2. Materials and Methods
Test specimens were printed using Electro-Optical Systems (EOS) MaragingSteel MS1 powder [
22] in an EOS M400 printer (EOS, Krailling, Germany). The powder had the composition of Maraging Steel 300 (18Ni-300, US; 1.2709, European; X3NiCoMoTi 19-9-5, German). The printer parameters are listed in
Table 1. Three groups of samples were printed in three different orientations (group names given in parentheses): in the XY-plane of the print stage (H); perpendicular to the print stage (V), and in a 45° direction relative to the print stage (45). Printed specimens were then machined into tensile testing specimens following the ASTM standard E8/E8M-16a [
23]. Completed samples were then tested “as-printed (AP)” or heat-treated following several different treatment conditions: 490 °C for six hours (HT490), 600 °C for six hours (HT600), or 900 °C for 45 min (HT900). All heat-treatments were completed in an argon atmosphere and air-cooled (with between 30–60 min cool-down time). Printed specimens were compared to 18Ni-300 maraging steel processed by the vacuum induction melting-vacuum arc remelting (VIM-VAR) method (MSC Industrial Supply Co., Cleveland, OH, USA), that was then machined following ASTM E8/E8M, hereafter referred to as CNC.
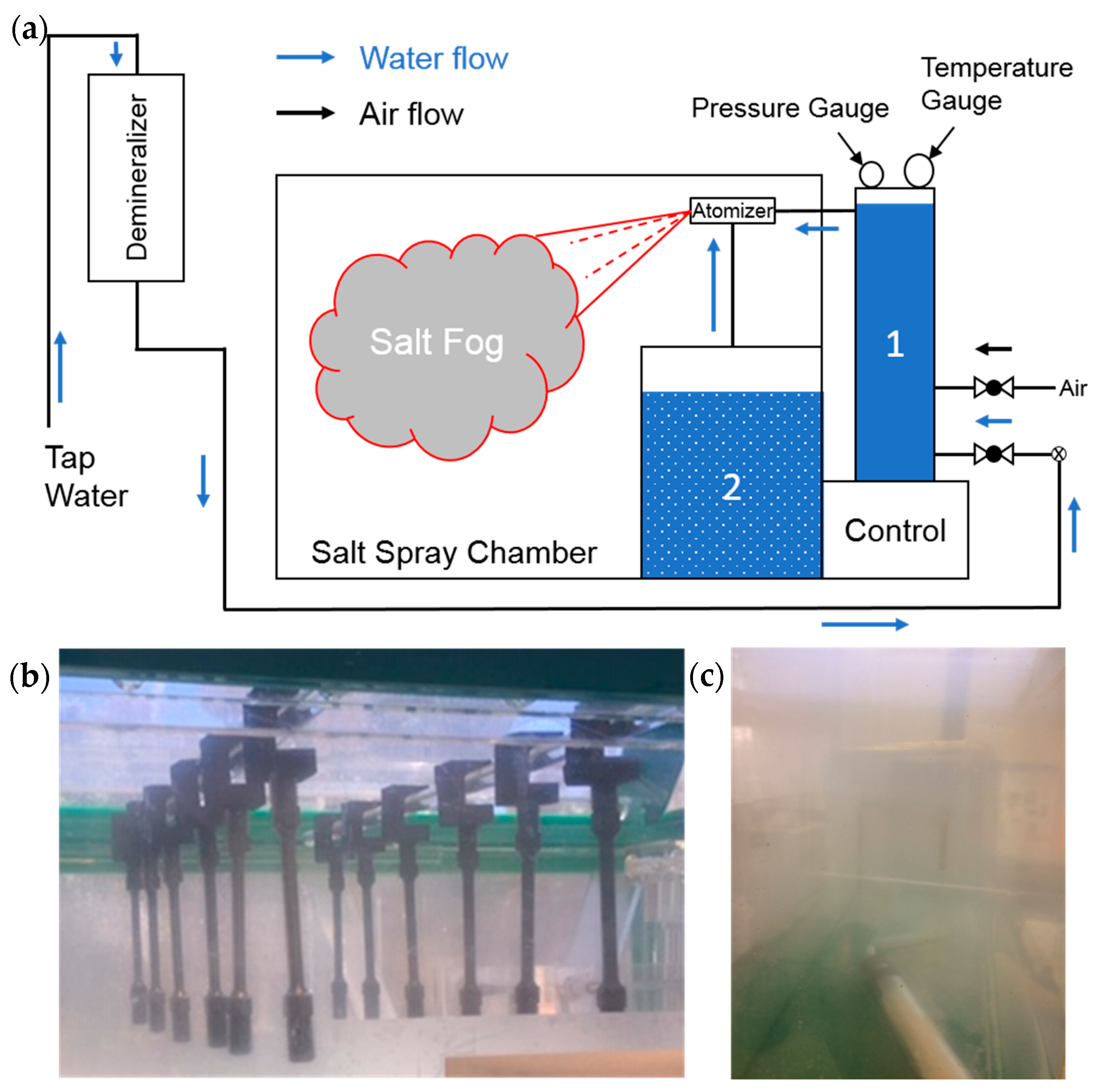
An MX-9204 Salt Spray Chamber (Associated Environmental Systems, Acton, MA, USA) was used for exposing samples to a simulated marine environment (a schematic is shown in
Figure 1a). First, a solution of 3.5 g NaCl per 100 mL of distilled water was prepared to approximately simulate the salinity found on average in the world’s oceans [
24]. The salt solution was then added to the salt solution reservoir. Compressed air was pumped into the saturation tower where pressure was maintained between 82.7–103 kPa (12–15 psi) while temperature was held between 41–43 °C during the test run. Tap water was pumped through a demineralizer before flowing into the saturation tower. Demineralized water was then routed to an atomizer. Saltwater from the reservoir was siphoned through a filter assembly (containing a nylon mesh strainer) up to the atomizer. The atomizer mixed and atomized the two water sources and sprayed the mixture into the salt spray chamber.
One end of the samples was threaded in custom-printed sample holders made of either carbon fiber reinforced nylon (CarbonX™ by 3DX Technology, Grand Rapids, MI, USA) or polyethylene terephthalate (Esun, Shenzhen, China). These were hung in the salt spray chamber.
Figure 1b shows samples placed in the salt spray chamber (colloquially “salt fog”) hanging down from printed sample holders. Salt fog is visible in
Figure 1c. Samples were exposed for three weeks in total (approximately 500 h).
The naming scheme for samples was chosen based on whether samples were exposed to a salt fog environment (simulated marine environment) or not, their printing orientation, and heat-treatment (or lack thereof). For example; samples exposed to a salt fog environment for three weeks, printed perpendicular to the stage, and heat-treated at 600 °C for six hours are called 3V600 (time of exposure in weeks, print orientation, and temperature of heat-treatment in °C). Another example; samples with no exposure, printed horizontally and with no heat-treatment, are called 0HAP.
Tensile test specimens were then installed in a tensile tester (Instron 5982 Tensile Tester, Norwood, MA, USA). The tensile test speed for all samples was 2 mm/min. Fractured samples were then prepared for further analysis using standard metallurgical processing involving cutting, mounting, polishing, and etching. Microhardness measurements were completed on a Durascan Microhardness Tester (Struers, Ballerup, Denmark).
Optical imaging of samples was performed on an Epiphot 200 reflective optical microscope (Nikon, Tokyo, Japan). Microstructural characterization was done on mounted specimens using a Neon 40 Dual-beam (Zeiss, Oberkochen, Germany) scanning electron microscope (SEM). Microanalysis was conducted by energy-dispersive spectroscopy (EDS) using an Octane Plus EDS detector (EDAX, Mahwah, NJ, USA). Samples were prepared for SEM/EDS analysis by first mounting specimens in epoxy mounts. Mounted samples were then placed in an automatic polisher (Buehler, Lake Bluff, IL, USA) and ground using 300-, 600-, and 1200-grit paper at a wheel speed of 26.2 rad/s (250 RPM) and a force of 22.2 N (5.0 lbs. f) for 15–60 min at each step. After grinding, samples were polished in the sample automatic polisher using long-napped synthetic polishing cloth (Buehler Microcloth PSA) and polishing fluid, 1 µm alumina suspension (Buehler MicroPolish). Prior to SEM microstructural analysis, samples were etched with a Nital etching solution (methanol was the alcohol in this case).
3. Results and Discussion
Raw powders used for printing consisted of mostly spherical particles with sizes ranging from 1 µm up to 84 µm and an average size of 14 ± 11 µm. A few of the particles observed in the SEM were irregularly shaped due to being formed by agglomerates that sintered. Those types of particulates are typically observed in powders produced by atomization. The composition of the powders, printed and heat-treated (at 490 °C) parts were studied using EDS. The elements found, in wt%, in the powders and printed parts are shown in
Table 2. The powder samples were rich in Ni and Co, but low in Mo as compared to the composition of 18Ni-300 found in the literature [
6,
22,
25]. The printed parts matched the expected composition. It is worth noting that EDS is only a semi-quantitative method of analysis that renders data from localized sections rather than a bulk analysis.
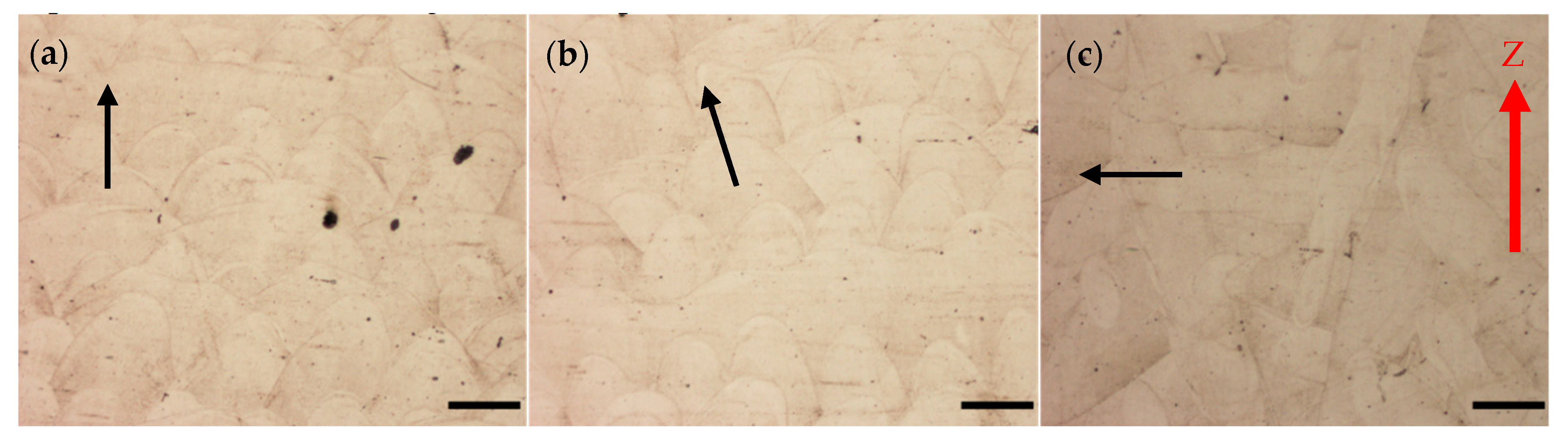
Printed metals exhibit a unique microstructure as a result of SLM printing in the form of track marks as seen in
Figure 2. The direction of the track marks indicates the orientation of a printed sample in relation to the build plate. Unaged maraging steel printed in the V (
Figure 2a), 45° (
Figure 2b), and H (
Figure 2c) directions. Arrows are placed in the figures to indicate the general direction of the apex of laser track marks. During SLM printing, the high-power laser melts the powders deposited on the build plate. As the laser passes any one point in the build plate, the melted powders begin to cool and fuse together. Once the printer completes one layer, the next layer of powder is deposited, and the laser begins its next pass.
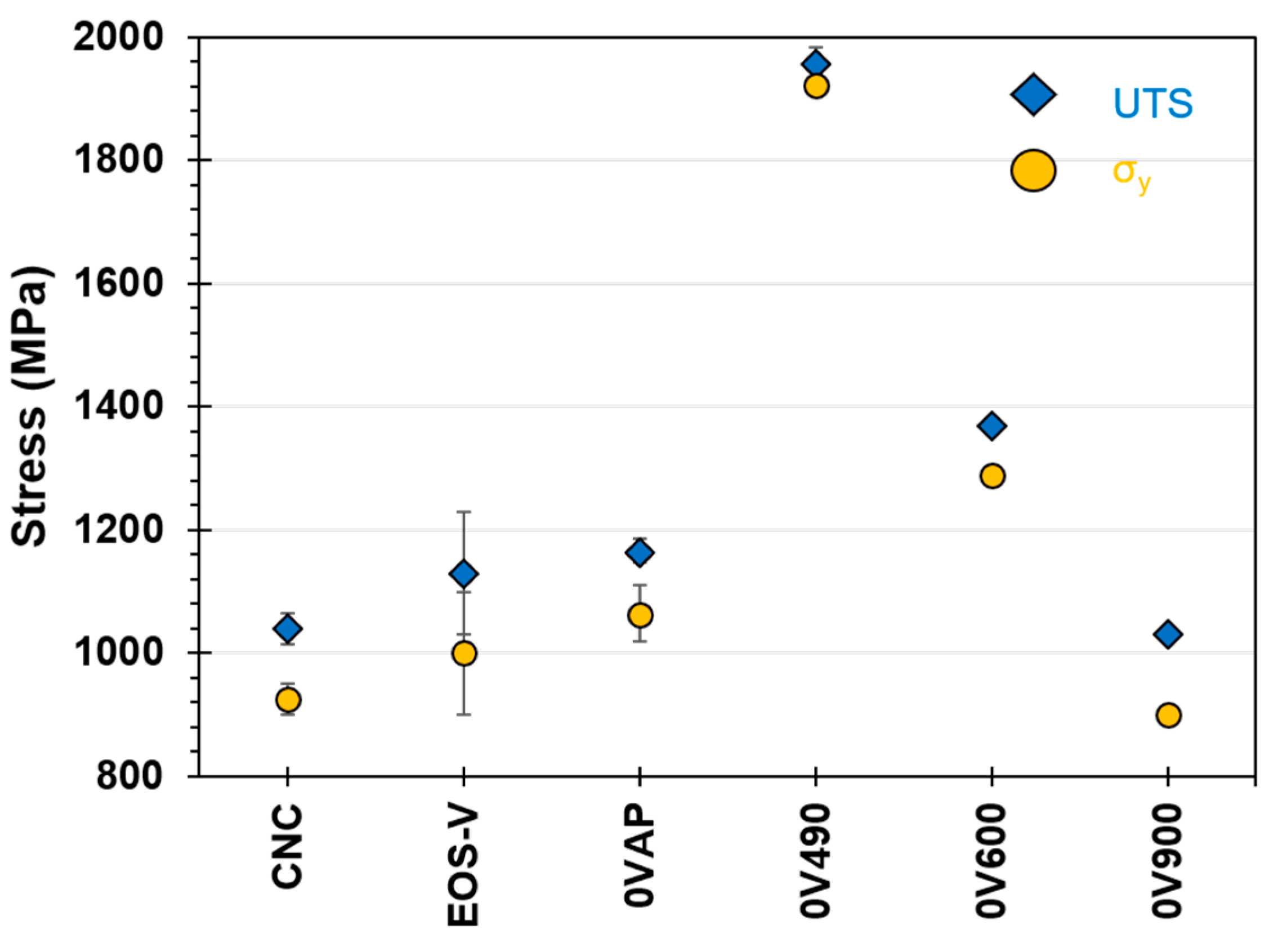
Shown in
Figure 3 are measurements of the ultimate tensile strength (UTS) of as-printed and heat-treated maraging steel with a V orientation. The as-printed samples met EOS benchmark values and exceeded the values of traditionally fabricated CNC parts measured here. Samples aged at 490 °C (0V490) saw an increase in UTS close to 2000 MPa. Tensile strength decreased below this maximum when printed samples were heat-treated at higher temperatures. At 600 °C, the 0V600 sample exhibited a UTS of 1370 MPa. When heat-treated at 900 °C, the 0V900 sample showed a further decrease in UTS down to 950 MPa; a value which was lower than UTS for as-printed or CNC samples. The behavior of yield strength closely matched the behavior of UTS for all samples.
These results are similar to those seen by Yasa and Kempen [
5,
6]. The tensile properties of as-printed 18Ni-300 exceeded those of wrought maraging steel by several hundred MPa while Young’s modulus and ductility were comparable and toughness values were reduced. The microstructure of printed maraging steel was found to be different from the traditional microstructure. When printed samples were aged, hardness and tensile strength were comparable to aged wrought maraging steel while ductility and toughness were reduced. Aged or heat-treated maraging steel properties closely matched those of aged wrought or other traditionally fabricated maraging steel [
6].
Figure 3 shows the same, that printed 18Ni-300 exhibited higher strength than CNC 18Ni-300 steel.
Strength is reduced when the heat-treatment temperature exceeds 500 °C. Maraging steel is precipitation hardened to obtain high-strength. In traditionally fabricated maraging steel, lath martensite will form during cooling from an initial melt. Age hardening causes the transformation of martensite to austenite if the temperature is increased above the austenite start temperature, A
S. How high the temperature is above A
S and how rapid the cooling will determine the amount of austenite formation and whether precipitates form during cooling. If the aging temperature is set between 455 °C and 510 °C, the transformation to austenite is slow enough to allow precipitates to form out of solution [
10]. At temperatures higher than 510 °C, the transformation rate of martensite to austenite is increased and precipitates do not form.
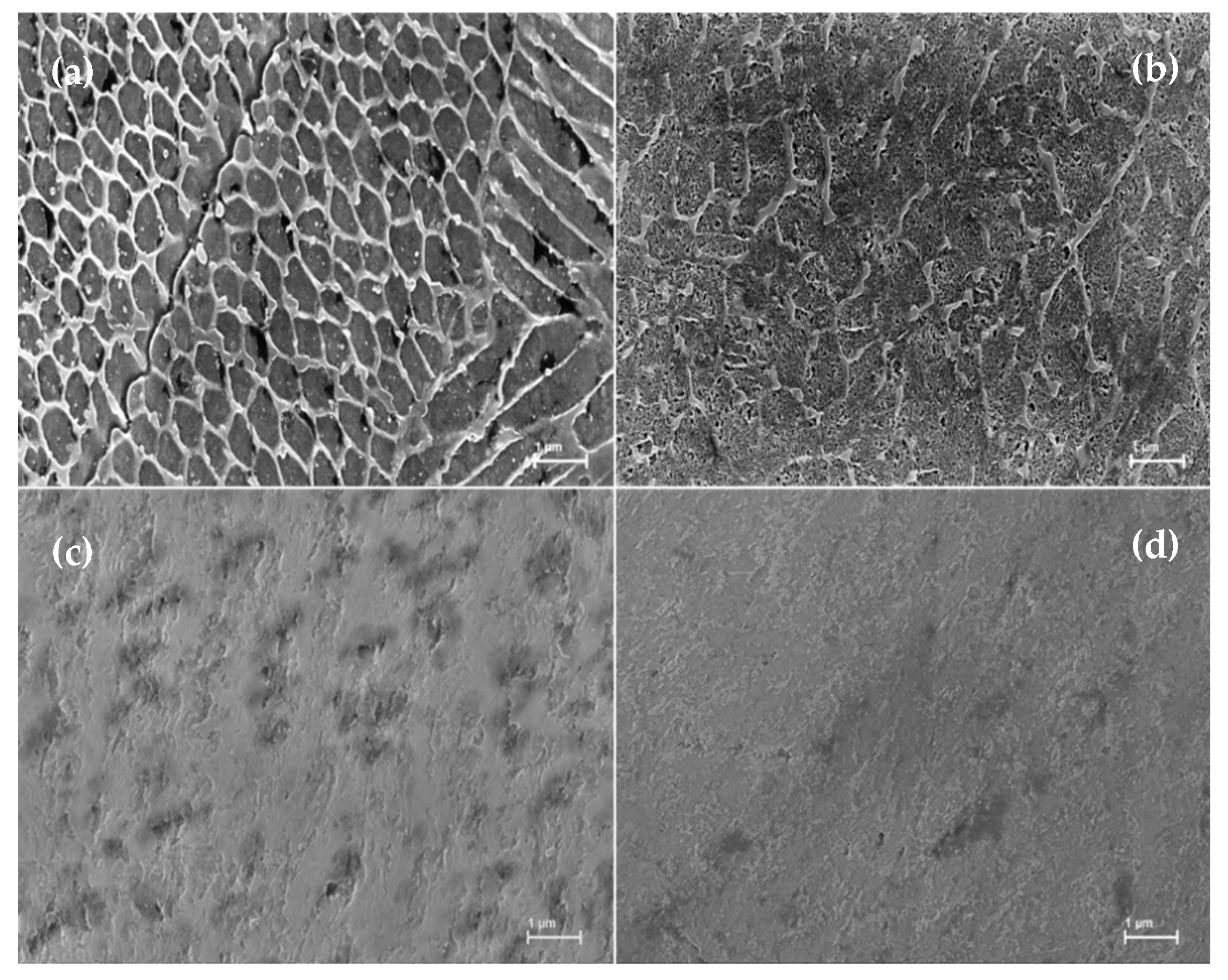
Microstructural analysis of vertically printed specimens with or without heat-treatment is shown in
Figure 4. The intercellular structure revealed in 0VAP samples (
Figure 4a) is believed to be caused by the rapid solidification of material following laser re-melting. As-printed samples exhibited a multi “domain” structure made of a combination of fine dendrites with short secondary arms, cellular regions, and columnar regions similar to the ones previously reported by [
5,
6,
17,
18,
19]. The onset of laser-induced rapid solidification prevents the formation of lath martensite [
6,
18] usually associated with maraging steel. This cellular structure is responsible for the improved strength of as-printed maraging steel as compared to traditionally fabricated maraging steel. Break-up of the cellular structure is seen in a sample of 0V490 (
Figure 4b). This is caused by the formation of austenite within the cells and through the cell boundaries in accordance with prior studies [
17,
19]. Further removal of the cells and enlarging of the austenite phase occurred with 600 °C and 900 °C heat-treatments as seen in
Figure 4c,d, which are images of 0V600 and 0V900, respectively.
The printing orientation also affected the tensile and yield strength of maraging steel. Shown in
Figure 5, samples printed in the H or 45° orientations exhibited increased tensile strength over V-printed samples. In printed samples where layering is perpendicular to the direction of the applied load, failure occurs before samples printed such that layers are parallel to the loading direction (H). Tan et al. found that similar to observations in this paper, the horizontally printed maraging steel exhibited higher tensile strength then vertically printed specimens [
21].
The changes induced in tensile strength after heat-treatment of V samples were also seen in H and 45° printed tensile specimens. Seen in
Figure 6, tensile strength increased for samples printed in all three orientations after the 600 °C heat-treatment. Then the tensile strength decreased for all print orientations upon the 900 °C heat-treatment.
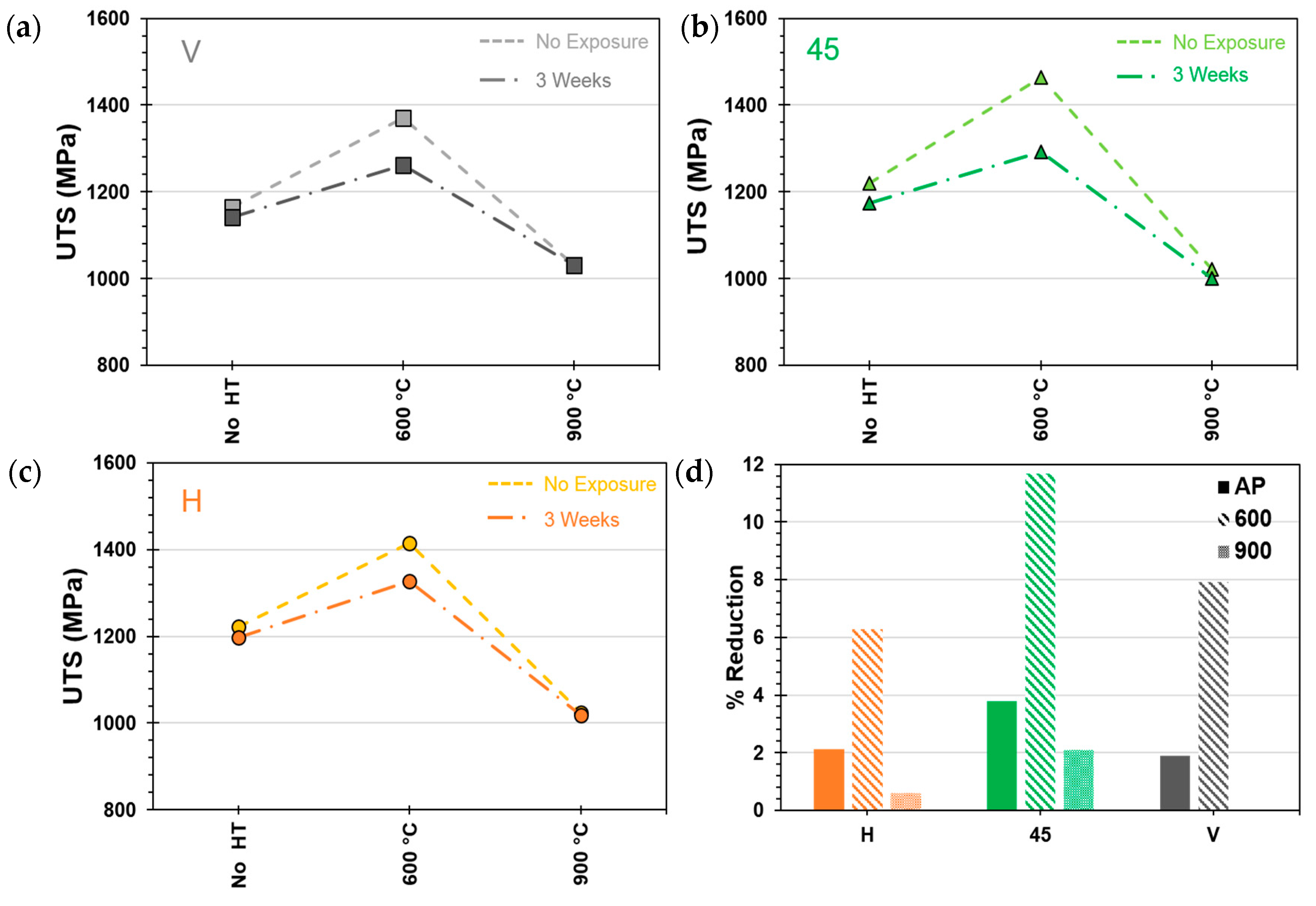
Samples placed in the salt fog for three weeks (~500 h) of simulated marine exposure experienced a decrease in tensile strength.
Figure 7 shows the change in tensile strength of samples before and after exposure. Testing results for V-printed samples are shown in
Figure 7a; 45°-printed samples in
Figure 7b; and H-printed samples in
Figure 7c. Surface corrosion degraded the tensile strength between 1.9% and 3.7% for as-printed specimens (1.9% for 3VAP and 3.7% for 345AP). HT600 samples experienced the highest reduction in strength with 6.3% for 3H600 and 11.7% reduction for the 345,600 sample. The smallest reduction in strength occurred for HT900 samples with a negligible reduction in V samples and a 2.1% reduction in UTS for the 345,900 samples.
Figure 7d presents the relative percent changes in UTS of all samples after 500 h of exposure. As will be seen later, corrosion penetration in samples causes the formation of cracking which may otherwise not appear in pristine samples. This may explain the reduction in strength of samples upon exposure to a marine environment.
As evidenced in
Figure 7, the as-printed samples (printed in all three orientations) exhibited relatively good corrosion resistance and little reduction in strength after exposure. Samples heat-treated at 600 °C saw larger reductions in strength after exposure. Samples heat-treated to 900 °C experienced a softening after cooling but also show better corrosion resistance. This was seen by a smaller reduction in strength after exposure as compared to the as-printed samples.
Figure 7d summarizes these results.
It was also seen in
Figure 7 that samples heat-treated at 600 °C were more affected by corrosion than as-printed samples in terms of tensile strength. Heat treatment at 490 °C also increased the susceptibility to corrosion in aged samples. Microhardness tests were conducted on AP and HT490 samples and shown in
Figure 8. Comparisons of hardness between 0VAP and 3VAP (bottom two sets of data) and between 0V490 and 3V490 (top two sets of data) are shown. The locations of hardness measurements of the 0V490 sample are shown in the inset. Similar positions were chosen for the hardness measurements conducted on the other samples where hardness was measured.
There was no difference in hardness away from the surface between as-printed specimens before and after exposure, 0VAP and 3VAP. There was a measurable difference (close to 10%) in the near-surface hardness between heat-treated at 490 °C samples 0V490 and 3V490. Results of hardness tests align with the results of tensile testing seen in the increased rate of decline of both in samples after exposure. Therefore, heat-treatment between 490 °C and 600 °C led to parts with a greater susceptibility to corrosion. Such an outcome is in agreement with previous reports [
26,
27,
28,
29] and it is believed to be a result of the formation of intermetallic precipitates. It has been shown that the existence of austenite with high Ni content leads to preferential pitting corrosion as shown in duplex stainless steels used for welding [
30]. Recall, during heat-treatments, if the temperature is higher than A
s, austenite reversion will take place [
10].
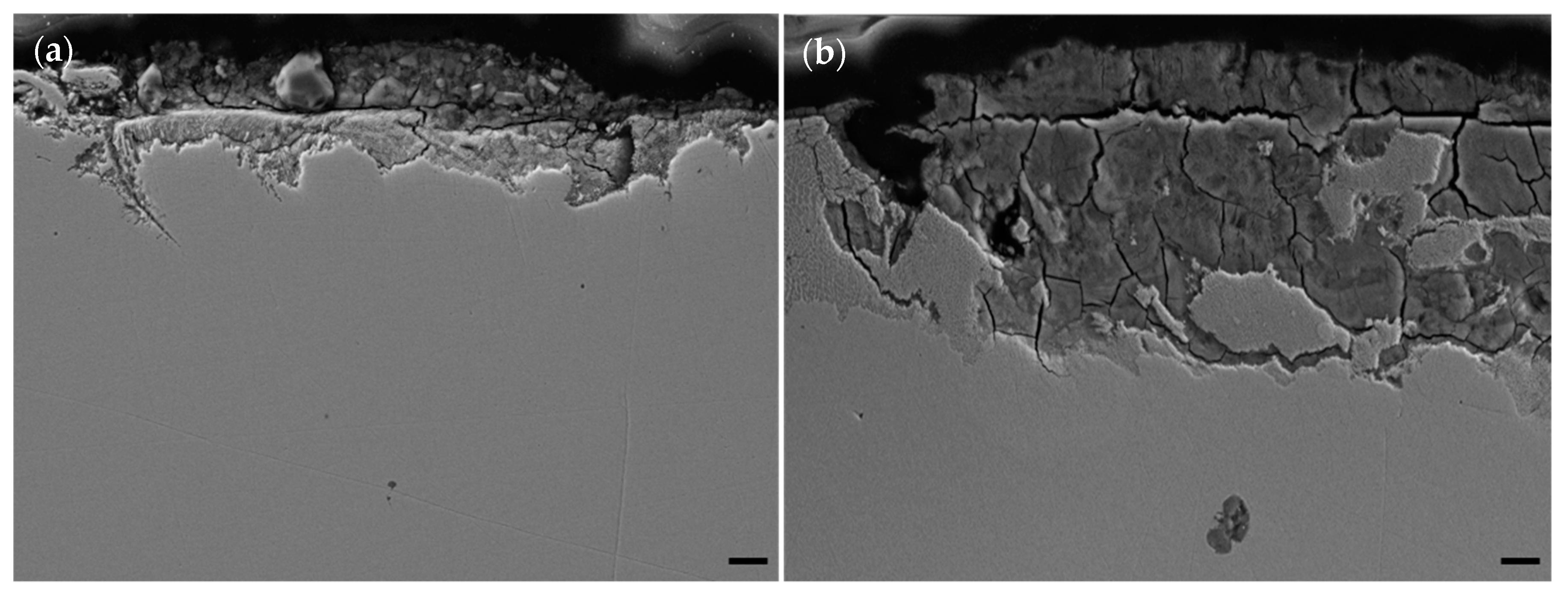
Microstructural analysis revealed the differences in susceptibility to general corrosion depending on heat-treatment (or lack thereof). SEM analysis of V printed maraging steel samples both unaged (
Figure 9a) and heat-treated at 600 °C for six hours (
Figure 9b) revealed pitting and corrosion by-products.
Figure 9 shows the differences in corrosion susceptibility between as-printed and heat-treated maraging steel. Note the deeper penetration of corrosion in the aged sample. Observed on the left-hand side of
Figure 9b, is a contrast difference between the base metal and corrosion affected metal. The contrast difference is due to exposure to the underlying microstructure. In effect, the environment in the salt spray chamber etched part of the metal near the surface. The increased corrosion susceptibility of heat-treated (490 °C and 600 °C) samples is likely due to the existence of precipitates and multiple phases. For samples heat-treated at 900 °C, corrosion susceptibility was like as-printed samples due to the existence of a one-phase only (austenite in this case).
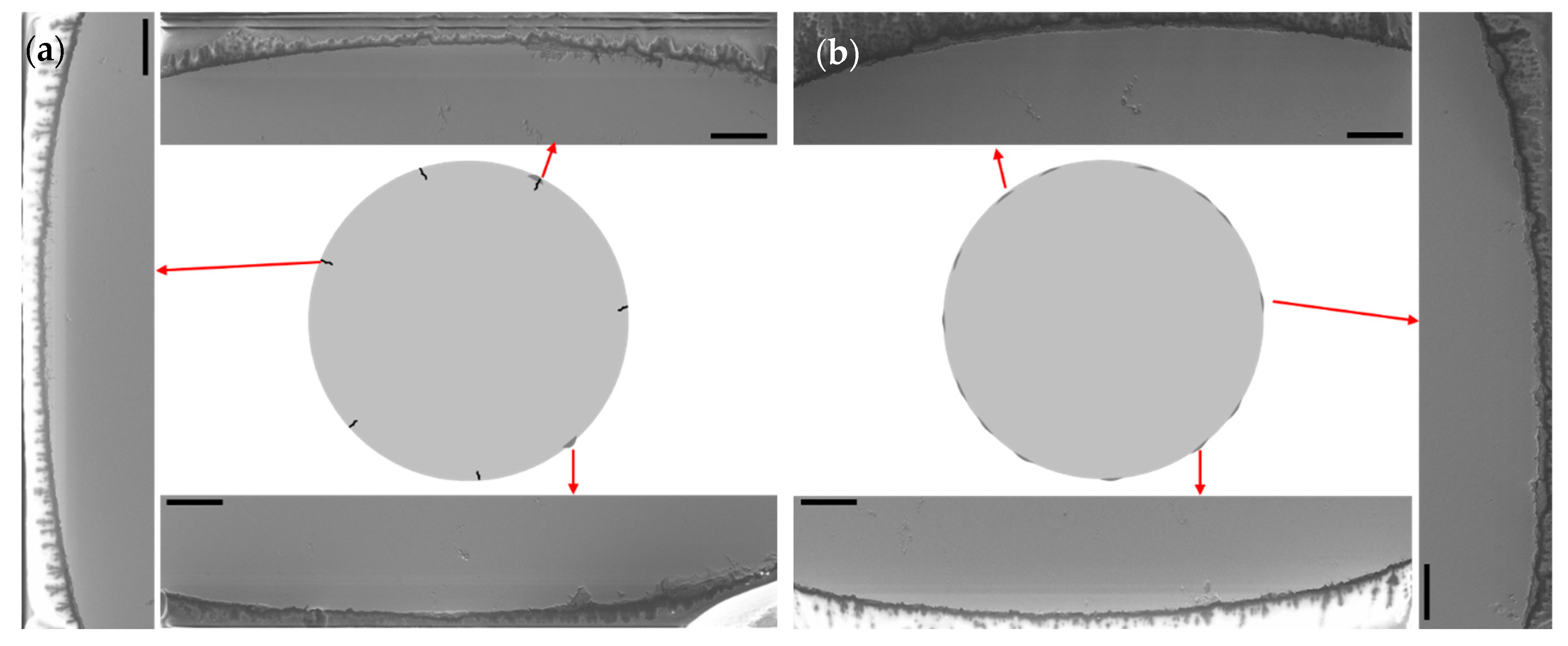
Corrosion induced a 4.7% decrease in the ultimate tensile strength of the as-fabricated CNC sample. This decrease was greater than in the as-printed specimens with decreases in UTS of 1.8% (between 0VAP and 3VAP), 2.1% (0HAP and 3HAP), and 3.8% (045AP and 345AP). After tensile testing, cross-sections were cut from corroded as-printed and CNC samples. Electron microscopy was performed on the surface of the cross-sections where corrosion formed. Microscopy of the exposed samples showed a greater volume of pitting corrosion in the surface of the as-printed specimens (
Figure 10a) as compared to the as-fabricated CNC specimens (
Figure 10b). Microscopy observations indicated a larger number of surface cracks in as-printed samples compared to the CNC sample.
Despite the extent of surface corrosion features identified in the SEM study in the as-printed parts, those features did not seem to be greater than the samples manufactured by traditional methods or have affected the overall UTS performance under the conditions explored herein. The 3D printed aged specimens; however, seemed greatly affected indicating heat-treated specimens should be protected by coatings or their exposure to marine environments be limited. It is worth noting that the surface cracks found in the additively manufactured parts could be exacerbated with longer exposure times, application of stress or more corrosive environments, thus, future studies should include a wider set of those variables.