Investigation of Decarburization Behaviour during the Sintering of Metal Injection Moulded 420 Stainless Steel
Abstract
1. Introduction
2. Experimental Procedures
3. Results
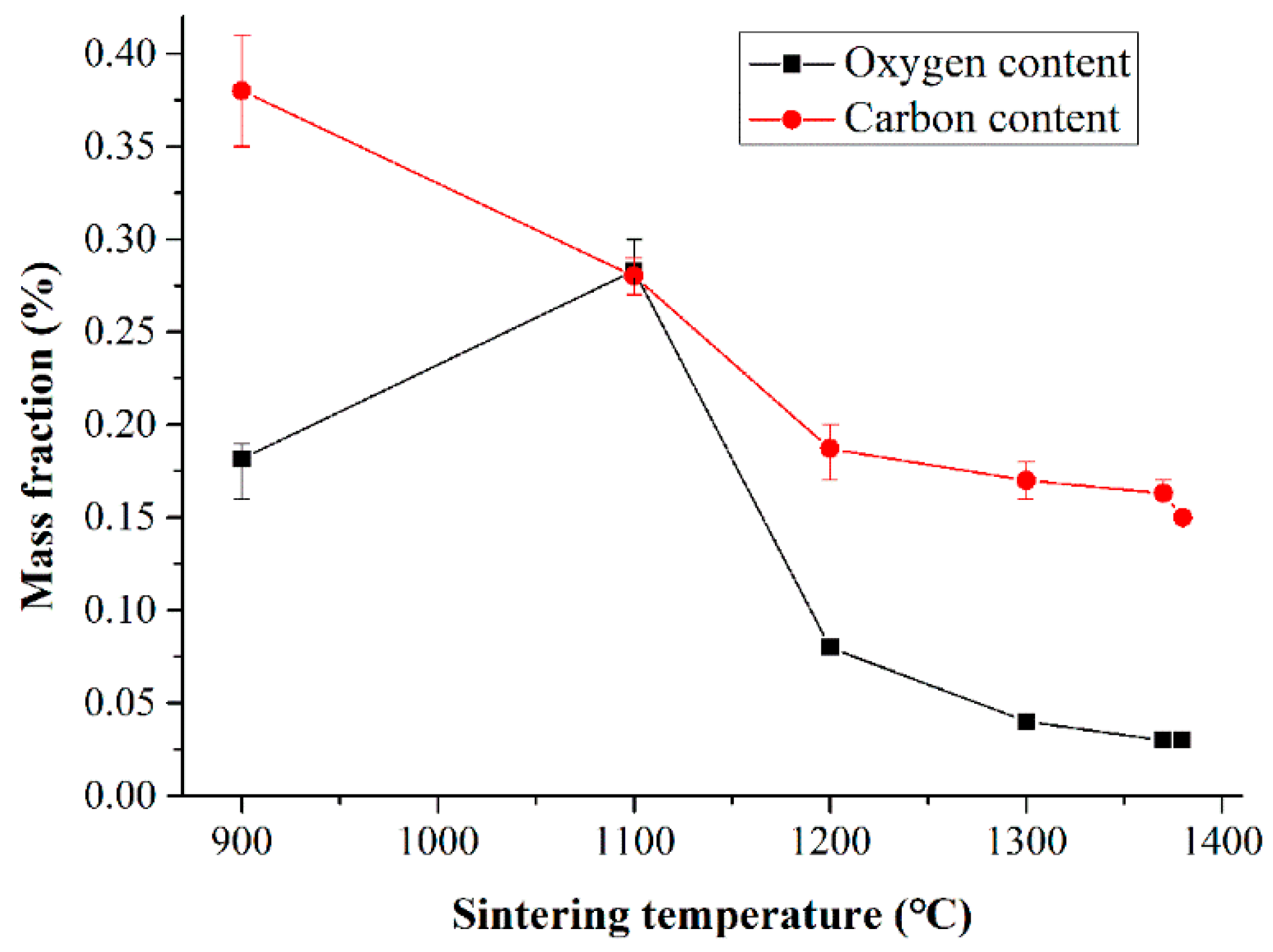
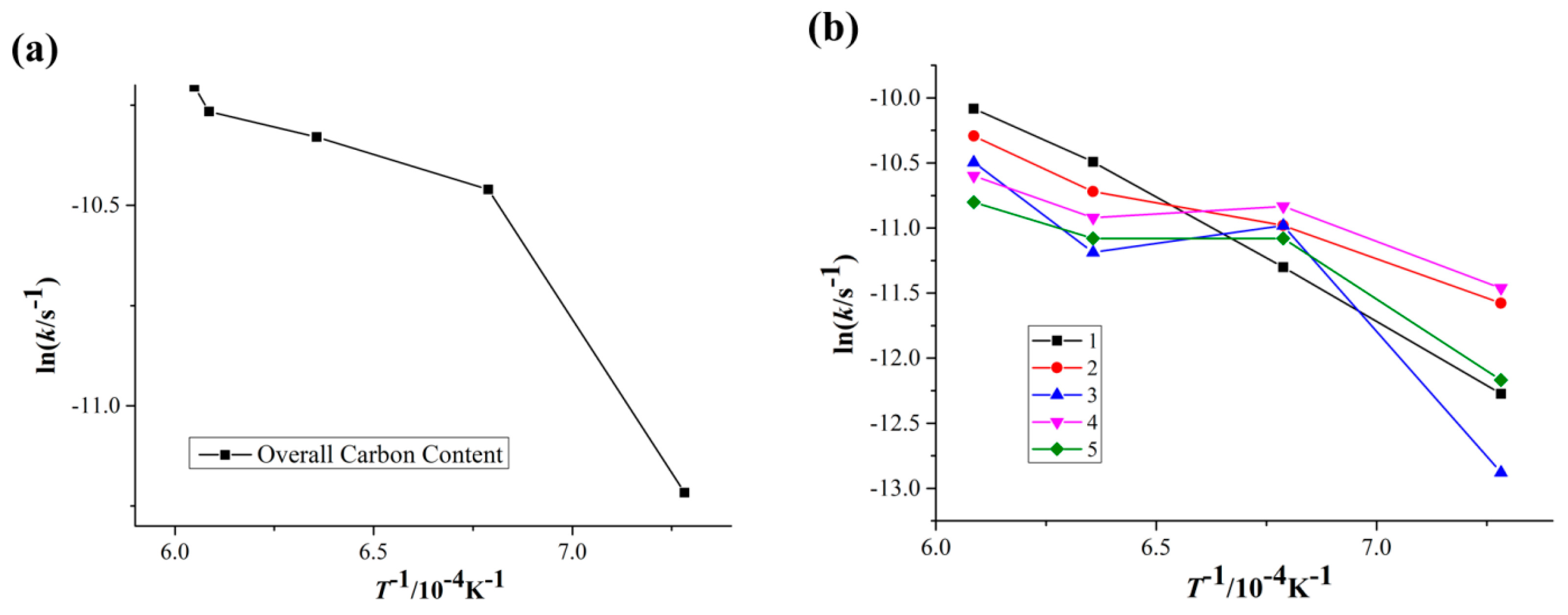
3.1. Overall Carbon and Oxygen Content
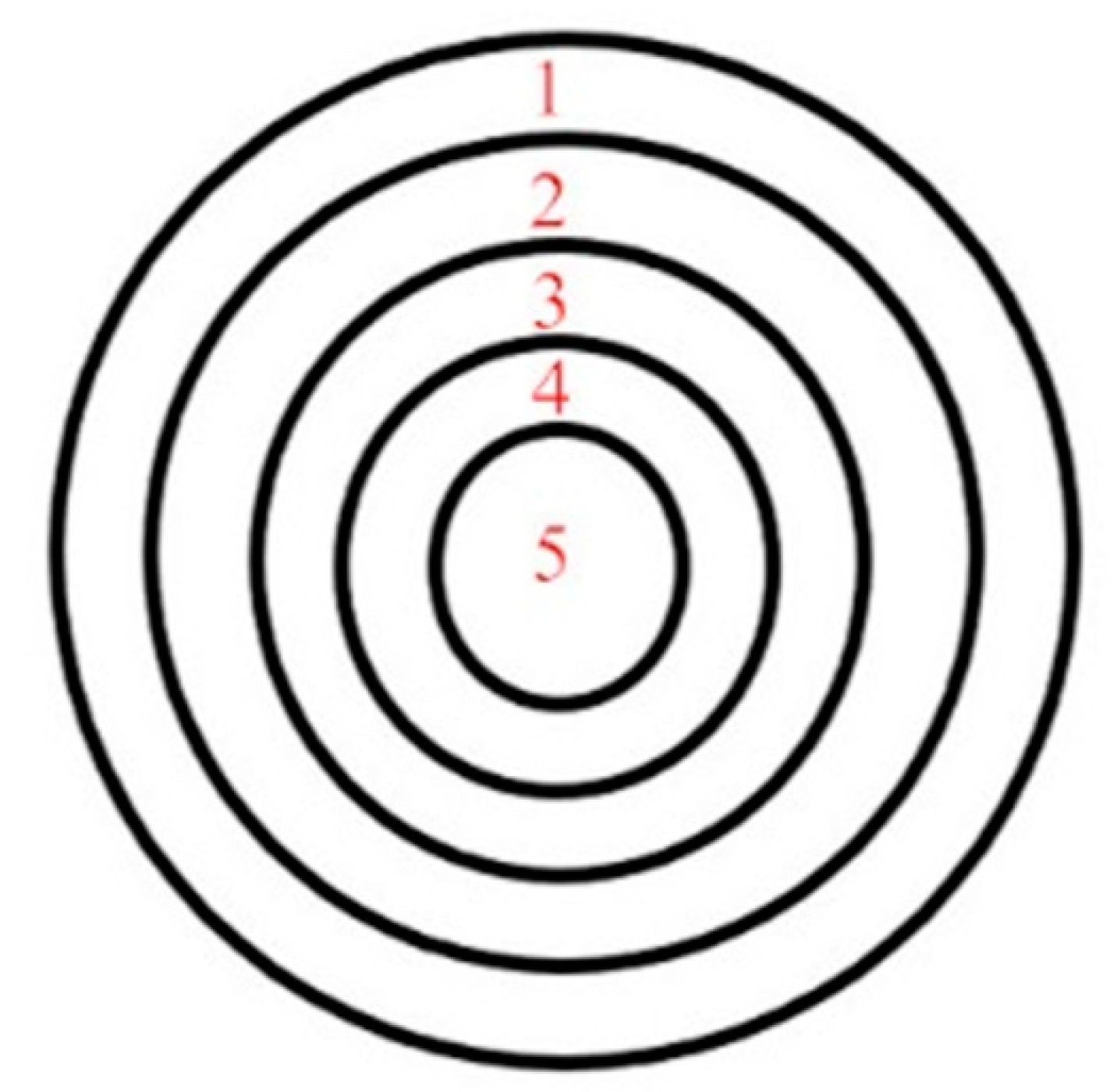
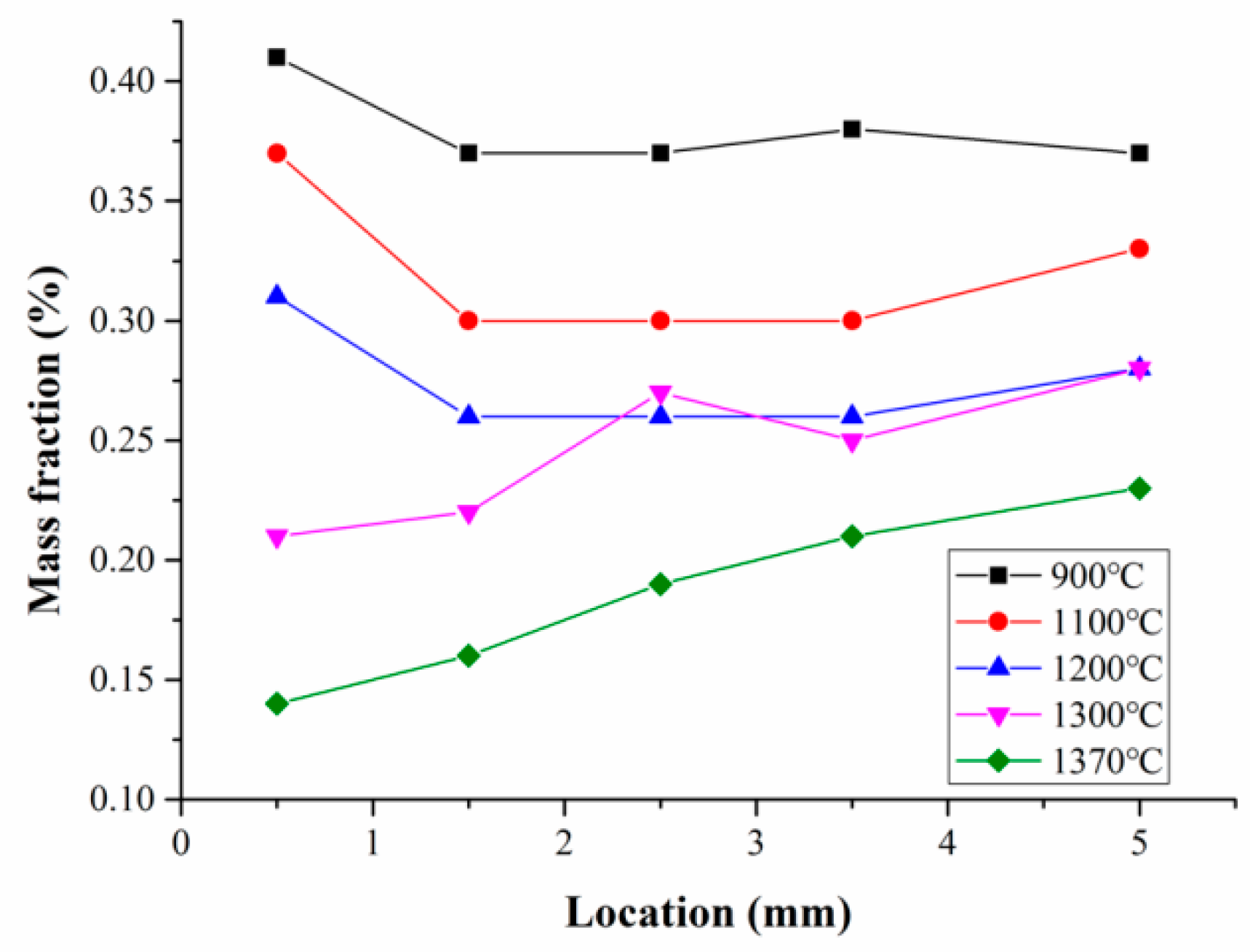
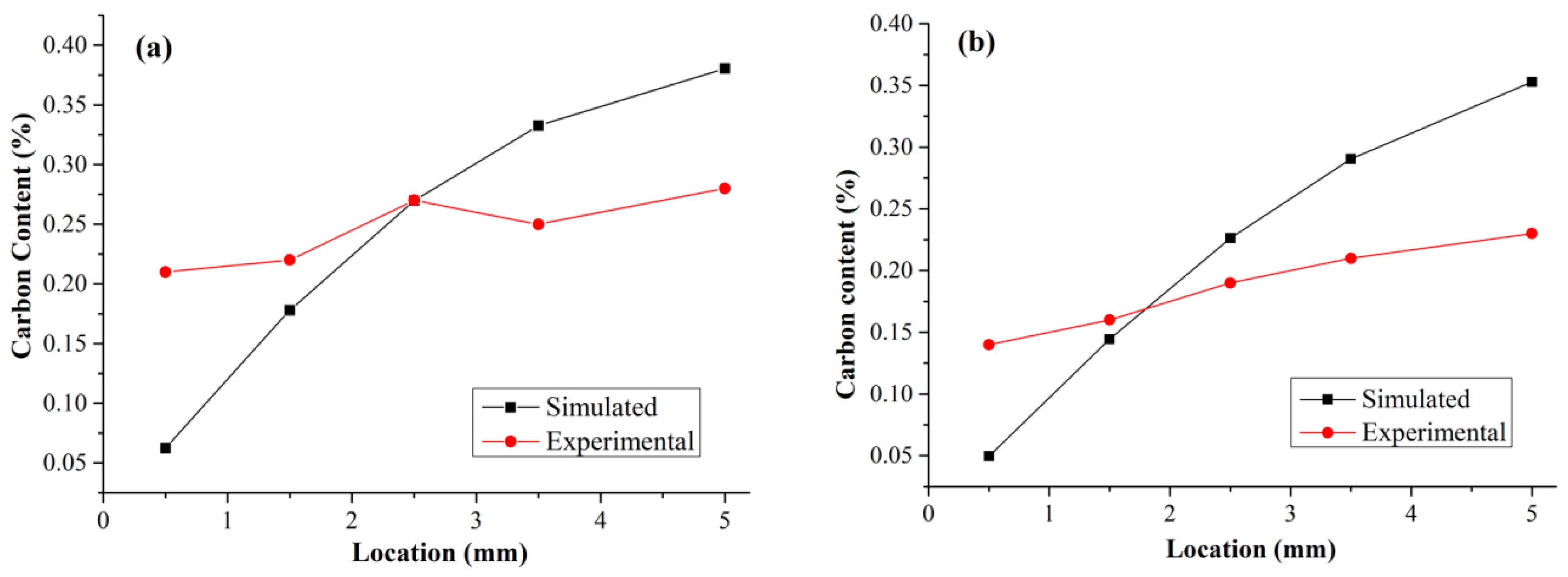
3.2. Carbon Content Distribution
3.3. Microstructure
4. Discussion
4.1. Effect of Sintering Temperature on the Decarburization Rate
4.2. Distribution of Carbon
4.3. Relationship between Decarburization and Densification
5. Conclusions
- The decarburization of MIM-produced 420 stainless steel could be separated into two stages.
- At lower temperatures, decarburization occurs at the powder particle surface. The oxygen in the atmosphere or surface oxides reacts with the carbon. At this time, the carbon content distribution of the sintered body is similar to that of the as-debound sample.
- The reduction of surface oxides promotes sintering. The densification speed is faster in the centre region of samples sintered at 1300 °C, where the interconnected pores are closed.
- At higher temperatures, carbon in the inner layer must migrate to the surface to react with the atmosphere. The decarburization speed is reduced, and the carbon content distribution of the as-sintered part is similar to that of the dense decarburized part.
Author Contributions
Funding
Conflicts of Interest
References
- Lou, J.; Li, Y.M.; He, H.; Li, D.Y.; Wang, G.Y.; Feng, J.; Liu, C. Interface development and numerical simulation of powder co-injection moulding. Part. I. Experimental results on the flow behaviour and die filling process. Powder Technol. 2017, 305, 405–410. [Google Scholar] [CrossRef]
- Lou, J.; Li, Y.M.; He, H.; Li, D.Y.; Wang, G.Y.; Feng, J.; Liu, C. Interface development and numerical simulation of powder co-injection moulding. Part II. Numerical simulation and experimental verification. Powder Technol. 2017, 305, 357–363. [Google Scholar] [CrossRef]
- Lou, J.; He, H.; Li, Y.M.; Zhu, C.Q.; Chen, Z.Y.; Liu, C. Effects of high O contents on the microstructure, phase-transformation behaviour, and shape-recovery properties of porous NiTi-based shape-memory alloys. Mater. Des. 2016, 106, 37–44. [Google Scholar] [CrossRef]
- Cesar, M.D.G.M.M.; Mantel, M.J. Effect of the temperature and dew point of the decarburization process on the oxide subscale of a 3% silicon steel. J. Magn. Magn. Mater. 2003, 254, 337–339. [Google Scholar] [CrossRef]
- Wei, J.H.; Zhu, D.P. Mathematical modeling of the argon-oxygen decarburization refining process of stainless steel: Part I. Mathematical model of the process. Metall. Mater. Trans. B 2002, 33, 111–119. [Google Scholar] [CrossRef]
- Boué-Bigne, F. Laser-induced breakdown spectroscopy applications in the steel industry: Rapid analysis of segregation and decarburization. Spectrochim. Acta, Part B. 2008, 63, 1122–1129. [Google Scholar] [CrossRef]
- Hao, X.J.; Yin, W.; Strangwood, M.; Peyton, A.J.; Morris, P.F.; Davis, C.L. Off-line measurement of decarburization of steels using a multifrequency electromagnetic sensor. Scr. Mater. 2008, 58, 1033–1036. [Google Scholar] [CrossRef]
- Auinger, M.; Vogel, A.; Praig, V.G.; Danninger, H.; Rohwerder, M. Thermogravimetry and insitu mass spectrometry at high temperatures compared to thermochemical modelling—The weight loss during selective decarburisation at 800 °C. Corros. Sci. 2014, 78, 188–192. [Google Scholar] [CrossRef]
- Szekely, J.; Asai, S. Decarburization of stainless steel: Part II. A mathematical model and a process optimization for industrial scale systems. Metall. Trans. 1974, 5, 1573–1580. [Google Scholar] [CrossRef]
- Zhao, X.; Song, B.; Zhang, Y.J.; Zhu, X.M.; Wei, Q.S.; Shi, Y.S. Decarburization of stainless steel during selective laser melting and its influence on Young’s modulus, hardness and tensile strength. Mater. Sci. Eng. A. 2015, 647, 58–61. [Google Scholar] [CrossRef]
- Rosa, C.E.F.D.L.; Trejo, M.H.; Román, M.C.; López, E.A. Effect of Decarburization on the Residual Stresses Produced by Shot Peening in Automotive Leaf Springs. J. Mater. Eng. Perform. 2016, 25, 1–8. [Google Scholar]
- Attia, U.M.; Alcock, J.R. A review of micro-powder injection moulding as a microfabrication technique. J. Micromech. Microeng. 2011, 21, 43001–43022. [Google Scholar] [CrossRef]
- Raza, M.R.; Ahmad, F.; Omar, M.A.; German, R.M.; Muhsan, A.S. Defect Analysis of 316LSS during the Powder Injection Moulding Process. Defect Diffus. Forum. 2012, 329, 35–43. [Google Scholar] [CrossRef]
- Do, T.; Chang, S.S.; Stetsko, D.; Vanconant, G.; Vartanian, A.; Pei, S.; Kwon, P. Improving Structural Integrity with Boron-based Additives for 3D Printed 420 Stainless Steel. Procedia Manuf. 2015, 1, 263–272. [Google Scholar] [CrossRef]
- He, H.; Lou, J.; Li, Y.M.; Zhang, H.; Yuan, S.; Zhang, Y.; Wei, X.S. Effects of oxygen contents on sintering mechanism and sintering-neck growth behaviour of Fe Cr powder. Powder Technol. 2018, 329, 12–18. [Google Scholar] [CrossRef]
- Li, X.B.; Qi, T.G.; Peng, Z.H.; Liu, G.H.; Zhou, Q.S. Kinetics of chromite ore in oxidation roasting process. Chin. J. Nonferrous Met. 2010, 20, 1822–1828. [Google Scholar]
- Li, X.G.; Chen, J.; Hao, J.J.; Han, P.D.; Liu, J.Y. Comparative research on solid state decarburization kinetics of high-carbon ferrochrome powder by microwave heating and conventional heating. Chin. J. Nonferrous Met. 2014, 24, 2181–2187. [Google Scholar]
- Chasoglou, D.; Hryha, E.; Norell, M.; Nyborg, L. Characterization of surface oxides on water-atomized steel powder by XPS/AES depth profiling and nano-scale lateral surface analysis. Appl. Surf. Sci. 2013, 268, 496–506. [Google Scholar] [CrossRef]
- Xu, Y.T.; Chen, Z.P.; Zhang, G. Kinetic Model of Decarburization and Denitrogenation in Vacuum Oxygen Decarburization Process for Ferritic Stainless Steel. Metall. Mater. Trans. B. 2009, 40, 345–352. [Google Scholar] [CrossRef]
- Li, D.; Hao, H.; Li, Y.; Jia, L. Inhomogeneous distribution of oxygen in sintered Ti–Nd alloy. Mater. Lett. 2017, 191, 85–88. [Google Scholar] [CrossRef]
- Duan, G. Effect of High Magnetic Field Annealing on Carbon Diffusion Coefficient in Pure Iron. Master’s Thesis, NorthEastern University, Shenyang, China, 28 June 2012. [Google Scholar]
- Lou, J.; Li, Y.M.; He, H.; Li, L.J. Effect of atomisation medium on sintering properties of austenitic stainless steel by eliminating influence of particle shape and particle size. Powder Metall. 2010, 53, 112–117. [Google Scholar] [CrossRef]
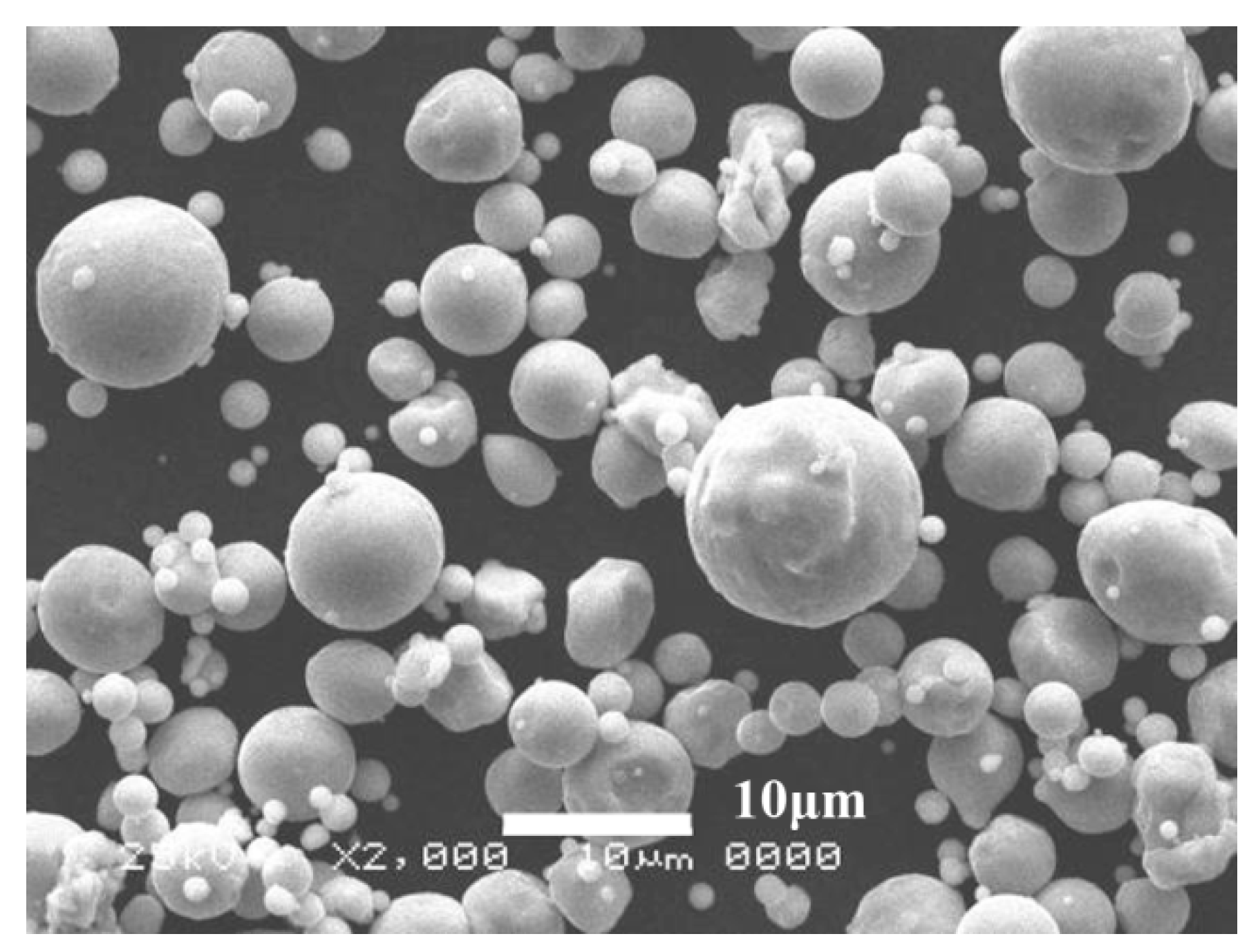


| Element | Cr | C | Mn | Si | N | O | S | Fe |
| Mass ratio (%) | 13.63 | 0.19 | 0.33 | 0.15 | 0.2 | 0.027 | 0.008 | Bal. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, J.; Liu, M.; He, H.; Wang, X.; Li, Y.; Ouyang, X.; An, C. Investigation of Decarburization Behaviour during the Sintering of Metal Injection Moulded 420 Stainless Steel. Metals 2020, 10, 211. https://doi.org/10.3390/met10020211
Lou J, Liu M, He H, Wang X, Li Y, Ouyang X, An C. Investigation of Decarburization Behaviour during the Sintering of Metal Injection Moulded 420 Stainless Steel. Metals. 2020; 10(2):211. https://doi.org/10.3390/met10020211
Chicago/Turabian StyleLou, Jia, Muyao Liu, Hao He, Xinming Wang, Yimin Li, Xiaoping Ouyang, and Chuanfeng An. 2020. "Investigation of Decarburization Behaviour during the Sintering of Metal Injection Moulded 420 Stainless Steel" Metals 10, no. 2: 211. https://doi.org/10.3390/met10020211
APA StyleLou, J., Liu, M., He, H., Wang, X., Li, Y., Ouyang, X., & An, C. (2020). Investigation of Decarburization Behaviour during the Sintering of Metal Injection Moulded 420 Stainless Steel. Metals, 10(2), 211. https://doi.org/10.3390/met10020211






