray, B.C. Morphological study of electrodeposited copper under the influence of ultrasound and low temperature. Thin Solid Films 2009, 517, 6612–6616. [Google Scholar] [CrossRef]
- Mallik, A.; Bankoti, A.; Ray, B.C. A study on the modification of Conventional Electrochemical crystallization under sonication: The Phenomena of secondary nucleation. Electrochem. Solid-State Lett. 2009, 12, F46–F49. [Google Scholar] [CrossRef]
- Hakamada, M.; Nakamoto, Y.; Matsumoto, H.; Iwasaki, H.; Chen, Y.; Kusuda, H.; Mabuchi, M. Relationship between hardness and grain size in electrodeposited copper films. Mater. Sci. Eng. A 2007, 457, 120–126. [Google Scholar] [CrossRef]
- Augustin, A.; Huilgol, P.; Udupa, K.R.; Bhat, K.U. Effect of current density during electrodeposition on microstructure and hardness of textured Cu coating in the application of antimicrobial Al touch surface. J. Mech. Behav. Biomed. Mater. 2016, 63, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.S.; Zhou, Y.C.; Long, S.G.; Lu, C. On the intrinsic hardness of a metallic film/substrate system: Indentation size and substrate effects. Int. J. Plast. 2012, 34, 1–11. [Google Scholar] [CrossRef]
- Muller, W.H.; Sbeiti, M.; Worrack, H. The effect of Dwell Time Variation during Microhardness Testing. In Proceedings of the 10th Youth Symposium on Experimental Solid Mechanics, Chemnitz, Germany, 25–28 May 2011. [Google Scholar]
- Fuguo, L.; Jinghui, L.; Bo, C.; Chengpeng, W.; Lei, W. Size effects at dwell stage of micro-indentation for pure aluminum. Rare Met. Mater. Eng. 2014, 12, 2931–2936. [Google Scholar] [CrossRef]
- Chicot, D.; Lesage, J. Absolute hardness of films and coatings. Thin Solid Films 1995, 254, 123–130. [Google Scholar] [CrossRef]
- Hong, S.H.; Kim, K.S.; Kim, Y.M.; Hahn, J.H.; Lee, C.S.; Park, J.H. Characterization of elastic moduli of Cu thin films using nanoindentation technique. Compos. Sci. Technol. 2005, 65, 1401–1408. [Google Scholar] [CrossRef]
- Saha, R.; Xue, Z.; Huang, Y.; Nix, W.D. Indentation of a soft metal film on a hard substrate: Strain gradient hardening effects. J. Mech. Phys. Solids 2011, 49, 1997–2014. [Google Scholar] [CrossRef]
- Zong, Z.; Lou, J.; Soboyejo, O.O.; Elmustafa, A.A; Hammada, F.; Soboyejo, W.O. Indentation size effects in the nano- and micro-hardness of fcc single crystal metals. Mater. Sci. Eng. A 2006, 434, 178–187. [Google Scholar] [CrossRef]
- Elmustafa, A.A.; Stone, D.S. Indentation size effect in polycrystalline F.C.C. metals. Acta Mater. 2002, 50, 3641–3650. [Google Scholar] [CrossRef]
- Beegan, D.; Chowdhury, S.; Laugier, M.T. Modification of composite hardness models to incorporate indentation size effects in thin films. Thin Solid Films 2008, 516, 3813–3817. [Google Scholar] [CrossRef]
- Bull, S.J.; Rickerby, D.S. New developments in the modeling of the hardness and scratch adhesion of thin films. Surf. Coat. Technol. 1990, 42, 149–164. [Google Scholar] [CrossRef]
- Burnett, P.J.; Rickerby, D.S. The mechanical properties of wear-resistant coatings. II: Experimental studies and interpretation of hardness. Thin Solid Films 1987, 148, 51–65. [Google Scholar] [CrossRef]
- Lesage, J.; Chicot, D. A model for hardness determination of thin coatings from standard microindentation test. Surf. Coat. Technol. 2005, 200, 886–889. [Google Scholar] [CrossRef]
- Benarioua, Y.; Mejias, A.; Roudet, F.; Lost, A.; Chicot, D. Hardness-load modeling applied to multilayer galvanized coatings. Surf. Eng. 2016, 32, 194–200. [Google Scholar] [CrossRef]
- Kriger, M.S.; Cook, K.D. Durable gold-coated fused silica capillaries for use in electrospray mass spectrometry. Anal. Chem. 1995, 67, 385–389. [Google Scholar] [CrossRef]
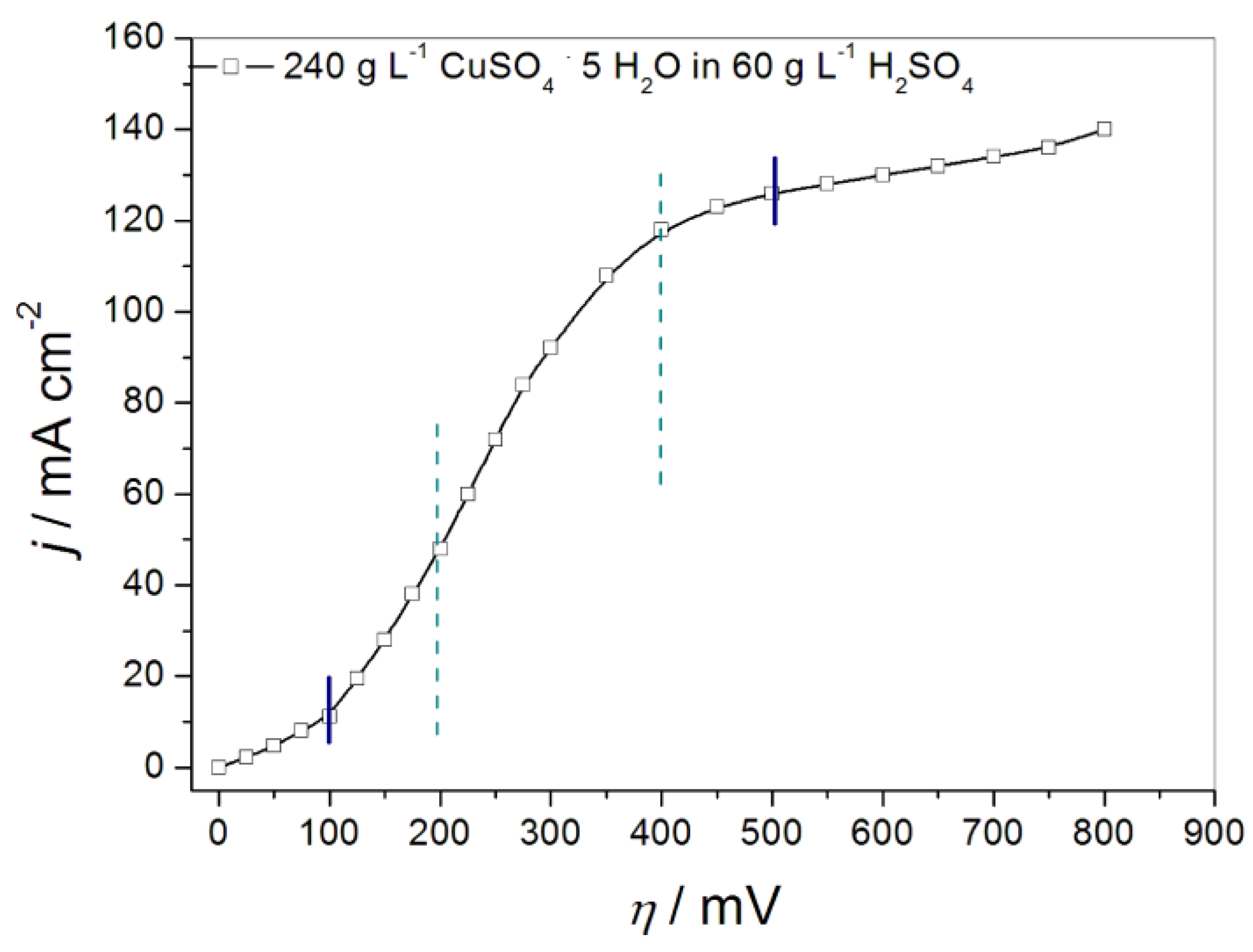
- Popov, K.I.; Živković, P.M.; Jokić, B.; Nikolić, N.D. The shape of the polarization curve and diagnostic criteria for the metal electrodeposition process control. J. Serb. Chem. Soc. 2016, 81, 291–306. [Google Scholar] [CrossRef]
- Berube, L.P.; Esperance, G.L. A Quantitative Method of Determining of the Degree of Texture of Zinc Electrodeposits. J. Electrochem. Soc. 1989, 136, 2314–2315. [Google Scholar] [CrossRef]
- Avramović, L.; Pavlović, M.M.; Maksimović, V.M.; Vuković, M.; Stevanović, J.S.; Bugarin, M.; Nikolić, N.D. Comparative Morphological and Crystallographic Analysis of Electrochemically- and Chemically-Produced Silver Powder Particles. Metals 2017, 7, 160. [Google Scholar] [CrossRef]
- Avramović, L.; Maksimović, V.M.; Baščarević, Z.; Ignjatović, N.; Bugarin, M.; Marković, R.; Nikolić, N.D. Influence of the Shape of Copper Powder Particles on the Crystal Structure and Some Decisive Characteristics of the Metal Powders. Metals 2019, 9, 56. [Google Scholar] [CrossRef]
- Popov, K.I.; Maksimović, M.D. Theory of the Effect of Electrodeposition at a Periodically Changing Rate on the Morphology of Metal Deposits. In Modern Aspects of Electrochemistry; Conway, B.E., Bockris, J.O’M., White, R.E., Eds.; Plenum Press: New York, NY, USA, 1989; Volume 19, pp. 193–250. [Google Scholar] [CrossRef]
- Popov, K.I.; Djokić, S.S.; Nikolić, N.D.; Jović, V.D. Morphology of Electrochemically and Chemically Deposited Metals; Springer International Publishing: New York, UY, USA, 2016. [Google Scholar]
- Li, H.; Bradt, R.C. Knoop microhardness anisotropy of single-crystal LaB6. Mater. Sci. Eng. A 1991, 142, 51–61. [Google Scholar] [CrossRef]
- Lamovec, J.; Jović, V.; Mladenović, I.; Stojanović, D.; Kojović, A.; Radojević, V. Indentation behavior of soft film on hard substrate composite system type. Mater. Prot. 2015, 56, 269–277. [Google Scholar]
- Buckle, H. The Science of Hardness Testing and Its Research Applications; Westbrook, J.W., Conrad, H., Eds.; American Society for Metals: Metals Park, OH, USA, 1973; p. 453. [Google Scholar]
- Nikolić, N.D.; Branković, G.; Maksimović, V.M. Morphology and internal structure of copper deposits electrodeposited by the pulsating current regime in the hydrogen co-deposition range. J. Solid State Electrochem. 2012, 16, 321–328. [Google Scholar] [CrossRef]
- Zhang, J.M.; Ma, F.; Xu, K.W. Calculation of the surface energy of FCC metals with modified embedded-atom method. Appl. Surf. Sci. 2004, 229, 34–42. [Google Scholar] [CrossRef]
- Wang, S.G.; Tian, E.K.; Lung, C.W. Surface energy of arbitrary crystal plane of bcc and fcc metals. J. Phys. Chem. Solids 2000, 61, 1295–1300. [Google Scholar] [CrossRef]
- Reddy, A.K.; Gamboa-Aldeco, M.E. Modern Electrochemistry 2A. In Fundamentals of Electrodics; Springer: Boston, MA, USA, 2000; p. 1333. [Google Scholar] [CrossRef]
- Nikolić, N.D.; Maksimović, V.M.; Branković, G. Morphological and crystallographic characteristics of electrodeposited lead from the concentrated electrolyte. RSC Adv. 2013, 3, 7466–7471. [Google Scholar] [CrossRef]
- Tao, S.; Li, D.Y. Trbological, mechanical and electrochemical properties of nanocrystalline copper deposits produced by pulse electrodeposition. Nanotechnology 2006, 17, 65–78. [Google Scholar] [CrossRef]
- Ibanez, A.; Fatas, E. Mechanical and structural properties of electrodeposited copper and their relation with the electrodeposition parameters. Surf. Coat. Technol. 2005, 191, 7–16. [Google Scholar] [CrossRef]
- Hall, E.O. The Deformation and Ageing of Mild Steel: III Discussion of Results. Proc. Phys. Soc. Sec. B 1951, 64, 747–753. [Google Scholar] [CrossRef]
- Petch, N.J. The cleavage strength of polycrystals. J. Iron Steel Inst. 1953, 174, 25–28. [Google Scholar]
- Lesage, J.; Pertuz, A.; Puchi-Cabrera, E.S.; Chicot, D. A model to determine the surface hardness of thin films from standard micro-indentation tests. Thin Solid Films 2006, 497, 232–238. [Google Scholar] [CrossRef]
- Lesage, J.; Pertuz, A.; Chicot, D. A New Method to Determine the Hardness of Thin Films. Matéria 2004, 9, 13–22. [Google Scholar]
- Nikolić, N.D.; Rakočević, Z.; Popov, K.I. Structural Characteristics of Bright Copper Surfaces. J. Electroanal. Chem. 2001, 514, 56–66. [Google Scholar] [CrossRef]
- Lamovec, J.; Jović, V.; Ranđelović, D.; Aleksić, R.; Radojević, V. Analysis of the composite and film hardness of electrodeposited nickel coatings on different substrates. Thin Solid Films 2008, 516, 8646–8654. [Google Scholar] [CrossRef]
- Callister, W.D., Jr. Fundamentals of Materials Science and Engineering; An Interactive E-text (5th Edition); John Wiley & Sons, Inc.: New York, NY, USA, 2001; p. S-166. [Google Scholar]
- Atkinson, M. Calculation of characteristic macro-hardness from low-load indentation test. Mater. Sci. Eng. A 1995, 197, 165–169. [Google Scholar] [CrossRef]
- Popov, K.I.; Pavlović, M.G. Electrodeposition of Metal Powders with Controlled Grain Size and Morphology. In Modern Aspects of Electrochemistry; White, R.E., Bockris, J.O’M., Conway, B.E., Eds.; Plenum Press: New York, NY, USA, 1993; Volume 24, pp. 299–391. [Google Scholar]


| Number of Samples | jA/mA·cm−2 | tc/ms | tp/ms | p | jav/mA·cm−2 | ν/Hz |
|---|---|---|---|---|---|---|
| 1 | 100 | 5 | 5 | 1 | 50 | 100 |
| 2 | 100 | 5 | 7.5 | 1.5 | 40 | 80 |
| 3 | 100 | 5 | 15 | 3 | 25 | 50 |
| 4 | 100 | 5 | 28.3 | 5.66 | 15 | 30 |
| 5 | 120 | 5 | 5 | 1 | 60 | 100 |
| 6 | 140 | 5 | 5 | 1 | 70 | 100 |
| Plane | R (in %) | Rs | TC | RTC (in %) | |||
|---|---|---|---|---|---|---|---|
| (hkl) | R30 Hz | R50 Hz | (in %) | TC30 Hz | TC50 Hz | RTC30 Hz | RTC50 Hz |
| (111) | 4.0 | 58.6 | 54.6 | 0.073 | 1.07 | 0.88 | 22.8 |
| (200) | 11.2 | 6.9 | 25.1 | 0.45 | 0.27 | 5.4 | 5.8 |
| (220) | 81.9 | 22.2 | 10.9 | 7.5 | 2.04 | 90 | 43.5 |
| (311) | 2.9 | 12.3 | 9.4 | 0.31 | 1.31 | 3.72 | 27.9 |
| Plane | R (in %) | Rs | TC | RTC (in %) | |||
|---|---|---|---|---|---|---|---|
| (hkl) | R80 Hz | R100 Hz | (in %) | TC80 Hz | TC100 Hz | RTC80 Hz | RTC100 Hz |
| (111) | 67.3 | 72.7 | 54.6 | 1.23 | 1.33 | 28.2 | 35.9 |
| (200) | 5.0 | 7.1 | 25.1 | 0.20 | 0.28 | 4.6 | 7.6 |
| (220) | 3.6 | 4.0 | 10.9 | 0.33 | 0.37 | 7.6 | 10 |
| (311) | 24.1 | 16.2 | 9.4 | 2.6 | 1.72 | 59.6 | 46.5 |
| Plane (hkl) | R (in %) | Rs | TC | RTC (in %) | |||
|---|---|---|---|---|---|---|---|
| R120 | R140 | (in %) | TC120 | TC140 | RTC120 | RTC140 | |
| (111) | 86.1 | 95.8 | 54.6 | 1.58 | 1.75 | 54.3 | 83.3 |
| (200) | 2.0 | 1.26 | 25.1 | 0.080 | 0.050 | 2.7 | 2.4 |
| (220) | 1.3 | 0.76 | 10.9 | 0.12 | 0.070 | 4.1 | 3.3 |
| (311) | 10.6 | 2.18 | 9.4 | 1.13 | 0.23 | 38.9 | 11.0 |
| Number of Samples | jav/mA·cm−2 | ν/Hz | jA/mA cm−2 | δ/μm | Ra/nm |
|---|---|---|---|---|---|
| 1 | 50 | 100 | 100 | 40 | 169.9 |
| 2 | 40 | 80 | 100 | 40 | 385 |
| 3 | 25 | 50 | 100 | 40 | 470.5 |
| 4 | 15 | 30 | 100 | 40 | 507.3 |
| 5 | 60 | 100 | 120 | 40 | 237 |
| 6 | 70 | 100 | 140 | 40 | 229.1 |
| 7 | 50 | 100 | 100 | 10 | 52.42 |
| 8 | 50 | 100 | 100 | 20 | 101.5 |
| 9 | 50 | 100 | 100 | 60 | 286.3 |
| The PC | ν/Hz | jA/mA·cm−2 | ||||
|---|---|---|---|---|---|---|
| Regime | 30 | 50 | 80 | 100 | 120 | 140 |
| ηA/mV | 99–135 | 135–200 | 210–290 | 290–350 | 320–425 | 380–500 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mladenović, I.O.; Lamovec, J.S.; Vasiljević Radović, D.G.; Vasilić, R.; Radojević, V.J.; Nikolić, N.D. Morphology, Structure and Mechanical Properties of Copper Coatings Electrodeposited by Pulsating Current (PC) Regime on Si(111). Metals 2020, 10, 488. https://doi.org/10.3390/met10040488
Mladenović IO, Lamovec JS, Vasiljević Radović DG, Vasilić R, Radojević VJ, Nikolić ND. Morphology, Structure and Mechanical Properties of Copper Coatings Electrodeposited by Pulsating Current (PC) Regime on Si(111). Metals. 2020; 10(4):488. https://doi.org/10.3390/met10040488
Chicago/Turabian StyleMladenović, Ivana O., Jelena S. Lamovec, Dana G. Vasiljević Radović, Rastko Vasilić, Vesna J. Radojević, and Nebojša D. Nikolić. 2020. "Morphology, Structure and Mechanical Properties of Copper Coatings Electrodeposited by Pulsating Current (PC) Regime on Si(111)" Metals 10, no. 4: 488. https://doi.org/10.3390/met10040488
APA StyleMladenović, I. O., Lamovec, J. S., Vasiljević Radović, D. G., Vasilić, R., Radojević, V. J., & Nikolić, N. D. (2020). Morphology, Structure and Mechanical Properties of Copper Coatings Electrodeposited by Pulsating Current (PC) Regime on Si(111). Metals, 10(4), 488. https://doi.org/10.3390/met10040488