Reducing-Effect of Chloride for the Dissolution of Black Copper
Abstract
1. Introduction
2. Experimental
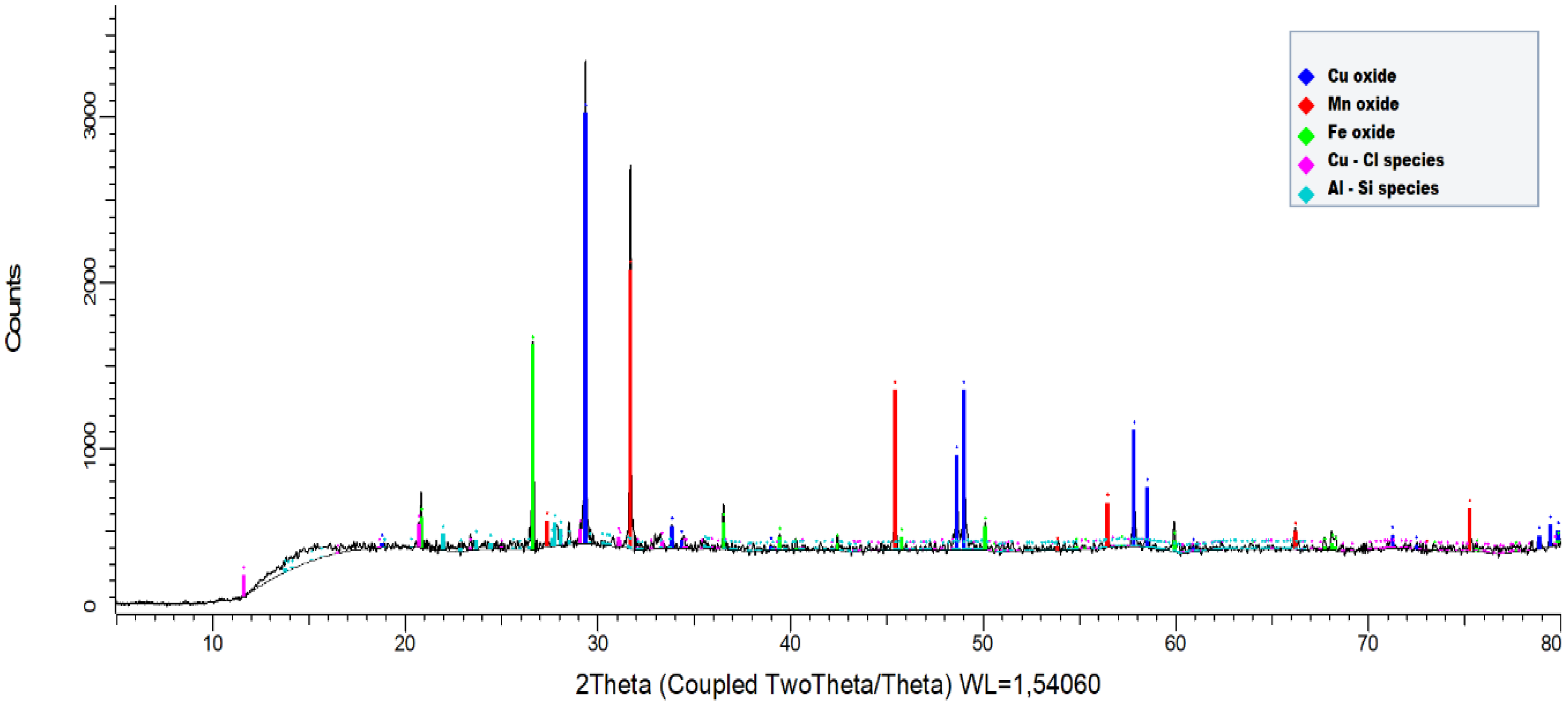
2.1. Black Copper Simple
2.2. Ferrous Ions
2.3. Pre-Treatment and Subsequent Leaching in reactors
2.4. Dissolution of Mn and Cu
2.5. Effect of NaCl Concentration and Cure Time
3. Results
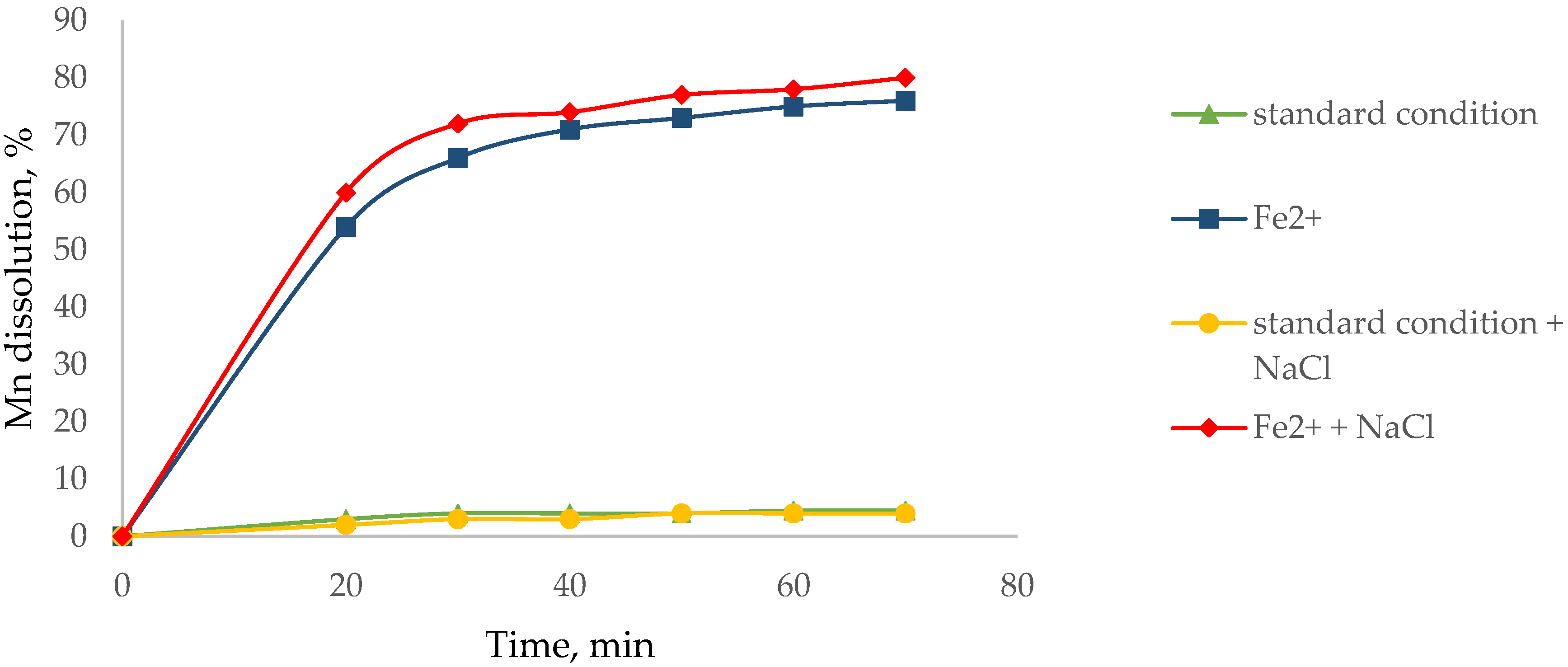
3.1. Effect of Agglomerate with NaCl to Dissolve Mn
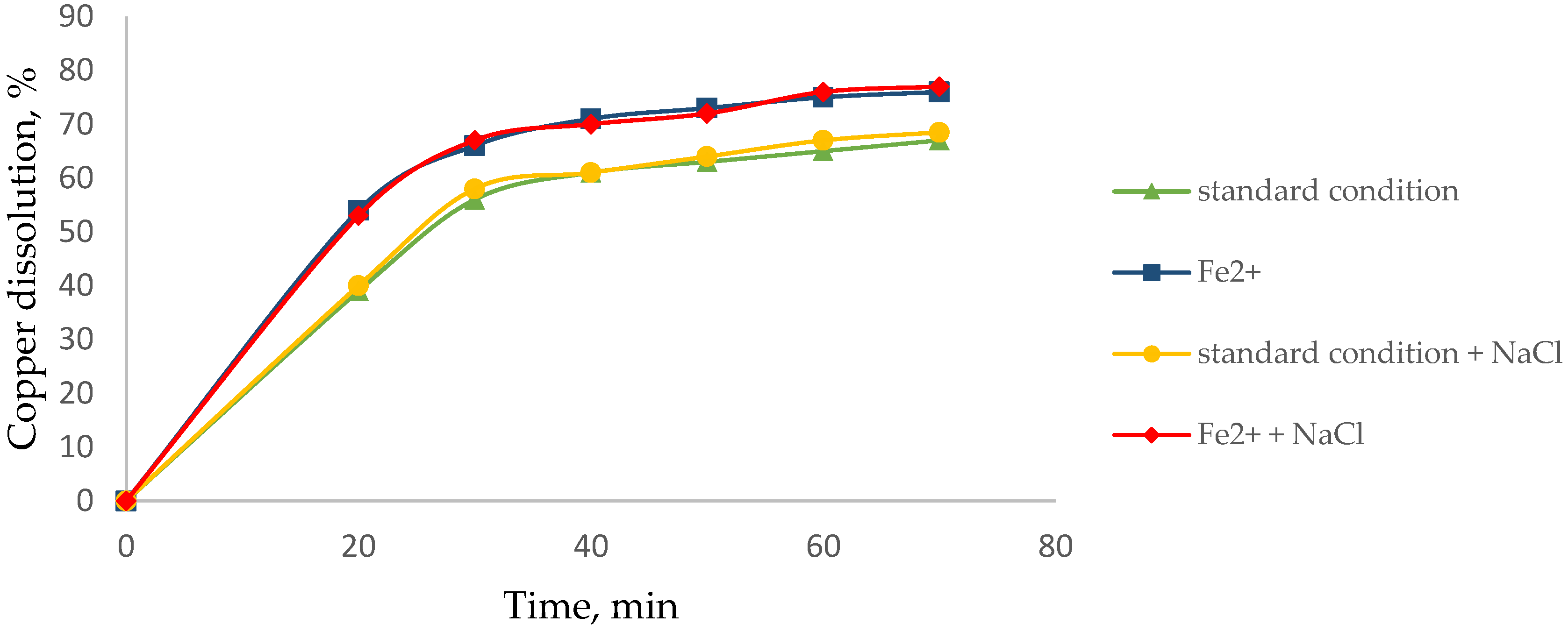
3.2. Effect of Agglomerate with NaCl to Dissolve Cu
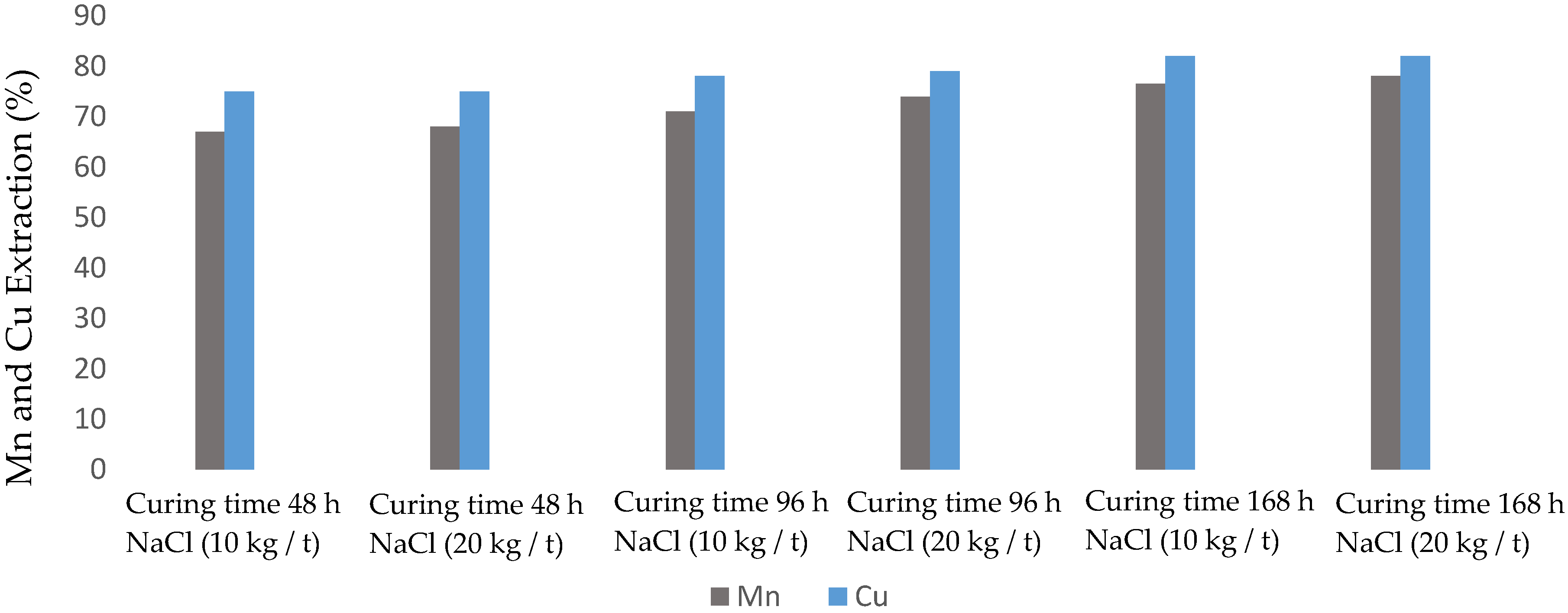
3.3. Effect of NaCl Concentration and Cure Time
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Cuadra, C.P.; Rojas, S.G. Oxide mineralization at the Radomiro Tomic porphyry copper deposit, Northern Chile. Econ. Geol. 2001, 96, 387–400. [Google Scholar]
- Pinget, M.; Dold, B.; Fontboté, L. Exotic mineralization at Chuquicamata, Chile: Focus on the copper wad enigma. In Proceedings of the 10th Swiss Geoscience Meeting, Bern, Switzerland, 16–17 November 2012; pp. 88–89. [Google Scholar]
- Kojima, S.; Astudillo, J.; Rojo, J.; Tristá, D.; Hayashi, K.I. Ore mineralogy, fluid inclusion, and stable isotopic characteristics of stratiform copper deposits in the coastal Cordillera of northern Chile. Miner. Depos. 2003, 38, 208–216. [Google Scholar] [CrossRef]
- Chavez, W.X., Jr. Supergene oxidation of copper deposits; zoning and distribution of copper oxide minerals. SEG Newsl. 2000, 41, 1–21. [Google Scholar]
- Pincheira, M.; Dagnini, A.; Kelm, U.; Helle, S. Copper pitch y copper wad: Contraste entre las fases presentes en las cabezas y en los ripios en pruebas de mina sur, Chuquicamata. In Proceedings of the X Congreso Geológico Chileno; 10° CONGRESO GEOLÓGICO CHILENO 2003, Concepción, Chile, 6–10 October 2003; p. 10. [Google Scholar]
- Pérez, K.; Toro, N.; Campos, E.; González, J.; Jeldres, R.I.; Nazer, A.; Rodriguez, M.H. Extraction of Mn from black copper using iron oxides from tailings and Fe2+ as reducing agents in acid medium. Metals (Basel) 2019, 9, 1112. [Google Scholar] [CrossRef]
- Benavente, O.; Hernández, M.C.; Melo, E.; Núñez, D.; Quezada, V.; Zepeda, Y. Copper dissolution from black copper ore under oxidizing and reducing conditions. Metals (Basel) 2019, 9, 799. [Google Scholar] [CrossRef]
- Helle, S.; Pincheira, M.; Jerez, O.; Kelm, U. Sequential extraction to predict the leaching potential of refractory black copper ores. In Proceedings of the XV Balkan Mineral Processing Congress, Sozopol, Bulgaria, 12–16 June 2013; pp. 109–111. [Google Scholar]
- Sahoo, R.N.; Naik, P.K.; Das, S.C. Leaching of manganese from low-grade manganese ore using oxalic acid as reductant in sulphuric acid solution. Hydrometallurgy 2001, 62, 157–163. [Google Scholar] [CrossRef]
- Furlani, G.; Pagnanelli, F.; Toro, L. Reductive acid leaching of manganese dioxide with glucose: Identification of oxidation derivatives of glucose. Hydrometallurgy 2006, 81, 234–240. [Google Scholar] [CrossRef]
- Bafghi, M.S.; Zakeri, A.; Ghasemi, Z.; Adeli, M. Reductive dissolution of manganese ore in sulfuric acid in the presence of iron metal. Hydrometallurgy 2008, 90, 207–212. [Google Scholar] [CrossRef]
- Su, H.; Wen, Y.; Wang, F.; Sun, Y.; Tong, Z. Reductive leaching of manganese from low-grade manganese ore in H2SO4 using cane molasses as reductant. Hydrometallurgy 2008, 93, 136–139. [Google Scholar] [CrossRef]
- Tian, X.; Wen, X.; Yang, C.; Liang, Y.; Pi, Z.; Wang, Y. Reductive leaching of manganese from low-grade manganese dioxide ores using corncob as reductant in sulfuric acid solution. Hydrometallurgy 2010, 100, 157–160. [Google Scholar] [CrossRef]
- Tang, Q.; Zhong, H.; Wang, S.; Li, J.Z.; Liu, G.Y. Reductive leaching of manganese oxide ores using waste tea as reductant in sulfuric acid solution. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2014, 24, 861–867. [Google Scholar] [CrossRef]
- Toro, N.; Saldaña, M.; Castillo, J.; Higuera, F.; Acosta, R. Leaching of manganese from marine nodules at room temperature with the use of sulfuric acid and the addition of tailings. Minerals 2019, 9, 289. [Google Scholar] [CrossRef]
- Toro, N.; Saldaña, M.; Gálvez, E.; Cánovas, M.; Trigueros, E.; Castillo, J.; Hernández, P.C. Optimization of parameters for the dissolution of Mn from manganese nodules with the use of tailings in an acid medium. Minerals 2019, 9, 387. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, C. Hydrometallurgical process for recovery of cobalt from zinc plant residue. Hydrometallurgy 2002, 63, 225–234. [Google Scholar] [CrossRef]
- Fuerstenau, D.W.; Han, K.N. Metallurgy and processing of marine manganese nodules. Miner. Process. Extr. Metall. Rev. 1983, 1, 1–83. [Google Scholar] [CrossRef]
- Senanayake, G. Acid leaching of metals from deep-sea manganese nodules—A critical review of fundamentals and applications. Miner. Eng. 2011, 24, 1379–1396. [Google Scholar] [CrossRef]
- Velásquez-yévenes, L.; Torres, D.; Toro, N. Hydrometallurgy Leaching of chalcopyrite ore agglomerated with high chloride concentration and high curing periods. Hydrometallurgy 2018, 181, 215–220. [Google Scholar] [CrossRef]
- Cerda, C.P.; Taboada, M.E.; Jamett, N.E.; Ghorbani, Y.; Hernández, P.C. Effect of pretreatment on leaching primary copper sulfide in acid-chloride media. Minerals 2018, 8, 1. [Google Scholar] [CrossRef]
- Bahamonde, F.; Gómez, M.; Navarro, P. Pre-treatment with sodium chloride and sulfuric acid of a bornitic concentrate and later leaching in chloride solution. In Proceedings of the Leaching and Bioleaching of Sulfide Concentrates and Minerals, Hydroprocess-ICMSE, Santiago, Chile, 22 June 2017. [Google Scholar]
- Toro, N.; Briceño, W.; Pérez, K.; Cánovas, M.; Trigueros, E.; Sepúlveda, R.; Hernández, P. Leaching of pure chalcocite in a chloride media using sea water and waste water. Metals (Basel) 2019, 9, 780. [Google Scholar] [CrossRef]
- Cruz, C.; Reyes, A.; Jeldres, R.I.; Cisternas, L.A.; Kraslawski, A. Using partial desalination treatment to improve the recovery of copper and molybdenum minerals in the Chilean mining industry. Ind. Eng. Chem. Res. 2019, 58, 8915–8922. [Google Scholar] [CrossRef]
- Zakeri, A.; Bafghi, M.; Shahriari, S. Dissolution kinetics of manganese dioxide ore in sulfuric acid in the presence of ferrous ion. Iran. J. Mater. Sci. Eng. 2007, 4, 22–27. [Google Scholar]
- Toro, N.; Herrera, N.; Castillo, J.; Torres, C.; Sepúlveda, R. Initial Investigation into the leaching of manganese from nodules at room temperature with the use of sulfuric acid and the addition of foundry slag—Part I. Minerals 2018, 8, 565. [Google Scholar] [CrossRef]
- Saldaña, M.; Toro, N.; Castillo, J.; Hernández, P.; Trigueros, E.; Navarra, A. Development of an analytical model for the extraction of manganese from marine nodules. Metals (Basel) 2019, 9, 903. [Google Scholar] [CrossRef]
- Torres, D.; Ayala, L.; Saldaña, M.; Cánovas, M.; Jeldres, R.I.; Nieto, S.; Castillo, J.; Robles, P.; Toro, N. Leaching manganese nodules in an acid medium and room temperature comparing the use of different Fe reducing agents. Metals (Basel) 2019, 9, 1316. [Google Scholar] [CrossRef]
- Komnitsas, K.; Bazdanis, G.; Bartzas, G.; Sahinkaya, E.; Zaharaki, D. Removal of heavy metals from leachates using organic/inorganic permeable reactive barriers. Desalin. Water Treat. 2013, 51, 3052–3059. [Google Scholar] [CrossRef]
| Mn (%) | Cu (%) | Fe (%) |
|---|---|---|
| 22.01 | 40.24 | 7.92 |
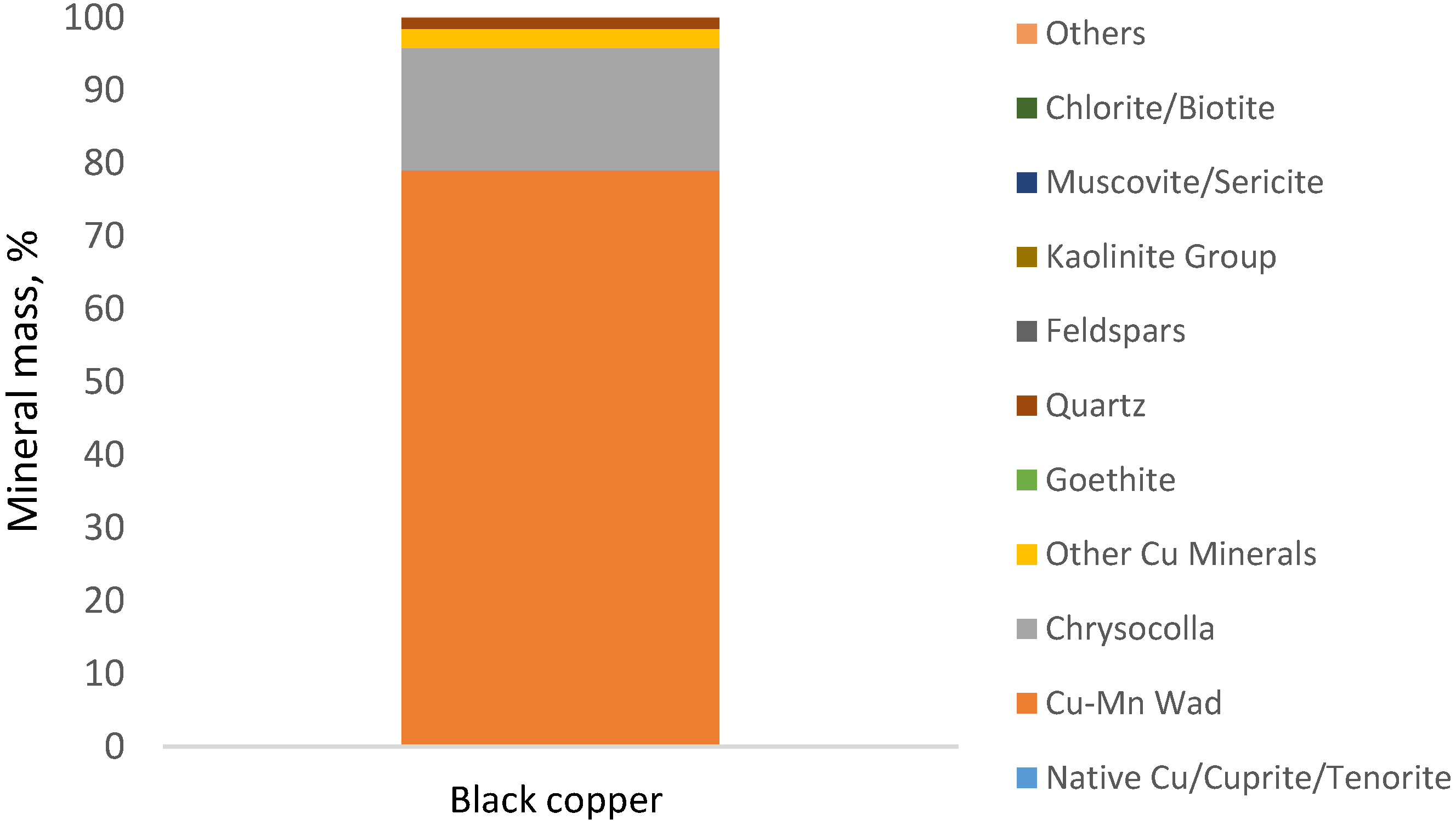
| Mineral (% Mass) | Black Copper |
|---|---|
| Native Cu/Cuprite/Tenorite | 0.12 |
| Cu-Mn Wad | 78.90 |
| Chrysocolla | 16.72 |
| Other Cu Minerals | 2.69 |
| Goethite | 0.01 |
| Quartz | 1.41 |
| Feldspars | 0.02 |
| Kaolinite Group | 0.01 |
| Muscovite/Sericite | 0.01 |
| Chlorite/Biotite | 0.01 |
| Others | 0.09 |
| Total | 100 |
| Test | Curing Time (h) | NaCl Concentration (kg/t) |
|---|---|---|
| 1 | 48 | 10 |
| 2 | 48 | 20 |
| 3 | 96 | 10 |
| 4 | 96 | 20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, D.; Pérez, K.; Trigueros, E.; I. Jeldres, R.; Salinas-Rodríguez, E.; Robles, P.; Toro, N. Reducing-Effect of Chloride for the Dissolution of Black Copper. Metals 2020, 10, 123. https://doi.org/10.3390/met10010123
Torres D, Pérez K, Trigueros E, I. Jeldres R, Salinas-Rodríguez E, Robles P, Toro N. Reducing-Effect of Chloride for the Dissolution of Black Copper. Metals. 2020; 10(1):123. https://doi.org/10.3390/met10010123
Chicago/Turabian StyleTorres, David, Kevin Pérez, Emilio Trigueros, Ricardo I. Jeldres, Eleazar Salinas-Rodríguez, Pedro Robles, and Norman Toro. 2020. "Reducing-Effect of Chloride for the Dissolution of Black Copper" Metals 10, no. 1: 123. https://doi.org/10.3390/met10010123
APA StyleTorres, D., Pérez, K., Trigueros, E., I. Jeldres, R., Salinas-Rodríguez, E., Robles, P., & Toro, N. (2020). Reducing-Effect of Chloride for the Dissolution of Black Copper. Metals, 10(1), 123. https://doi.org/10.3390/met10010123








