Antimicrobial Resistance: The Impact from and on Society According to One Health Approach
Abstract
1. Introduction
2. Methods
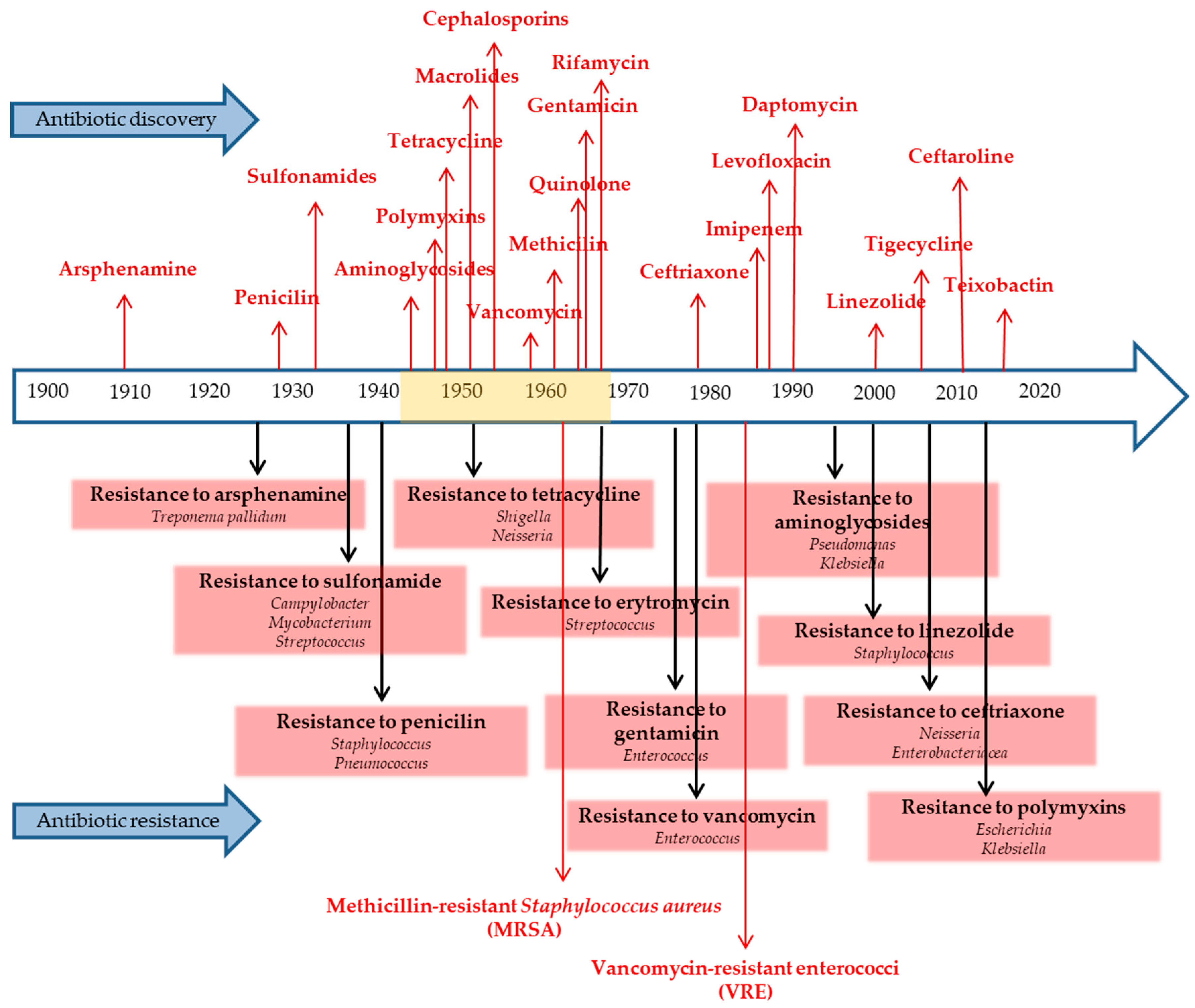
3. Chronology of Key Antibiotic Discoveries and the Rise of Antibiotic Resistance
4. Factors Contributing to AMR
4.1. Antibiotic Misuse
4.2. Inappropriate Prescription Practices
4.3. Scarcity of Next-Generation Antibiotics
4.4. Livestock Use of Antibiotics
4.5. Mobility
4.6. Lack of Knowledge
5. Strategies to Combat AMR
5.1. Antimicrobial Stewardship Programs
5.2. Reduced Use of Antibiotics in Animals
5.3. Creating New Medications and Vaccines
5.4. One Health Approach
6. Antimicrobial Resistance and Society
6.1. General Considerations
6.2. AMR in Developing Countries
6.3. AMR in Elderly Centers
7. Discussion
8. Conclusions and Future Directions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial resistance |
| ASPs | Antimicrobial stewardship programs |
| CDC | Centers for Disease Control and Prevention |
| CoNS | Coagulase-negative Staphylococci |
| ESBLs | Extended-spectrum β-lactamases |
| MDR | Multidrug-resistant |
| MDR-TB | Multidrug-resistant tuberculosis |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| TB | Tuberculosis |
| US | United States |
| VISA | Vancomycin-intermediate Staphylococcus aureus |
| VRE | Vancomycin-resistant Enterococcus |
| VRSA | Vancomycin-resistant Staphylococcus aureus |
| WHO | World Health Organization |
| XDR-TB | Extensively drug-resistant tuberculosis |
References
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Munoz-Davila, M.J. Role of Old Antibiotics in the Era of Antibiotic Resistance. Highlighted Nitrofurantoin for the Treatment of Lower Urinary Tract Infections. Antibiotics 2014, 3, 39–48. [Google Scholar] [CrossRef]
- Moo, C.L.; Yang, S.K.; Yusoff, K.; Ajat, M.; Thomas, W.; Abushelaibi, A.; Lim, S.H.; Lai, K.S. Mechanisms of Antimicrobial Resistance (AMR) and Alternative Approaches to Overcome AMR. Curr. Drug Discov. Technol. 2020, 17, 430–447. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.Y.; Xu, S.X.; Tang, Z.X.; Li, Z.K.; Zhang, L. Use of antimicrobials in food animals and impact of transmission of antimicrobial resistance on humans. Biosaf. Health 2021, 3, 32–38. [Google Scholar] [CrossRef]
- Castanon, J.I. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 2007, 86, 2466–2471. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Food Animals from 2017 to 2030. Antibiotics 2020, 9, 918. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Assessing the Health Burden of Infections with Antibiotic-Resistant Bacteria in the EU/EEA, 2016–2020; European Centre for Disease Prevention and Control: Solna, Sweden, 2022.
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.M.R.M.; Mitra, S.; Bin Emran, T.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef]
- White, A.; Hughes, J.M. Critical Importance of a One Health Approach to Antimicrobial Resistance. Ecohealth 2019, 16, 404–409. [Google Scholar] [CrossRef]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Kirchhelle, C.; Podolsky, S.H. An Awkward Fit: Antimicrobial Resistance and the Evolution of International Health Politics (1945–2022). J. Law. Med. Ethics 2022, 50, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Collignon, P.; Beggs, J.J. Socioeconomic Enablers for Contagion: Factors Impelling the Antimicrobial Resistance Epidemic. Antibiotics 2019, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Perales-Adan, J.; Rubino, S.; Martinez-Bueno, M.; Valdivia, E.; Montalban-Lopez, M.; Cebrian, R.; Maqueda, M. LAB Bacteriocins Controlling the Food Isolated (Drug-Resistant) Staphylococci. Front. Microbiol. 2018, 9, 1143. [Google Scholar] [CrossRef]
- Chokshi, A.; Sifri, Z.; Cennimo, D.; Horng, H. Global Contributors to Antibiotic Resistance. J. Glob. Infect. Dis. 2019, 11, 36–42. [Google Scholar] [CrossRef]
- Shrestha, P.; Cooper, B.S.; Coast, J.; Oppong, R.; Do Thi Thuy, N.; Phodha, T.; Celhay, O.; Guerin, P.J.; Wertheim, H.; Lubell, Y. Enumerating the economic cost of antimicrobial resistance per antibiotic consumed to inform the evaluation of interventions affecting their use. Antimicrob. Resist. Infect. Control 2018, 7, 98. [Google Scholar] [CrossRef]
- Weist, K.; Hogberg, L.D. ECDC publishes 2015 surveillance data on antimicrobial resistance and antimicrobial consumption in Europe. Euro Surveill 2016, 21, 30401. [Google Scholar] [CrossRef]
- Silva, V.; Canica, M.; Capelo, J.L.; Igrejas, G.; Poeta, P. Diversity and genetic lineages of environmental staphylococci: A surface water overview. FEMS Microbiol. Ecol. 2020, 96, fiaa191. [Google Scholar] [CrossRef]
- Gwenzi, W.; Chaukura, N.; Muisa-Zikali, N.; Teta, C.; Musvuugwa, T.; Rzymski, P.; Abia, A.L.K. Insects, Rodents, and Pets as Reservoirs, Vectors, and Sentinels of Antimicrobial Resistance. Antibiotics 2021, 10, 68. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Antimicrobial Resistance, C. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Goncalves, E.; Carvalhal, R.; Mesquita, R.; Azevedo, J.; Coelho, M.J.; Magalhaes, R.; Ferraz, M.P.; Manso, M.C.; Gavinha, S.; Pina, C.; et al. Detection of Staphylococcus aureus (MRSA/MSSA) in surfaces of dental medicine equipment. Saudi J. Biol. Sci. 2020, 27, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 9 May 2024).
- World Health Organization. Multidrug-Resistant Tuberculosis (MDR-TB). Available online: https://www.who.int/docs/default-source/documents/tuberculosis/multidrug-resistant-tuberculosis-mdr-tb.pdf (accessed on 9 May 2024).
- Ghimpeteanu, O.M.; Pogurschi, E.N.; Popa, D.C.; Dragomir, N.; Dragotoiu, T.; Mihai, O.D.; Petcu, C.D. Antibiotic Use in Livestock and Residues in Food-A Public Health Threat: A Review. Foods 2022, 11, 1430. [Google Scholar] [CrossRef]
- Founou, R.C.; Founou, L.L.; Essack, S.Y. Clinical and economic impact of antibiotic resistance in developing countries: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0189621. [Google Scholar] [CrossRef]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Aminov, R.I. A brief history of the antibiotic era: Lessons learned and challenges for the future. Front. Microbiol. 2010, 1, 134. [Google Scholar] [CrossRef]
- Tan, S.Y.; Tatsumura, Y. Alexander Fleming (1881–1955): Discoverer of penicillin. Singapore Med. J. 2015, 56, 366–367. [Google Scholar] [CrossRef]
- Chavada, J.; Muneshwar, K.N.; Ghulaxe, Y.; Wani, M.; Sarda, P.P.; Huse, S. Antibiotic Resistance: Challenges and Strategies in Combating Infections. Cureus 2023, 15, e46013. [Google Scholar] [CrossRef]
- Srinivasan, A.; Dick, J.D.; Perl, T.M. Vancomycin resistance in staphylococci. Clin. Microbiol. Rev. 2002, 15, 430–438. [Google Scholar] [CrossRef]
- Hourigan, D.; Stefanovic, E.; Hill, C.; Ross, R.P. Promiscuous, persistent and problematic: Insights into current enterococcal genomics to guide therapeutic strategy. BMC Microbiol. 2024, 24, 103. [Google Scholar] [CrossRef]
- Parmar, A.; Lakshminarayanan, R.; Iyer, A.; Mayandi, V.; Leng Goh, E.T.; Lloyd, D.G.; Chalasani, M.L.S.; Verma, N.K.; Prior, S.H.; Beuerman, R.W.; et al. Design and Syntheses of Highly Potent Teixobactin Analogues against Staphylococcus aureus, Methicillin-Resistant Staphylococcus aureus (MRSA), and Vancomycin-Resistant Enterococci (VRE) In Vitro and In Vivo. J. Med. Chem. 2018, 61, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- Grossman, T.H. Tetracycline Antibiotics and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025387. [Google Scholar] [CrossRef] [PubMed]
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A Review on Antibiotic Resistance: Alarm Bells are Ringing. Cureus 2017, 9, e1403. [Google Scholar] [CrossRef]
- Suay-Garcia, B.; Perez-Gracia, M.T. Present and Future of Carbapenem-resistant Enterobacteriaceae (CRE) Infections. Antibiotics 2019, 8, 122. [Google Scholar] [CrossRef]
- Parmanik, A.; Das, S.; Kar, B.; Bose, A.; Dwivedi, G.R.; Pandey, M.M. Current Treatment Strategies Against Multidrug-Resistant Bacteria: A Review. Curr. Microbiol. 2022, 79, 388. [Google Scholar] [CrossRef]
- Abushaheen, M.A.; Muzaheed; Fatani, A.J.; Alosaimi, M.; Mansy, W.; George, M.; Acharya, S.; Rathod, S.; Divakar, D.D.; Jhugroo, C.; et al. Antimicrobial resistance, mechanisms and its clinical significance. Dis. Mon. 2020, 66, 100971. [Google Scholar] [CrossRef]
- Michael, C.A.; Dominey-Howes, D.; Labbate, M. The antimicrobial resistance crisis: Causes, consequences, and management. Front. Public Health 2014, 2, 145. [Google Scholar] [CrossRef]
- Chang, Q.; Wang, W.; Regev-Yochay, G.; Lipsitch, M.; Hanage, W.P. Antibiotics in agriculture and the risk to human health: How worried should we be? Evol. Appl. 2015, 8, 240–247. [Google Scholar] [CrossRef]
- Carter, R.R.; Sun, J.; Jump, R.L. A Survey and Analysis of the American Public’s Perceptions and Knowledge About Antibiotic Resistance. Open Forum Infect. Dis. 2016, 3, ofw112. [Google Scholar] [CrossRef]
- Castro-Sanchez, E.; Moore, L.S.; Husson, F.; Holmes, A.H. What are the factors driving antimicrobial resistance? Perspectives from a public event in London, England. BMC Infect. Dis. 2016, 16, 465. [Google Scholar] [CrossRef]
- DiMasi, J.A.; Grabowski, H.G.; Hansen, R.W. Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ. 2016, 47, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Woolhouse, M.; Waugh, C.; Perry, M.R.; Nair, H. Global disease burden due to antibiotic resistance-state of the evidence. J. Glob. Health 2016, 6, 010306. [Google Scholar] [CrossRef] [PubMed]
- Arcilla, M.S.; van Hattem, J.M.; Haverkate, M.R.; Bootsma, M.C.J.; van Genderen, P.J.J.; Goorhuis, A.; Grobusch, M.P.; Lashof, A.M.O.; Molhoek, N.; Schultsz, C.; et al. Import and spread of extended-spectrum beta-lactamase-producing Enterobacteriaceae by international travellers (COMBAT study): A prospective, multicentre cohort study. Lancet Infect. Dis. 2017, 17, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Chaw, P.S.; Hopner, J.; Mikolajczyk, R. The knowledge, attitude and practice of health practitioners towards antibiotic prescribing and resistance in developing countries-A systematic review. J. Clin. Pharm. Ther. 2018, 43, 606–613. [Google Scholar] [CrossRef]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef]
- Pulia, M.; Kern, M.; Schwei, R.J.; Shah, M.N.; Sampene, E.; Crnich, C.J. Comparing appropriateness of antibiotics for nursing home residents by setting of prescription initiation: A cross-sectional analysis. Antimicrob. Resist. Infect. Control 2018, 7, 74. [Google Scholar] [CrossRef]
- Bokhary, H.; Pangesti, K.N.A.; Rashid, H.; Abd El Ghany, M.; Hill-Cawthorne, G.A. Travel-Related Antimicrobial Resistance: A Systematic Review. Trop. Med. Infect. Dis. 2021, 6, 11. [Google Scholar] [CrossRef]
- Dutescu, I.A.; Hillier, S.A. Encouraging the Development of New Antibiotics: Are Financial Incentives the Right Way Forward? A Systematic Review and Case Study. Infect. Drug Resist. 2021, 14, 415–434. [Google Scholar] [CrossRef]
- Laur, C.; Sribaskaran, T.; Simeoni, M.; Desveaux, L.; Daneman, N.; Mulhall, C.; Lam, J.; Ivers, N.M. Improving antibiotic initiation and duration prescribing among nursing home physicians using an audit and feedback intervention: A theory-informed qualitative analysis. BMJ Open Qual. 2021, 10, e001088. [Google Scholar] [CrossRef]
- McCubbin, K.D.; Anholt, R.M.; de Jong, E.; Ida, J.A.; Nobrega, D.B.; Kastelic, J.P.; Conly, J.M.; Gotte, M.; McAllister, T.A.; Orsel, K.; et al. Knowledge Gaps in the Understanding of Antimicrobial Resistance in Canada. Front. Public Health 2021, 9, 726484. [Google Scholar] [CrossRef]
- Sridhar, S.; Turbett, S.E.; Harris, J.B.; LaRocque, R.C. Antimicrobial-resistant bacteria in international travelers. Curr. Opin. Infect. Dis. 2021, 34, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Iskandar, K.; Murugaiyan, J.; Halat, D.H.; Hage, S.E.; Chibabhai, V.; Adukkadukkam, S.; Roques, C.; Molinier, L.; Salameh, P.; Van Dongen, M. Antibiotic Discovery and Resistance: The Chase and the Race. Antibiotics 2022, 11, 182. [Google Scholar] [CrossRef]
- Smit, C.C.H.; Lambert, M.; Rogers, K.; Djordjevic, S.P.; Van Oijen, A.M.; Keighley, C.; Taxis, K.; Robertson, H.; Pont, L.G. One Health Determinants of Antimicrobial Resistance in Humans in the Community: An Umbrella Review. Int. J. Mol. Sci. 2023, 24, 17204. [Google Scholar] [CrossRef]
- Bo, L.T.; Sun, H.D.; Li, Y.D.; Zhu, J.A.T.; Wurpel, J.N.D.; Lin, H.L.; Chen, Z.S. Combating antimicrobial resistance: The silent war. Front. Pharmacol. 2024, 15, 1347750. [Google Scholar] [CrossRef]
- de Barros Fernandes, T.; Ramos, S.F.; Leitzke, L.R.F.; Júnior, R.G.A.; de Araújo, J.M.; de Souza Júnior, A.S.; da Silva, A.R.O.; Heineck, I.; de França Fonteles, M.M.; Bracken, L.E.; et al. Use of antimicrobials in pediatric wards of five Brazilian hospitals. BMC Pediatr. 2024, 24, 177. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Antimicrobial Resistance. Available online: https://www.fao.org/antimicrobial-resistance/key-sectors/animal-production/en/ (accessed on 1 May 2024).
- Gattinger, D.; Schlenz, V.; Weil, T.; Sattler, B. From remote to urbanized: Dispersal of antibiotic-resistant bacteria under the aspect of anthropogenic influence. Sci. Total Environ. 2024, 924, 171532. [Google Scholar] [CrossRef]
- Koenig, E.; Kriegl, L.; Pux, C.; Uhlmann, M.; Schippinger, W.; Avian, A.; Krause, R.; Zollner-Schwetz, I. Implementation of an antimicrobial stewardship program for urinary tract infections in long-term care facilities: A cluster-controlled intervention study. Antimicrob. Resist. Infect. Control 2024, 13, 43. [Google Scholar] [CrossRef]
- Netea, S.A.; Messina, N.L.; Gardiner, K.; Pittet, L.F.; Curtis, N.; Consortium, M.B.T. Inappropriate prescribing contributes to high antibiotic exposure in young children in Australia. J. Antimicrob. Chemoth. 2024, 79, 1289–1293. [Google Scholar] [CrossRef]
- Pires, A.J.; Pereira, G.; Fangueiro, D.; Bexiga, R.; Oliveira, M. When the solution becomes the problem: A review on antimicrobial resistance in dairy cattle. Future Microbiol. 2024, 19, 903–929. [Google Scholar] [CrossRef]
- Robles-Jimenez, L.E.; Ghavipanje, N.; Hernandez, J.C.A.; Gonzalez-Ronquillo, M. Recent developments in antibiotic contamination of animal products, soil, and water worldwide. Ann. Anim. Sci. 2024. [Google Scholar] [CrossRef]
- Singh, V.P.; Jha, D.; Rehman, B.U.; Dhayal, V.S.; Dhar, M.S.; Sharma, N. A mini-review on the burden of antimicrobial resistance and its regulation across one health sectors in India. J. Agr. Food Res. 2024, 15, 100973. [Google Scholar] [CrossRef]
- Sunpuwan, M.; Punpuing, S.; Jaruruengpaisan, W.; Wertheim, H. Understanding antibiotic use in the community setting in Thailand: Does communication matter? PLoS ONE 2024, 19, e0298972. [Google Scholar] [CrossRef]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report: 2022. Available online: https://www.who.int/publications/i/item/9789240062702 (accessed on 14 May 2024).
- Yang, Y.Y.; Xie, S.Y.; He, F.J.; Xu, Y.D.; Wang, Z.F.; Ihsan, A.; Wang, X. Recent development and fighting strategies for lincosamide antibiotic resistance. Clin. Microbiol. Rev. 2024, 37, e00161-23. [Google Scholar] [CrossRef] [PubMed]
- Zarske, M.; Luu, H.Q.; Deneke, C.; Knüver, M.T.; Thieck, M.; Hoang, H.T.; Bretschneider, N.; Pham, N.T.; Huber, I.; Stingl, K. Identification of knowledge gaps in whole-genome sequence analysis of multi-resistant thermotolerant spp. BMC Genom. 2024, 25, 156. [Google Scholar] [CrossRef]
- Zhang, X.X.; Lederman, Z.; Han, L.F.; Schurer, J.M.; Xiao, L.H.; Zhang, Z.B.; Chen, Q.L.; Pfeiffer, D.; Ward, M.P.; Sripa, B.; et al. Towards an actionable One Health approach. Infect. Dis. Poverty 2024, 13, 28. [Google Scholar] [CrossRef]
- Pandey, S.; Doo, H.; Keum, G.B.; Kim, E.S.; Kwak, J.; Ryu, S.; Choi, Y.; Kang, J.; Kim, S.; Lee, N.R.; et al. Antibiotic resistance in livestock, environment and humans: One Health perspective. J. Anim. Sci. Technol. 2024, 66, 266–278. [Google Scholar] [CrossRef]
- Cella, E.; Giovanetti, M.; Benedetti, F.; Scarpa, F.; Johnston, C.; Borsetti, A.; Ceccarelli, G.; Azarian, T.; Zella, D.; Ciccozzi, M. Joining Forces against Antibiotic Resistance: The One Health Solution. Pathogens 2023, 12, 1074. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan on Antimicrobial Resistance. Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 25 August 2024).
- Guisado-Gil, A.B.; Mejias-Trueba, M.; Penalva, G.; Aguilar-Guisado, M.; Molina, J.; Gimeno, A.; Alvarez-Marin, R.; Praena, J.; Bueno, C.; Lepe, J.A.; et al. Antimicrobial Stewardship in the Emergency Department Observation Unit: Definition of a New Indicator and Evaluation of Antimicrobial Use and Clinical Outcomes. Antibiotics 2024, 13, 356. [Google Scholar] [CrossRef]
- Fiallo, P.; Williams, T.; Bush, L.M. When Antimicrobial Treatment and Surgical Prophylaxis Collide: A Stewardship Opportunity. Hosp. Pharm. 2024, 59. [Google Scholar] [CrossRef]
- Pant, S.; Corwin, A.; Adhikari, P.; Acharya, S.P.; Acharya, U.; Silwal, S.; Dawadi, P.; Poudyal, A.; Paudyal, V.; Bhumiratana, A. Evaluating Antibiotic Treatment Guideline Adherence to Ongoing Antibiotic Stewardship in a Tertiary Care Setting: A Retrospective Observational Study. Can. J. Infect. Dis. Med. 2024, 2024, 6663119. [Google Scholar] [CrossRef]
- Balm, M.; Bupha-Intr, O.; Sinha, T.; Kelly, M.; Stewart, L.; Stephen, R.; Blackmore, T.; Bloomfield, M. Incorporating patient, nursing and environmental factors into antimicrobial stewardship: Effects of simplifying treatment from cefuroxime to ceftriaxone. N. Z. Med. J. 2024, 137, 31–42. [Google Scholar] [CrossRef]
- Smith, D.R.; Olivier, A.K.; Thurlow, M.H. Enhancing the health of animals through antimicrobial stewardship research. Am. J. Vet. Res. 2024, 85. [Google Scholar] [CrossRef]
- Courtenay, M.; Hawker, C.; Gallagher, R.; Castro-Sanchez, E.; Gould, D.J.; Al Salti, F.; Bate, J.; Cooper, D.; Cooper, R.; Craig, R.; et al. The application of antimicrobial stewardship knowledge to nursing practice: A national survey of United Kingdom pre-registration nursing students. J. Adv. Nurs. 2024. [Google Scholar] [CrossRef]
- Hadi, H.A.; Eltayeb, F.; Al Balushi, S.; Daghfal, J.; Ahmed, F.; Mateus, C. Evaluation of Hospital Antimicrobial Stewardship Programs: Implementation, Process, Impact, and Outcomes, Review of Systematic Reviews. Antibiotics 2024, 13, 253. [Google Scholar] [CrossRef]
- Madaline, T.; Classen, D.C.; Eby, J.C. Building the Future of Infectious Diseases: A Call to Action for Quality Improvement Research and Measurement. J. Infect. Dis. 2024. [Google Scholar] [CrossRef]
- Nampoothiri, V.; Mbamalu, O.; Mendelson, M.; Singh, S.; Charani, E. Pharmacist roles in antimicrobial stewardship: A qualitative study from India, South Africa and the United Kingdom. JAC-Antimicrob. Resis 2024, 6, dlae047. [Google Scholar] [CrossRef]
- Zini, T.; Miselli, F.; D’Esposito, C.; Fidanza, L.; Costantini, R.C.; Corso, L.; Mazzotti, S.; Rossi, C.; Spaggiari, E.; Rossi, K.; et al. Sustaining the Continued Effectiveness of an Antimicrobial Stewardship Program in Preterm Infants. Trop. Med. Infect. Dis. 2024, 9, 59. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Implementation of Antibiotic Stewardship Core Elements at Small and Critical Access Hospitals. Available online: https://www.cdc.gov/antibiotic-use/core-elements/small-critical.html (accessed on 14 May 2024).
- Pinto Ferreira, J.; Battaglia, D.; Dorado Garcia, A.; Tempelman, K.; Bullon, C.; Motriuc, N.; Caudell, M.; Cahill, S.; Song, J.; LeJeune, J. Achieving Antimicrobial Stewardship on the Global Scale: Challenges and Opportunities. Microorganisms 2022, 10, 1599. [Google Scholar] [CrossRef]
- Hu, Y.J.; Cowling, B.J. Reducing antibiotic use in livestock, China. Bull. World Health Organ. 2020, 98, 360–361. [Google Scholar] [CrossRef]
- Aidara-Kane, A.; Angulo, F.J.; Conly, J.M.; Minato, Y.; Silbergeld, E.K.; McEwen, S.A.; Collignon, P.J.; Group, W.H.O.G.D. World Health Organization (WHO) guidelines on use of medically important antimicrobials in food-producing animals. Antimicrob. Resist. Infect. Control 2018, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Simoni, M.; Quaini, L.; Bignamini, D.A.; Costa, A.; Righi, F. Impact of pre-partum nutraceutical or monensin intraruminal boluses on colostrum quality and Holstein dairy cows’ performance: Exploratory field study. Ital. J. Anim. Sci. 2024, 23, 479–491. [Google Scholar] [CrossRef]
- Ludwiczak, A.; Skladanowska-Baryza, J.; Cieslak, A.; Stanisz, M.; Skrzypczak, E.; Sell-Kubiak, E.; Slósarz, P.; Racewicz, P. Effect of prudent use of antimicrobials in the early phase of infection in pigs on the performance and meat quality of fattening pigs. Meat Sci. 2024, 212, 109471. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.; Jessen, L.R.; Tompson, A.; Rutland, C.; Singleton, D.; Battersby, I.; Gajanayake, I.; Mosher, M.; Pfleger, S.; Gemmill, T.; et al. Influencing attitudes towards antimicrobial use and resistance in companion animals-the impact on pet owners of a short animation in a randomized controlled trial. JAC-Antimicrob. Resis 2024, 6, dlae065. [Google Scholar] [CrossRef]
- Clift, C.; Salisbury, D.M. Enhancing the role of vaccines in combatting antimicrobial resistance. Vaccine 2017, 35, 6591–6593. [Google Scholar] [CrossRef]
- Alghamdi, S. The role of vaccines in combating antimicrobial resistance (AMR) bacteria. Saudi J. Biol. Sci. 2021, 28, 7505–7510. [Google Scholar] [CrossRef]
- Micoli, F.; Bagnoli, F.; Rappuoli, R.; Serruto, D. The role of vaccines in combatting antimicrobial resistance. Nat. Rev. Microbiol. 2021, 19, 287–302. [Google Scholar] [CrossRef]
- Mullins, L.P.; Mason, E.; Winter, K.; Sadarangani, M. Vaccination is an integral strategy to combat antimicrobial resistance. PLoS Pathog. 2023, 19, e1011379. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. One Health. Available online: https://www.cdc.gov/one-health/about/?CDC_AAref_Val=https://www.cdc.gov/onehealth/basics/index.html (accessed on 14 May 2024).
- Van Camp, P.J.; Haslam, D.B.; Porollo, A. Prediction of Antimicrobial Resistance in Gram-Negative Bacteria From Whole-Genome Sequencing Data. Front. Microbiol. 2020, 11, 1013. [Google Scholar] [CrossRef]
- Velazquez-Meza, M.E.; Galarde-Lopez, M.; Carrillo-Quiroz, B.; Alpuche-Aranda, C.M. Antimicrobial resistance: One Health approach. Vet. World 2022, 15, 743–749. [Google Scholar] [CrossRef]
- Davies, D.S.C. One health: Coming of age and antimicrobial resistance. Epidemiol. Infect. 2024, 152, e71. [Google Scholar] [CrossRef] [PubMed]
- Coque, T.M.; Canton, R.; Perez-Cobas, A.E.; Fernandez-de-Bobadilla, M.D.; Baquero, F. Antimicrobial Resistance in the Global Health Network: Known Unknowns and Challenges for Efficient Responses in the 21st Century. Microorganisms 2023, 11, 1050. [Google Scholar] [CrossRef] [PubMed]
- Frid-Nielsen, S.S.; Rubin, O.; Baekkeskov, E. The state of social science research on antimicrobial resistance. Soc. Sci. Med. 2019, 242, 112596. [Google Scholar] [CrossRef]
- Bassetti, M.; Giacobbe, D.R. A look at the clinical, economic, and societal impact of antimicrobial resistance in 2020. Expert Opin. Pharmaco 2020, 21, 2067–2071. [Google Scholar] [CrossRef]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef]
- Barros, J.; Monteiro, F.J.; Ferraz, M.P. Bioengineering Approaches to Fight against Orthopedic Biomaterials Related-Infections. Int. J. Mol. Sci. 2022, 23, 11658. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- Majumder, M.A.A.; Rahman, S.; Cohall, D.; Bharatha, A.; Singh, K.; Haque, M.; Gittens-St Hilaire, M. Antimicrobial Stewardship: Fighting Antimicrobial Resistance and Protecting Global Public Health. Infect. Drug Resist. 2020, 13, 4713–4738. [Google Scholar] [CrossRef]
- Jasovsky, D.; Littmann, J.; Zorzet, A.; Cars, O. Antimicrobial resistance-a threat to the world’s sustainable development. Ups. J. Med. Sci. 2016, 121, 159–164. [Google Scholar] [CrossRef]
- Chinemerem Nwobodo, D.; Ugwu, M.C.; Oliseloke Anie, C.; Al-Ouqaili, M.T.S.; Chinedu Ikem, J.; Victor Chigozie, U.; Saki, M. Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef]
- Byarugaba, D.K. A view on antimicrobial resistance in developing countries and responsible risk factors. Int. J. Antimicrob. Agents 2004, 24, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. Infect. Control 2017, 6, 47. [Google Scholar] [CrossRef]
- Agyare, E.; Acolatse, J.E.E.; Dakorah, M.P.; Akafity, G.; Chalker, V.J.; Spiller, O.B.; Schneider, K.A.; Yevutsey, S.; Aidoo, N.B.; Blankson, S.; et al. Antimicrobial stewardship capacity and antibiotic utilisation practices in the Cape Coast Teaching Hospital, Ghana: A point prevalence survey study. PLoS ONE 2024, 19, e0297626. [Google Scholar] [CrossRef]
- Diarra, B.; Guindo, I.; Koné, B.; Dembélé, M.; Cissé, I.; Thiam, S.; Konate, K.; Tékété, M.; Maiga, A.; Maiga, O.; et al. High frequency of antimicrobial resistance in and causing diarrheal diseases at the Yirimadio community health facility, Mali. BMC Microbiol. 2024, 24, 35. [Google Scholar] [CrossRef]
- Mata, T.M.; Felgueiras, F.; Martins, A.A.; Monteiro, H.; Ferraz, M.P.; Oliveira, G.M.; Gabriel, M.F.; Silva, G.V. Indoor Air Quality in Elderly Centers: Pollutants Emission and Health Effects. Environments 2022, 9, 86. [Google Scholar] [CrossRef]
- Nguyen, H.Q.; Nguyen, N.T.Q.; Hughes, C.M.; O’Neill, C. Trends and impact of antimicrobial resistance on older inpatients with urinary tract infections (UTIs): A national retrospective observational study. PLoS ONE 2019, 14, e0223409. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, G.A.; Lee, S.H.; Park, Y.H. A systematic review on the causes of the transmission and control measures of outbreaks in long-term care facilities: Back to basics of infection control. PLoS ONE 2020, 15, e0229911. [Google Scholar] [CrossRef]
- Bouza, E.; Garcia Navarro, J.A.; Alonso, S.; Duran Alonso, J.C.; Escobar, C.; Fontecha Gomez, B.J.; Galva Borras, M.I.; Garcia Rojas, A.J.; Gomez Pavon, F.J.; Gracia, D.; et al. Infection control in long term care institutions for the elderly: A reflection document on the situation in Spain. Rev. Esp. Quimioter. 2023, 36, 346–379. [Google Scholar] [CrossRef]
- Schramm, L.; Byrne, M.K.; Sweetnam, T. Antibiotic Misuse Behaviours of Older People: Confirmation of the Factor Structure of the Antibiotic Use Questionnaire. Antibiotics 2023, 12, 718. [Google Scholar] [CrossRef]
- Biguenet, A.; Bouxom, H.; Bertrand, X.; Slekovec, C. Antibiotic resistance in elderly patients: Comparison of Enterobacterales causing urinary tract infections between community, nursing homes and hospital settings. Infect. Dis. Now. 2023, 53, 104640. [Google Scholar] [CrossRef]
- Morrill, H.J.; Caffrey, A.R.; Jump, R.L.; Dosa, D.; LaPlante, K.L. Antimicrobial Stewardship in Long-Term Care Facilities: A Call to Action. J. Am. Med. Dir. Assoc. 2016, 17, 183.e1–183.e16. [Google Scholar] [CrossRef] [PubMed]
- Shelke, Y.P.; Bankar, N.J.; Bandre, G.R.; Hawale, D.V.; Dawande, P. An Overview of Preventive Strategies and the Role of Various Organizations in Combating Antimicrobial Resistance. Cureus 2023, 15, e44666. [Google Scholar] [CrossRef] [PubMed]
- Pantano, D.; Friedrich, A.W. Hub and Spoke: Next level in regional networks for infection prevention. Int. J. Med. Microbiol. 2024, 314, 151605. [Google Scholar] [CrossRef]
- Review on Antimicrobial Resistance. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations; Review on Antimicrobial Resistance: London, UK, 2015. [Google Scholar]
- World Health Organization. One Health Initiative. Available online: https://www.who.int/teams/one-health-initiative (accessed on 14 May 2024).
- Burnham, C.D.; Leeds, J.; Nordmann, P.; O’Grady, J.; Patel, J. Diagnosing antimicrobial resistance. Nat. Rev. Microbiol. 2017, 15, 697–703. [Google Scholar] [CrossRef]
- Andersen, B.; Hair, L.; Groshek, J.; Krishna, A.; Walker, D. Understanding and Diagnosing Antimicrobial Resistance on Social Media: A Yearlong Overview of Data and Analytics. Health Commun. 2019, 34, 248–258. [Google Scholar] [CrossRef]
- Adebisi, Y.A. Balancing the risks and benefits of antibiotic use in a globalized world: The ethics of antimicrobial resistance. Glob. Health 2023, 19, 27. [Google Scholar] [CrossRef]
- Leung, E.; Weil, D.E.; Raviglione, M.; Nakatani, H.; World Health Organization World Health Day Antimicrobial Resistance Technical Working Group. The WHO policy package to combat antimicrobial resistance. Bull. World Health Organ. 2011, 89, 390–392. [Google Scholar] [CrossRef]
- Ferraz, M.P.; Oliveira, G.M. Climate Change and Transmissible Diseases. In Climate Change and Health Hazards. Climate Change Management; Leal Filho, W., Vidal, D.G., Dinis, M.A.P., Eds.; Springer: Cham, Switzerland. [CrossRef]

| Contributing Factors | Main Drivers | References |
|---|---|---|
| Antibiotic misuse | Humans (patients, health workers, pharmacists, and veterinarians) | [16,47,48,64,65,67,69] |
| Inappropriate prescription practices | Medical doctors and veterinarians | [40,41,44,45,50,52,53,59,60,62,63,66] |
| Scarcity of next-generation antibiotics | Pharmaceutical companies | [41,44,52,56,58,60] |
| Livestock use of antibiotics | Farmers and livestock producers and veterinarians | [26,43,46,49,60,63] |
| Mobility | Humans, animals, and goods | [46,51,54,55,57] |
| Lack of knowledge | Healthcare workers and the general public | [42,45,61,68,70,71] |
| Strategy | Main Drivers | References |
|---|---|---|
| Antimicrobial stewardship programs | Regulatory agencies | [75,78,79,80,81,82,83,84,85,86] |
| Reduced use of antibiotics in animals | Veterinarians, farmers, and livestock producers | [65,88,89,90,91] |
| Creating new medications and vaccines | Pharmaceutical companies, research institutes, and universities | [68,92,93,94,95] |
| One Health approach | Farmers and livestock producers, veterinarians, pharmaceutical companies, regulatory agencies, industry organizations, and consumers | [68,96,97,98,99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraz, M.P. Antimicrobial Resistance: The Impact from and on Society According to One Health Approach. Societies 2024, 14, 187. https://doi.org/10.3390/soc14090187
Ferraz MP. Antimicrobial Resistance: The Impact from and on Society According to One Health Approach. Societies. 2024; 14(9):187. https://doi.org/10.3390/soc14090187
Chicago/Turabian StyleFerraz, Maria Pia. 2024. "Antimicrobial Resistance: The Impact from and on Society According to One Health Approach" Societies 14, no. 9: 187. https://doi.org/10.3390/soc14090187
APA StyleFerraz, M. P. (2024). Antimicrobial Resistance: The Impact from and on Society According to One Health Approach. Societies, 14(9), 187. https://doi.org/10.3390/soc14090187






