Abstract
Flotation tailings rich in carbonate minerals from the tailings deposit of the copper mine Majdanpek (Serbia) were applied for neutralization of the water taken from the extremely acidic Lake Robule (Bor, Serbia). Tests conducted in Erlenmeyer flasks showed that after neutralization of the lake water to pH 7, over 99% of aluminum (Al), iron (Fe), and copper (Cu) precipitated, as well as 92% of Zn and 98% of Pb. In order to remove residual Mn and Ag, the water was further treated with NaOH. After treatment with NaOH, all concentrations of the metals in the lake water samples were below discharge limits for municipal wastewater according to the national legislation of the Republic of Serbia. The results of this work suggest that mining waste could be used for active neutralization of the acid mine drainage. The use of the mining waste instead of lime could reduce the costs of the active treatment of the acid mine drainage.
1. Introduction
Environmental pollution by acid mine drainage (AMD) is a widespread problem in mining impacted areas [1,2]. One such area is located near the town of Bor, in the eastern part of Serbia. Mining activities in the region of Bor began in 1903. Approximately 7 × 108 tons of mining waste (overburden and flotation tailings) have been deposited in the close proximity of the town [3]. An extremely acidic water body named Lake Robule was formed at the foot of the overburden deposit, just a few kilometers from the center of the town. The length of the lake is approximately 400 m, and the width at its widest part is approximately 130 m. The water is characterized by a low pH (approximately 2–2.5) and very high concentrations of ferric iron, causing the deep red color of the lake. High concentrations of Cu, Zn, Al, Mn, and other metals were also detected in the lake water [4,5,6]. Approximately 10 L s−1 of acidic water flows from the lake to the Bor River through the drainage pipe [7]. One of the attempts to find an environmentally and economically feasible method to treat this accumulated AMD was conducted by Pavlović et al. [8]. They used a laboratory scale cascade line system with three reactors for selective precipitation of metals such as iron, copper, nickel, and arsenic from the synthetic solution that resembled acidic effluent from the open pit mine from the Bor area. The neutralizing agent was 1 M NaOH. Recently, Masuda et al. [9] applied hydrated lime (Ca(OH)2) for neutralization of acidity and selective precipitation of metals from the water collected from Lake Robule using semi-industrial scale equipment for active AMD treatment with two chemical reactors. Stopic et al. [10] used red mud from Greece and Germany firstly for neutralization of AMD (pH value of 2.3) from South Africa and for precipitation of copper. Mwewa et al. [11] performed precipitation of poly-alumino-ferric sulfate coagulant for wastewater treatment using AMD solution from South Africa.
There are active and passive methods in the treatment of AMD [12,13]. The conventional method for active neutralization of the acid mine drainage is the application of Ca(OH)2. This process is fast and efficient; hydrated lime increases the pH of the solution, resulting in precipitation of the dissolved metals in the form of metal hydroxides [14]. The main disadvantage of this technology is generation of the voluminous sludge. The price of the lime and need for the dehydration, solidification, and stabilization of the sludge after treatment increase the capital and operational costs of the AMD neutralization process [14,15,16]. More recently, passive methods have been attracting more attention due to reduced energy and maintenance costs [13]. With a view toward sustainable development, many studies have focused on finding alternative materials for AMD neutralization. Kaur et al. [17] investigated the application of the waste material from the alumina refining industry (Bayer liquor and precipitates formed by sea water neutralization of the Bayer liquor) to treat AMD from mine pit water. Kefeni et al. [18] reviewed technologies for AMD treatment, including alternative approaches for AMD neutralization by waste materials, such as the application of coal combustion by-products (fly ash, bottom ash, flue gas desulphurization materials), recycled concrete aggregates, and cryptocrystalline magnesite tailings from the gold extraction industry. Moodley et al. [19] also reviewed alternative methods for AMD remediation with a focus on industrial by-products, such as by-products from the paper mill and steel mill industries, meat industry, tire manufacturing, and phosphate waste rock.
The aim of the research is to investigate the ability of flotation tailings, a significant voluminous waste of the same industry that causes the formation of AMD in the Bor area, to neutralize and purify water collected from the acidic Lake Robule. Flotation tailings are a waste material generated during the production of mineral concentrate by froth flotation. Approximately 99 wt. % of the processed ore becomes flotation tailings [20]. Flotation tailings are consisted mostly of gangue minerals, but a considerable amount of carbonate minerals (calcite and dolomite) identified in particular flotation tailings samples collected from the dump of Copper Mine Majdanpek indicates a possible acid neutralization potential of the material. The motivation of the research is not only to investigate the ability of flotation tailings to neutralize and purify water collected from the acidic Lake Robule, but also to remove iron in order to prepare a solution for recovery of valuable elements such as copper, gold, aluminum, and silver in our future work. In order to predict the behavior of metals and especially their precipitation, the geochemical software will be tested and discussed with experimental results.
2. Materials and Methods
2.1. Sampling
Copper Mine Majdanpek is a part of the mining and smelting industry in Bor, Serbia. The mining operations started in 1963; and since then, 378 million tons of flotation tailings have been deposited in a large dump. Flotation tailings samples were dug out from cubes of a 0.04 m3 volume (0.2 m × 0.2 m × 1 m) at 25 equidistant spatial locations (the distance between locations was 50 m), forming a square shaped network, and put in the separate bags. In the laboratory, the composite sample was prepared by the quartering method.
A total of 10 water samples was collected in March 2018 from the pipe that drains water from Lake Robule. The main physicochemical parameters (pH, Eh, temperature) were measured on the spot (Hanna Instruments HI98196, Hanna Instruments Deutschland GmbH, Vöhringen, Germany). Containers (1 L, high density polyethylene) were rinsed with concentrated HNO3 (Tehnohemija, Belgrade, Serbia) and deionized water before collecting samples. The filled containers were immediately transported to the laboratory the same day. Containers were stored at 4 °C in the refrigerator. A schematic geographic map of Serbia with the location of Copper Mine Majdanpek and the extremely acidic Lake Robule is given in Figure 1.
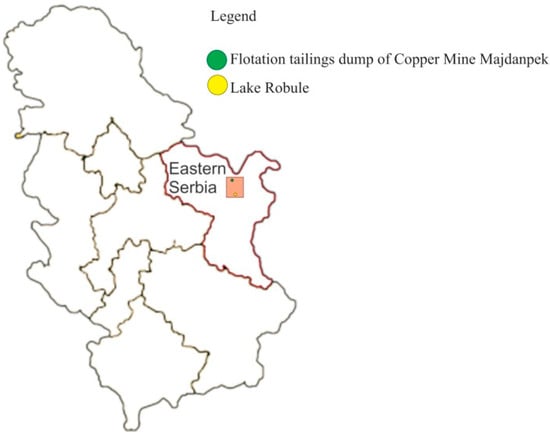
Figure 1.
Schematic geographic map of Serbia with the location of Copper Mine Majdanpek and the extremely acidic Lake Robule.
2.2. Characterization of the Flotation Tailings
The chemical characterization of the flotation tailings was performed by dissolving a sample of the material in aqua regia and measuring the concentration of the selected elements by the Atomic Absorption Spectroscopy (AAS) method using Perkin Elmer Aanalyst 300 (PerkinElmer, Inc, Norwalk, CT, USA).
The concentration of the selected chemical elements in the water samples was measured by the Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) method (Spectro Genesis, Spectro Analytical Instruments, Kleve, Germany).
The sulfate concentration in the lake water sample was determined gravimetrically with BaCl2. Carbonates and hydrogen carbonates were determined by titration with 0.1 M HCl using phenol phthalein and methyl orange (Tehnohemija, Belgrade, Serbia) as indicators.
Mineralogical characterization of the tailings was performed by the microscopy and XRD (X-Ray Diffraction, Zeiss-Jena, Oberkochen, Germany) methods. The polarizing microscope Carl-Zeiss, Model “JENAPOL-U” equipped with 10×, 20×, 50×, and 100× (oil immersion) objectives and a system for photomicrography (“Axiocam105 color” camera and “Carl Zeiss Axio Vision SE64 Rel. 4.9.1.” software package with Multiphase module), was used for microscope investigations in reflected light. The XRD patterns were obtained using a Philips PW-1710 automated diffractometer with a Cu tube operated at 40 kV and 30 mA. The instrument was equipped with a diffracted beam curved graphite monochromator and an Xe filled proportional counter. The diffraction data were collected in the 2θ Bragg angle range of 4–65°, counting for 1 s. Semi-quantitative analysis of the data obtained by XRD was performed by “Powder Cell” computer software [21], (Federal Institute for Materials Research and Testing, Berlin, Germany).
2.3. Acid Neutralization Capacity
Acid Neutralization Capacity (ANC) represents the ability of a material to neutralize acid and remain stable under the external effluence of the environment [22]. In order to investigate and compare the ANC of flotation tailings before and after the treatment with the lake water, both samples of the tailings (fresh and treated) were subjected to the ANC test described by Stegemann and Cote [23]. The test procedure consisted of a series of leaching tests with increasing concentrations of nitric acid (HNO3) and measuring the pH values of the leachates. Samples and solutions with different nitric acid contents, in the range from 0 (distilled water) to 2 H+ eq/kg, with a solid to liquid ratio of 10, were rotated in polyethylene bottles (V = 50 mL) for 48 h prior to centrifugation and measurement of pH. Titration curves were obtained by plotting measured pH values versus acid content.
2.4. Determination of the Optimal Quantity of Flotation Tailings Required for Neutralization of the Lake Water Samples and Metal Precipitation
The neutralization test was performed with increasing quantities of the flotation tailings in order to identify the optimal solid to liquid ratio for neutralization of the lake water. The tests were conducted in eight 100 mL Erlenmeyer flasks containing 50 mL of water with increasing pulp density: 1, 3, 5, 10, 15, 20, 25, 30, and 40%. The Erlenmeyer flasks were put in an incubated orbital shaker (Heidolph Unimax 1010, Heidolph, Shwabach, Germany) at a temperature of 25 °C and a shaking speed of 250 rpm. After two hours of shaking, the samples were put to rest for seven days at room temperature.
After determination of the optimal quantity of the flotation tailings for the neutralization of the lake water, the precipitation of metal cations as a function of time and pH was determined. Nine 100 mL Erlenmeyer flasks were filled with 50 mL of the lake water, and 7.5 g of the flotation tailings were added to each flask. Flasks were placed in an orbital shaker under the same conditions as in the previous test. After agitation was stopped, the solution was left to stand for 30 min in order to let the particles settle out. Samples were collected after 5, 10, 15, and 30 min and 2, 4, 24, 72, and 168 h. The collected samples were filtered by using filter paper and analyzed by ICP-OES.
2.5. Treatment of the Lake Water Samples with Hydrated Lime and NaOH
After treatment of lake water samples with flotation tailings, the water was further treated with hydrated lime Ca(OH)2 in order to increase its pH to 10, as is shown in Figure 2. A flask with 50 mL of the lake water previously treated with flotation tailings was put on a magnetic stirrer; the pH of the water was measured during the experiment by a laboratory pH meter (Hach Sension + MM340). Hydrated lime was carefully added until the pH of the solution reached a value of 10.
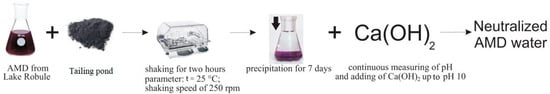
Figure 2.
Schematic description of AMD water treatment with flotation tailings from the dump of Copper Mine Majdanpek.
In order to compare the results obtained by lake water neutralization with flotation tailings, the lake water was also neutralized with NaOH. Two Erlenmeyer flasks with 50 mL of the lake water were put on a magnetic stirrer and treated with NaOH and flotation tailings until the pH value of the water reached 7.
2.6. Simulation of Metal Precipitation Using PHREEQC Software
PHREEQC is a computer program designed to perform a variety of aqueous geochemical calculations [24], and in numerous research works [12,13,25,26], it was used to explain and support the experimental results addressed to the behavior of contaminants during a specific treatment. Here, the PHREEQC software (USGS, Reston, VA, USA) was applied for the modelling of the aqueous speciation of the water from Lake Robule and the saturation index calculations of the solid phases formed during the treatment of lake water by flotation tailings based on the pH and concentrations of elements in the solutions. An individual simulation was made for each defined time during the experiment according to the measured values of pH and the concentration of metals as input values for the calculations. Water volume, solid to liquid ratio, and temperature, as constant parameters during the experiment, were also input values for the calculation. Saturation Indices (SI) are defined as the logarithm of the ratio of the Ion Activity Product (IAP) to the solid solubility product (Ks), Equation (1).
SI = log(IAP/Ks)
A positive SI indicates precipitation of the mineral, while a negative value of SI indicates mineral dissolution. Values of SI ≥ 0 were chosen to specify the controlling mineral for the constituent element’s precipitation.
3. Results and Discussion
3.1. Physical and Chemical Properties of the Water Collected from Lake Robule
The physical and chemical properties of the water collected from Lake Robule are presented in Table 1. The acidic nature of the Lake Robule water was confirmed by a pH value of 2.47. The most dominant ions in the water samples were sulfate ions (7.5 g/L), Al2+ (1017.62 mg/L), and Fe ions (287 mg/L), almost completely in the form of Fe3+ (286.9 mg/L).

Table 1.
Physico-chemical properties of the water collected from Lake Robule.
3.2. Characterization of the Flotation Tailings’ Sample from Copper Mine Majdanpek
The chemical composition of the flotation tailings’ composite sample collected from Copper Mine Majdanpek is presented in Table 2, while the results of the X-ray diffraction analysis are presented on Figure 3.

Table 2.
Chemical composition of the flotation tailings from Copper Mine Majdanpek.
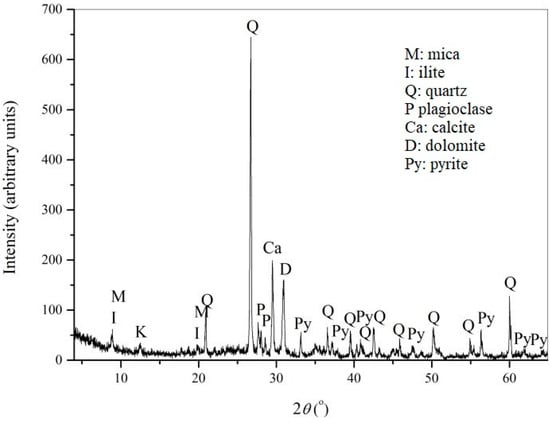
Figure 3.
Diffractogram of the flotation tailings sample from Copper Mine Majdanpek.
The most abundant minerals in the analyzed flotation tailings sample identified by X-ray diffraction analysis were quartz, pyrite, and carbonates (calcite and dolomite), followed by feldspar, clay minerals, mica, and illite, which were less abundant. The results of the semi-quantitative analysis of the data obtained by XRD were: quartz ≈ 50–55%, total carbonates ≈ 20–25%, kaolinite ≈ 5–10%, pyrite ≈ 5%, and illite ≈ 5%. High pyrite content supported the results of chemical analysis and also indicated the high potential of exposed tailings to generate AMD. The content of carbonate minerals (20–25%) indicated the acid neutralization capacity of the material. The concentrations of Mn (0.138%), Cu (0.072%), Zn (0.086%), and Pb (0.0079%) indicated the possibility of leaching of these metals from tailings in contact with acidic water from lake Robule.
3.3. Results of the Acid Neutralization Capacity Test
Remediation of the acid mine drainage is based on neutralization of the solution’s acidity, which results in the precipitation of the metal cations, mostly in the form of the insoluble hydroxides and carbonates [8,14,27,28,29,30]. The acid neutralization capacity of mining waste, such as flotation tailings, depends on the relative reactivity of the contained minerals to interact with H+ ions and undergo the neutralization process. According to Sverdrup [31], minerals can be divided into several groups based on their relative reactivity in the acid neutralization process: dissolving (calcite, dolomite, magnesite), fast weathering (anorthite, nepheline, forsterite, olivine, garnet, jadeite, leucite, spodumene, diopside, wollastonite), intermediate weathering (sorosilicates, pyroxenes, amphiboles, phyllosilicates), slow weathering (plagioclase, kaolinite), very slow weathering (K-feldspar), and inert (quartz). The most relevant minerals as potential neutralization agents contained in flotation tailings are carbonates, hydroxides and silicates [32,33]. The results of the ANC test of fresh and treated flotation tailings are shown in Figure 4. Common to both materials is a wide plateau, from 0.2 to 1.4 H+ eq/kg, at pH values between 5 and 6 associated with the dissolution of carbonate minerals [34]. Carbonates, primarily calcite, are the main minerals involved in the acid neutralization reaction described by Equation (2) at pH > 6.3 and Equation (3) at pH < 6.3 [33].
CaCO3 + H+ ⇔ Ca2+ + HCO3−
CaCO3 + 2H+ ⇔ Ca2+ + H2CO3
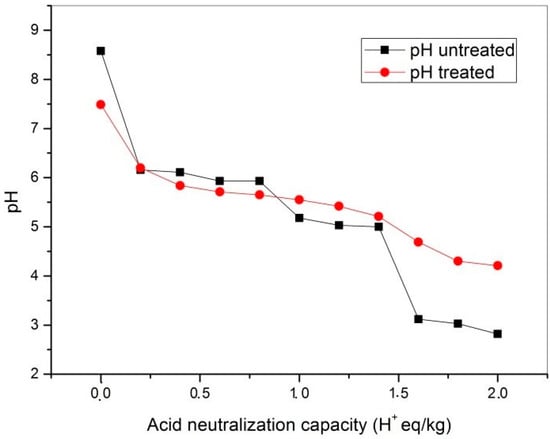
Figure 4.
Results of the Acid Neutralization Capacity (ANC) test. Untreated: flotation tailings before neutralization of the lake water. Treated: flotation tailings after neutralization of the lake water.
An interesting observation is that the treatment did not significantly reduce the buffering capacity of the material and that the amount of acid that the carbonates could neutralize was in the same range for both samples. Carbonate consumption for acid neutralization was reflected in the initial pH values after leaching with distilled water (0 H+ eq/kg): 8.6 for fresh tailings and 7.5 for treated. Products of the treatment and their effect on the ANC were reflected in the position of the last plateau on the titration curves at a higher H+ concentration for treated tailings (pH ≈ 4.5) than for fresh tailings (pH ≈ 3.0). This was associated with the formation of solid hydroxide minerals of metal cations with a valence of 3+ (Fe3+ and Al3+) during the treatment. These metal hydroxides hydrolyzed with high ionic potential, thus representing important buffers controlling the pH at ≈ 4.3 (Al(OH)3) and ≈ 3.5 (Fe(OH)3) [22,33,34]. It can be concluded from the results of the ANC test that flotation tailings from Copper Mine Majdanpek had significant acid neutralization capacity and that these flotation tailings could be used multiple times for AMD neutralization. Furthermore, an important finding was that the buffering capacity of the tailings actually increased after treatment, due to the hydrolysis of the precipitated metal hydroxides.
3.4. Treatment of Water from Lake Robule by Using Flotation Tailings
3.4.1. Determination of the Optimal Quantity of Flotation Tailings Required for Neutralization of the Lake Water Samples
The next step was to find the optimal solid to liquid ratio (S:L) for neutralization of the water from Lake Robule. The results of the neutralization test with gradually increased amounts of flotation tailings are presented in Table 3.

Table 3.
Determination of the optimal quantity of flotation tailings required for neutralization of the lake water samples to pH 7.
The results of the experiment presented in Table 3 showed that following the increase in pulp density, the pH value dropped faster, but after seven days of settling, the difference in the final pH values between flasks with pulp densities of 15%, 20%, 25%, 30%, and 40% was not significant. Based on these results, a pulp density of 15% was identified as optimal for further experiments.
3.4.2. Experimental Results of the Treatment
Changes in the concentrations of the selected metals in solution during the treatment are presented in Table 4. Beside concentrations, the removal percentage of each element was calculated according to Equation (4).

Table 4.
Concentration of elements in solution during treatment time.
Experimental results showed that the concentration of all elements, except Mn and Cd, decreased during the treatment with the increasing value of pH. After 168 h of treatment, when the pH of the solution reached 7.01, the concentrations of all elements, except Mn, were below the discharge limits for municipal wastewaters prescribed by the national legislation of the Republic of Serbia [35], with over 99% of Fe, Al, and Cu precipitation, 98% removal of Pb, and 92% of Zn. Unlike all other metals, the concentrations of Mn and Cd in the solution increased during the treatment, indicating that these metals might be leached from the tailings.
3.5. Results of the PHREEQC Software Simulation of the Water Treatment
The results of the treatment simulation obtained by the PHREEQC program are given in Table 5. The results were based on the measured concentrations of the elements and pH values of the obtained solutions over time, as well as on the defined water volume (50 mL), solid to liquid ratio (15%), and temperature (18 °C) as constant values during the experiment. The results are presented in the form of calculated Saturation Indices (SI) of the corresponding mineral phases. The saturation indices of certain mineral phases, noted in the Table 5, increased and became positive at specific pH values. Such SI denote minerals whose constituent elements achieved thermodynamic equilibrium concentration in the solution during the neutralization reaction (SI = 0), after which they began to precipitate (SI > 0). The results of PHREEQC modeling partially complemented the experimental results and gave an additional explanation of the mechanism of metals’ removal during the treatment.

Table 5.
Results of the PHREEQC software simulation.
3.6. Mechanism of Acid Neutralization and Precipitation of Metals during the Treatment
Figure 5, Figure 6 and Figure 7 present the changes in the concentrations of the metal cations as a function of the pH. After two hours of mixing, the pH of the solution increased to 4.78, and after seven days of settling, the pH of the solution further increased to 7. In samples collected from Lake Robule, over 99% of the total iron was in the ferric state. Deeper anoxic sections of the lake, which have not been disturbed nor sampled, could have iron in the ferrous (Fe2+) state. During the first 5 min of the treatment, the pH value of the solution increased due to the carbonate dissolution and acid neutralization process and reached a value of 4.3, when the solution was supersaturated with Fe3+ ions that began to precipitate. The removal of iron was relatively rapid with a sharp decline in iron concentration in the pH range between 2 and 4. Approximately 99.8% of iron from the water samples was removed. Iron in the ferric state should readily precipitate as oxyhydroxide compounds (FeO(OH)), as shown in Equation (5), at pH values greater than 3.5 [17].
Fe3+(aq) + 2OH−(aq) ↔ FeO(OH)(s) + H+(aq)
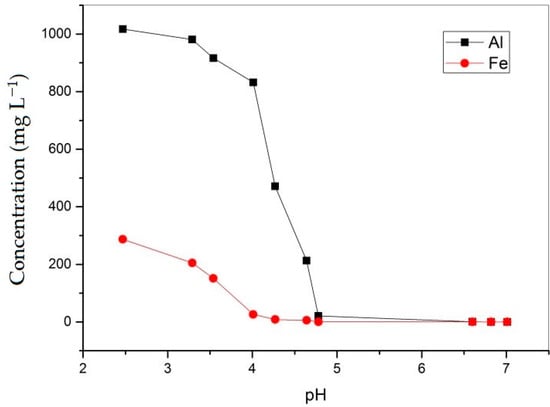
Figure 5.
Changes in the concentrations of Al and Fe as a function of the pH.
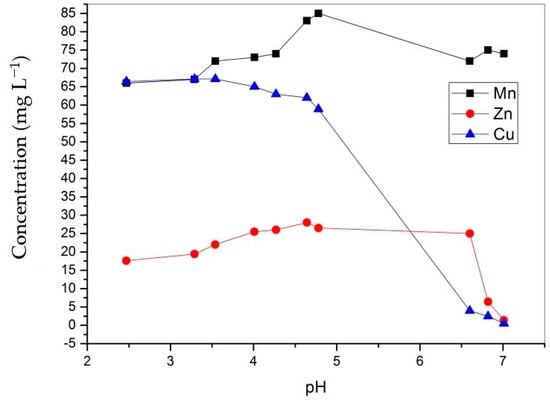
Figure 6.
Changes in the concentrations of Mn, Zn, and Cu as a function of pH.
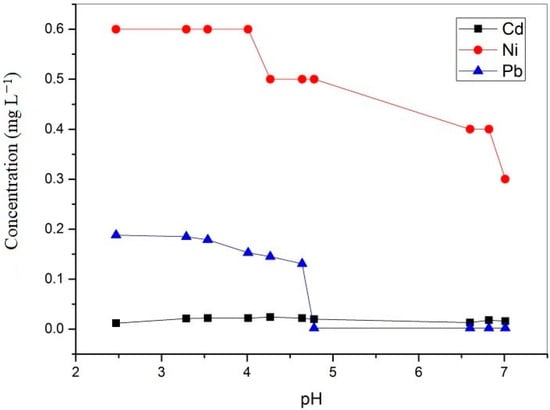
Figure 7.
Changes in the concentrations of Cd, Ni, and Pb as a function of pH.
Equation (5) includes the dynamic information about the formation of goethite, but the analysis of the software included the equilibrium information, where the maximal possibility for precipitation was in the case of the hematite. Generally, as shown in Table 5, an increase of time and pH-values increased the possibility of the precipitation of iron hydroxide, goethite, and hematite with different values of calculated Saturation Indices (SI). In the case of the other elements such as zinc, cadmium, lead, and manganese, these SI-values were negative with small possibilities for precipitation.
Park et al. [36] concluded that in synthetic multimetal solutions, ferric iron precipitated as an amorphous ferric oxyhydroxide and then was transformed by a slow dehydration reaction and internal atomic rearrangement to hematite. These two transformation processes compete with each other, and one of them is dominant depending on the reaction conditions [37].
| Fe2+ |  | Fe3+ |  | α−tFeO(OH) |  | α−tFe2O3 |
| Oxidation | Precipitation | Dehydration |
After 30 min of the process, the pH value of the solution was 4.7, at which the Al3+ ions began to precipitate in the form of gibbsite (Al(OH)3). This was consistent with the fact that in the presence of carbonate ions, the trivalent metals began to precipitate in the form of their hydroxides, Fe at pH ≥ 3.5 and Al at pH ≥ 4.5 [10]. Park et al. [36] also found that Al3+ from complex multimetal AMD solutions precipitated as amorphous AlOHSO4. In the experiment presented in this paper, aluminum precipitated in the pH range from 3.5 to 5 (Figure 5).
The experimental results showed a significant decrease in Zn and Cu concentrations in the lake water samples during the treatment, although simulation results complemented only the precipitation of Zn in the form of smithsonite (ZnCO3) and hydroxide (Zn(OH)2) at pH values above 7, which was not achieved during the treatment. The majority of copper cations precipitated in the pH range between 5 and 7, and over 99% of copper was removed. Software simulation did not predict the precipitation of copper. Precipitation of copper from complex solutions depends on the interactions with other ions. Park et al. [36] investigated the precipitation of metals from quaternary mixtures Fe/Al/Cu/Zn and Fe/Al/Cu/Ni and discovered that metals precipitated in the order Fe-Al-Cu-Zn or Ni. In these solutions, copper probably forms brochantite in the pH range from 5.0 to 6.6 and transforms to tenorite (CuO) above a pH of 6.6 [36,38]. Therefore, in this experiment, the forms of Cu precipitates were expected to be brochantite and tenorite. Theoretically, brochantite can be transformed to tenorite as follows [39]:
4Cu2+ + 6OH− + SO42− ↔ Cu4(SO4)(OH)6
Cu4(SO4)(OH)6 + 2OH−↔4CuO + SO42− + 2H2O
Cu4(SO4)aq +2 OH− ↔ CuO + SO42− + H2O
During the neutralization experiment, the concentration of zinc increased between pH 3.29 and 6.6 and then dropped to a minimal value of 1.4 mg L−1, leading to the removal of 92% of Zn. The increase in zinc concentration was probably the consequence of the leaching of this metal from flotation tailings. Park et al. [36] concluded that in solutions that mimic AMD, zinc probably precipitates as hydrozincite Zn5(CO3)2(OH)6. The removal of Zn and Cu from multimetal solutions can be also attributed to the effect of co-precipitation or adsorption onto amorphous Fe and Al hydroxides [40]. At pH values between 6 and 7, the PHREEQC simulation predicted a precipitation of Pb in the form of carbonate mineral cerussite (PbCO3) since Pb(OH)2 precipitates at higher pH values between 9 and 10; as a result, 98% of lead precipitated at a pH value of approximately 4.8.
According to the software simulation, manganese should precipitate as MnCO3, but no precipitation of manganese occurred during neutralization with flotation tailings. Instead, the concentration of manganese actually increased during the experiment probably due to leaching of Mn from the flotation tailings. The possible explanation is that the formation of MnCO3 was not thermodynamically favorable under the experimental conditions and that also manganese hydroxide (Mn(OH)2) precipitated at a pH above 10.
The concentration of cadmium also increased after neutralization because flotation tailings probably released some cadmium into the solution. The software simulation did not predict the precipitation of cadmium, which is an accordance with the experimental results. Fifty percent of nickel precipitated during the neutralization test. Park et al. [36] found that in the quaternary solution, Ni completely precipitated at pH 8.4. Precipitation of nickel started at pH 7, and the pH required for complete precipitation of Ni was not reached in this experiment. Nickel probably did not precipitate as Ni(OH)2 because nickel hydroxide precipitates at approximately pH 10, but more likely as NiCO3.
3.7. Comparison of the Lake Water Neutralization with Flotation Tailings and NaOH
In order to determine if other mechanisms of metal removal were included, such as adsorption of metal cations on the surface of the minerals that constituted flotation tailings, neutralization of the lake water to pH 7 was performed by application of flotation tailings and NaOH. The most significant differences were observed for the concentrations of Mn and Ag (Table 6). The concentrations of these metal cations were higher in water treated with flotation tailings than in water treated with NaOH. The most probable explanation is that these metals were leached from flotation tailings during neutralization. Changes in metal concentrations during neutralization with flotation tailings were the result of the increased pH of the solution; adsorption on the surface of the minerals had no significant effect on the metal removal efficiency.

Table 6.
Comparison of the metals’ precipitation efficiency after treatment with Flotation Tailings (FT) and NaOH.
3.8. Post-Treatment of the Lake Water Samples with Hydrated Lime
In order to purify water from residual Mn and Ag, the sample of water that was treated with flotation tailings was further treated with hydrated lime, and the pH of the water was increased to 10. The concentrations of manganese and silver were reduced to 0.062 mg L−1 and 0.013 mg L−1 (Table 7). After this treatment, the concentrations of all metals in the water were below the discharge limits for municipal wastewaters according to national legislation of the Republic of Serbia [35].

Table 7.
Results of the treatment of the lake water sample with Ca(OH)2 after neutralization with flotation tailings. Results were compared with national discharge limits for municipal wastewater of the Republic of Serbia [35].
3.9. Characterization of the Solid Residue after the Treatment of Water from Lake Robule with Flotation Tailings
Results of the XRD analysis of the flotation tailings residue after the neutralization experiment with 15% pulp density are presented in Figure 8. The content of carbonate minerals (calcite and dolomite) only slightly decreased after water treatment. The results of the semi-quantitative analysis of the XRD data were: quartz 55–60%, total carbonates 15–20%, mica/illite ≈ 10%, pyrite ≈ 5%, and kaolinite ≈ 5%. These results confirmed that the same sample of the flotation tailings could be used multiple times for neutralization of the water from Lake Robule.
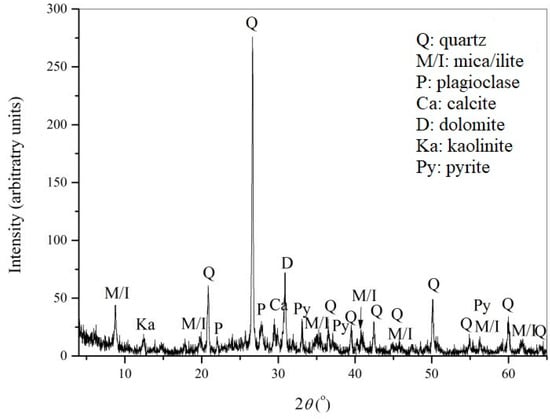
Figure 8.
Diffractogram of the flotation tailings residue after neutralization of the lake water sample.
4. Conclusions
- Flotation tailings’ samples collected from the flotation tailings dump of Copper Mine Majdanpek were rich in carbonate minerals (calcite and dolomite) and possessed significant acid neutralization capacity.
- Laboratory experiments confirmed that the flotation tailings could be applied in order to neutralize water from the extremely acidic Lake Robule located near the town of Bor (Serbia).
- After neutralization with flotation tailings, over 99% of Al, Fe, and Cu precipitated, 98% of Pb, and 92% of Zn. The concentrations of Cd and Mn increased due to leaching of these metals from flotation tailings.
- In order to remove Mn below discharge limits, application of hydrated lime was required in order to increase the pH up to 10.
- The flotation tailings could be applied in the active treatment of AMD in combination with hydrated lime. The results of this research were a step further in the implementation of the principles of the sustainable development including minimization, treatment, and reuse of waste within the same industry.
- Our future activities shall be performed in order to propose a continuous neutralization process for the application of the flotation tailings as an alternative material for acid mine drainage remediation.
- Furthermore, this methodology will be studied in scale-up conditions, in order to obtain more realistic data on the consumption of flotation tailing amount and percent of acid mine water
Author Contributions
Conceptualization, investigation, and experimental design, N.P. and S.S.; methodology and editing, B.M.; formal analysis and editing, D.R.; writing, review and editing, S.R.S.; supervision, conceptualization, and editing, M.S. and Z.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was realized in the frame of the projects TR 34023 and TR 34033, supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dimitrijević, M.D. Pyrite Oxidation and Acid Mine Drainage; University of Belgrade: Belgrade, Serbia, 2013; pp. 75–95. (In Serbian) [Google Scholar]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.; Seno, K.; Ito, M.; Hiroyoshi, N. A review of recent strategies for acid mine drainage prevention and mine tailings recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Bogdanović, G.; Trumić, M.; Trumić, M.; Antić, D.V. Mining waste management: Genesis and possibility of processing. Recycl. Sustain. Dev. 2011, 4, 37–43. (In Serbian) [Google Scholar]
- Korać, M.; Kamberović, Ž. Characterization of wastewater streams from Bor site. Metall. Mater. Eng. 2007, 13, 41–51. [Google Scholar]
- Stevanović, Z.; Obradović, L.; Marković, R.; Jonović, R.; Avramović, L.; Bugarin, M.; Stevanović, J. Mine waste water management in the Bor municipality in order to protect the Bor River water. In Waste Water—Treatment Technologies and Recent Analytical Developments; Einschlag, F.S.G., Carlos, L., Eds.; InTech: Rijeka, Croatia, 2013. [Google Scholar] [CrossRef]
- Stanković, S.; Morić, I.; Pavić, A.; Vasiljević, B.; Johnson, D.B.; Cvetković, V. Investigation of the microbial diversity of an extremely acidic metal-rich water body (Lake Robule, Bor, Serbia). J. Serb. Chem. Soc. 2014, 79, 729–741. [Google Scholar] [CrossRef]
- Beškoski, V.P.; Papić, P.; Dragišić, V.; Matić, V.; Vrvić, M.M. Long term studies on the impact of thionic bacteria on the global pollution of waters with toxic ions. Adv. Mater. Res. 2009, 71, 105–108. [Google Scholar] [CrossRef]
- Pavlović, J.; Stopić, S.; Friedrich, B.; Kamberović, Z. Selective removal of heavy metals from metal-bearing wastewater in a cascade line reactors. Environ. Sci. Pollut. Res. Int. 2007, 14, 518–522. [Google Scholar] [CrossRef]
- Masuda, N.; Marković, R.; Bozić, D.; Bessho, M.; Inoue, T.; Hoshino, K.; Ishiyama, D.; Stevanović, Z. Experimental results of metal recovery by a two-step neutralization process from AMD from a copper mine in Serbia. In Proceedings of the IMWA 2019, Conference “Mine Water: Technological and Ecological Challenges”, Perm, Russia, 15–19 July 2019; Wolkersdorfer, C., Khayrulina, E., Polyakova, S., Bogush, A., Eds.; Perm State University: Perm, Russia, 2019; pp. 232–237. [Google Scholar]
- Stopic, S.; Dertmann, C.; Xakalashe, B.; Lucas, H.; Alkan, G.; Aygmurlu, B.; Friedrich, B. A near zero waste valorization vision for bauxite residue through experimental results. In Proceedings of the XXI YuCorr International Conference, Tara Mountain, Serbia, 17–20 September 2019; Pavlović, M., Pavlović, M., Eds.; Serbian Society of Corrosion and Materials Protection (UISKoZaM): Belgrade, Serbia, 2019; pp. 120–125. [Google Scholar]
- Mwewa, B.; Stopic, S.; Ndlovu, S.; Simate, G.; Xakalashe, B.; Friedrich, B. Synthesis of poly-alumino-ferric sulphate coagulant from acid mine drainage by precipitation. Metals 2019, 9, 1166. [Google Scholar] [CrossRef]
- Masindi, V.; Ndiritu, J.G.; Maree, J.P. Fractional and step-wise recovery of chemical species from acid mine drainage using calcined cryptocrystalline magnesite nano-sheets: An experimental and geochemical modelling approach. J. Environ. Chem. Eng. 2018, 6, 1634–1650. [Google Scholar] [CrossRef]
- Pérez-López, R.; Castillo, J.; Quispe, D.; Nieto, J.M. Neutralization of acid mine drainage using the final product from CO2 emissions capture with alkaline paper mill waste. J. Hazard. Mater. 2010, 177, 762–772. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total. Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef]
- Aube, B.; Zinck, J. Lime treatment of acid mine drainage in Canada. In Proceedings of the Brazil-Canada Seminar on Mine Rehabilization: Techological innovations, Florianopolis, Brazil, 1–3 December 2003; Barbosa, J.P., Ed.; CETEM/MCT: Rio de Janiero, Brazil, 2003; pp. 26–30. [Google Scholar]
- Ivšić-Bajčeta, D.; Kamberović, Ž.; Korać, M.; Gavrilovski, M. A solidification/stabilization process for wastewater treatment of sludge from primary copper smelter. J. Serb. Chem. Soc. 2013, 78, 725–739. [Google Scholar] [CrossRef]
- Kaur, G.; Couperthwaite, S.J.; Hatton-Johnes, B.W.; Millar, G.J. Alternative neutralisation materials for acid mine drainage treatment. J. Water Process. Eng. 2018, 22, 46–58. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Msagati, T.A.M.; Mamba, B.B. Acid mine drainage: Prevention, treatment options, and resource recovery: A review. J. Clean. Prod. 2017, 151, 475–493. [Google Scholar] [CrossRef]
- Moodley, I.; Sheridan, C.M.; Kappelmeyer, U.; Akcil, A. Environmentally sustainable acid mine drainage remediation: Research developments with a focus on waste/by-products. Miner. Eng. 2018, 126, 207–220. [Google Scholar] [CrossRef]
- Dold, B. Sustainability in metal mining: From exploration, over processing to mine waste management. Rev. Environ. Sci. Bio/Technol. 2008, 7, 275–285. [Google Scholar] [CrossRef]
- Kraus, W.; Noize, G. POWDER CELL—A program for the representation and manipulation of crystal structures and calculation of the resulting X-ray powder patterns. J. Appl. Cryst. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- Wahlström, M.; Laine-Ylijoki, J.; Kaartinen, T.; Hjelmar, O.; Bendz, D. Acid Neutralization Capacity of Waste—Specification of Requirement Stated in Landfill Regulations; Nordic Council of Ministers: Copenhagen, Denmark, 2009; p. 51. [Google Scholar]
- Stegemann, J.A.; Cote, P.L. Summary of an investigation of test methods for solidified waste evaluation. Waste Manag. 1990, 10, 41–52. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appel, C.A.J. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; CreateSpace Independent Publishing Platform: Scotts Valley, CA, USA, 2014; p. 497. [Google Scholar]
- Štulović, M.; Radovanović, D.; Kamberović, Ž.; Korać, M.; Anđić, Z. Assessment of leaching characteristics of solidified products containing secondary alkaline lead slag. Int. J. Environ. Res. Publi. Health 2019, 16, 2005. [Google Scholar] [CrossRef]
- Madzivire, G.; Gitari, W.M.; Vadapalli, V.R.K.; Ojumu, T.V.; Petrik, L.F. Fate of sulphate removed during the treatment of circumneutral mine water and acid mine drainage with coal fly ash: Modelling and experimental approach. Miner. Eng. 2011, 24, 1467–1477. [Google Scholar] [CrossRef]
- Rodriguez, J.; Schweda, M.; Stopić, S.; Friedrich, B. Techno-economical comparison between chemical precipitation and electrocoagulation for heavy metal removal in industrial wastewater. Met. Berl. 2007, 61, 208–214. [Google Scholar]
- Dimitrijević, M.D. Acid mine drainage. Bakar 2012, 37, 33–44. (In Serbian) [Google Scholar]
- Dimitrijević, M.D.; Alagić, S.Č. Passive treatment of acid mine drainage. Bakar 2012, 37, 57–68. (In Serbian) [Google Scholar]
- Dimitrijević, M.D.; Nujkić, M.M.; Milić, S.M. The lime Treatment of acid mine drainage. Bakar 2012, 37, 45–56. (In Serbian) [Google Scholar]
- Sverdrup, H.U. The Kinetics of Base Cation Release Due to Chemical Weathering; Lund University Press: Lund, Sweden, 1990; p. 245. [Google Scholar]
- Lawrence, R.W.; Scheske, M. A method to calculate the neutralization potential of mining waste. Environ. Geol. 1997, 32, 100–106. [Google Scholar] [CrossRef]
- Dold, B. Acid rock drainage prediction: A critical review. J. Geochem. Explor. 2017, 172, 120–132. [Google Scholar] [CrossRef]
- Komonweeraket, K.; Cetin, B.; Benson, C.H.; Aydilek, A.H.; Edil, T.B. Leaching characteristics of toxic constituents from coal fly ash mixed soils under the influence of pH. Waste Manag. 2015, 38, 174–184. [Google Scholar] [CrossRef]
- Masuda, N.; Marković, R.; Bessho, M.; Božić, D.; Obradović, L.; Marinković, V.; Ishiyama, D.; Stevanović, Z. A new approach to recover dissolved metals in AMD by two-step pH control on the neutralization method. In Proceedings of the 13th International Mine Water Association Congress “Mine Water and Circular Economy” (IMWA 2017), Lappeenranta, Finland, 25–30 June 2017; Wolkersdrofer, C., Sartz, L., Silanpaa, M., Hakkinen, A., Eds.; Lappeenranta University of Technology: Lappeenranta, Finland, 2017; pp. 1111–1118. [Google Scholar]
- Park, S.M.; Yoo, J.C.; Ji, S.W.; Yang, J.S.; Baek, K. Selective recovery of dissolved Fe, Al, Cu and Zn in acid mine drainage based on modeling to predict precipitation pH. Environ. Sci. Pollut. Res. Int. 2015, 22, 3013–3022. [Google Scholar] [CrossRef]
- Cudennec, Y.; Lecerf, A. The transformation of ferrihydrite into goethite or hematite revisited. J. Solid State Chem. 2006, 179, 716–722. [Google Scholar] [CrossRef]
- Giannopoulou, I.; Panias, D. Differential precipitation of copper and nickel from acidic polymetallic aqueous solutions. Hydrometallurgy 2008, 90, 137–146. [Google Scholar] [CrossRef]
- Park, S.M.; Yoo, J.C.; Ji, S.W.; Yang, J.S.; Baek, K. Selective recovery of Cu, Zn, and Ni from acid mine drainage. Environ. Geochem. Health 2013, 35, 735–743. [Google Scholar] [CrossRef]
- Patterson, J.W.; Allen, H.E.; Scala, J.J. Carbonate precipitation for heavy metals pollutants. J. Water Pollut. Control. Fed. 1977, 49, 2397–2410. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).