Use, Practices and Attitudes of Elite and Sub-Elite Athletes towards Tart Cherry Supplementation
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Participant Demographics
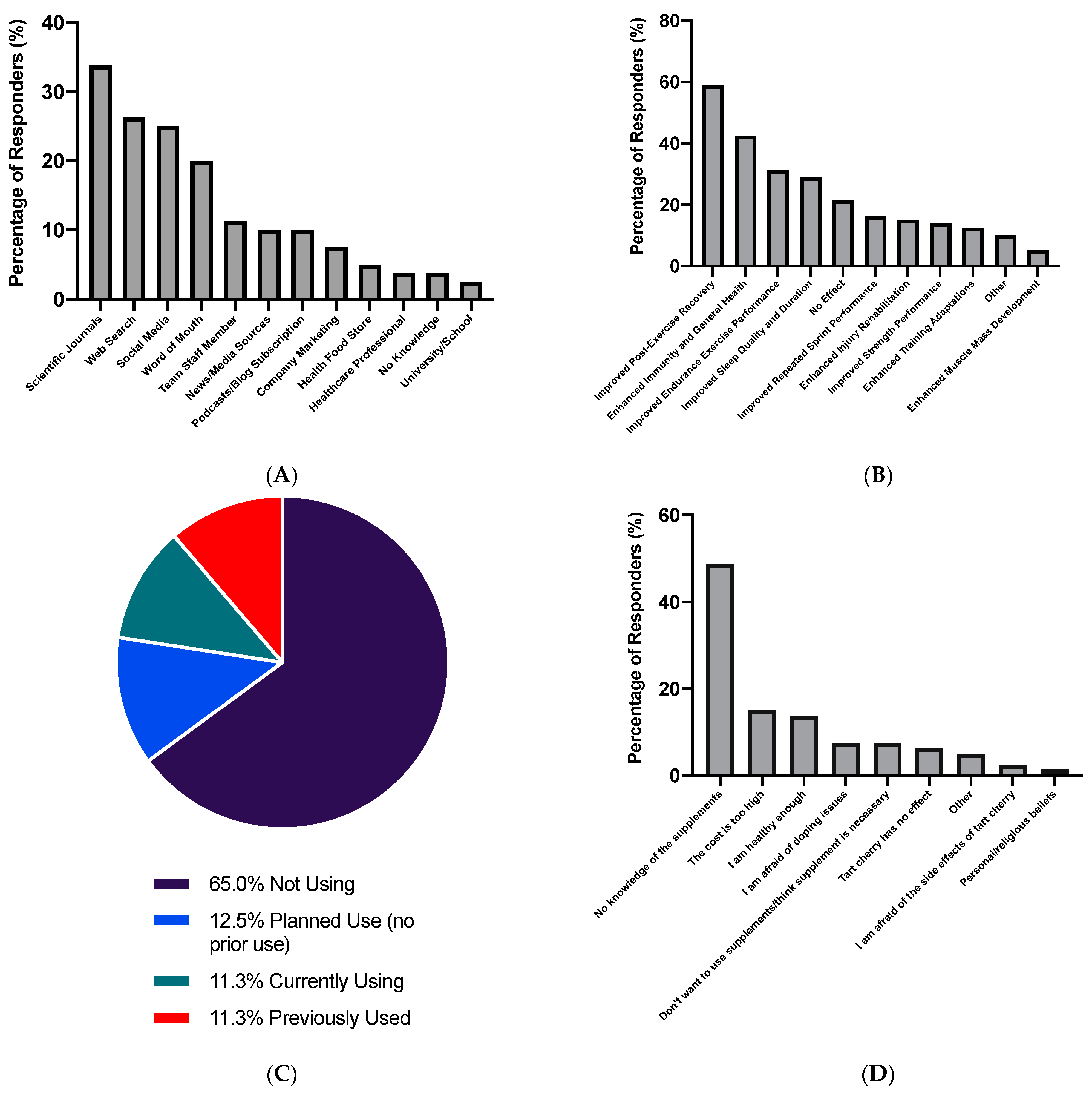
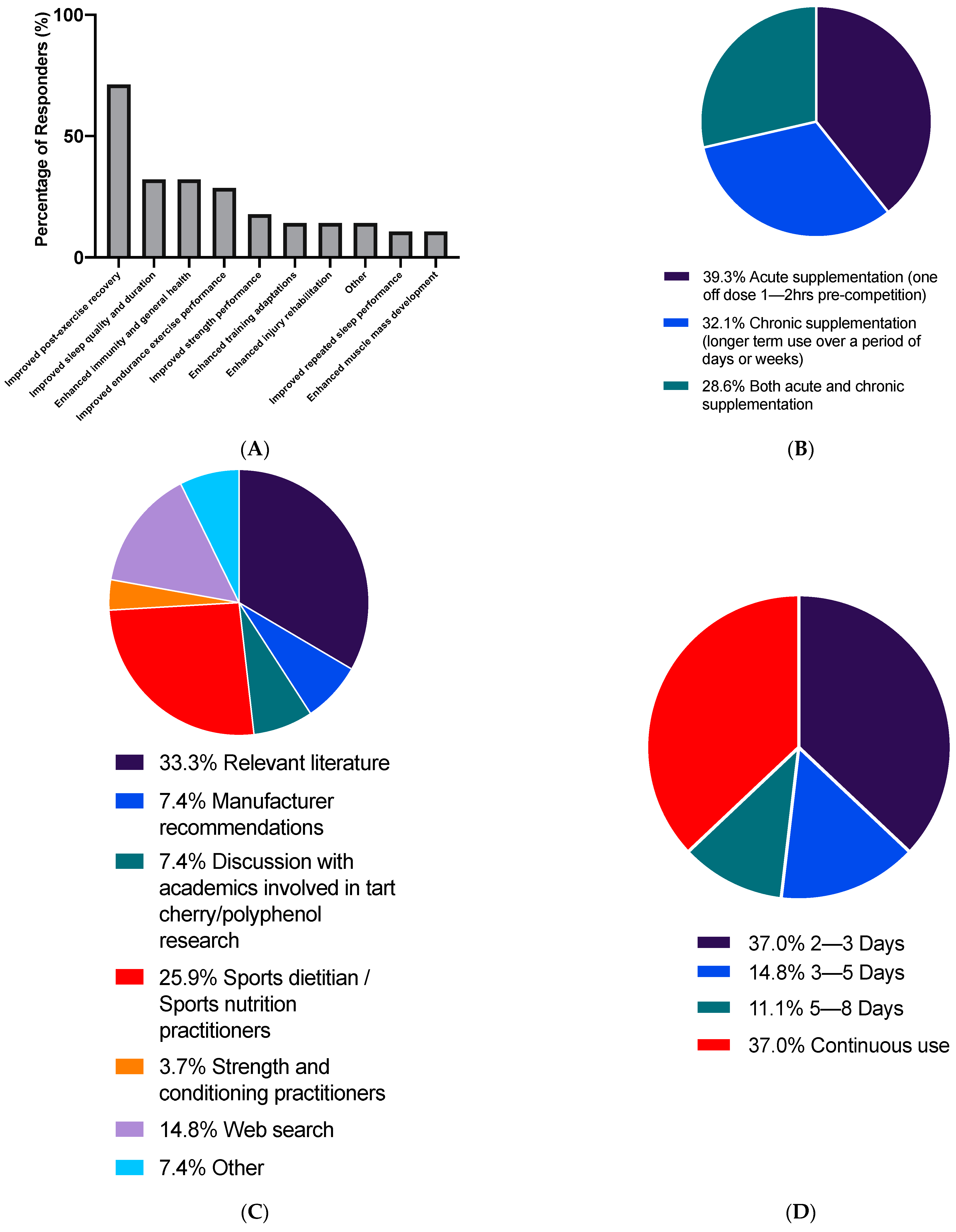
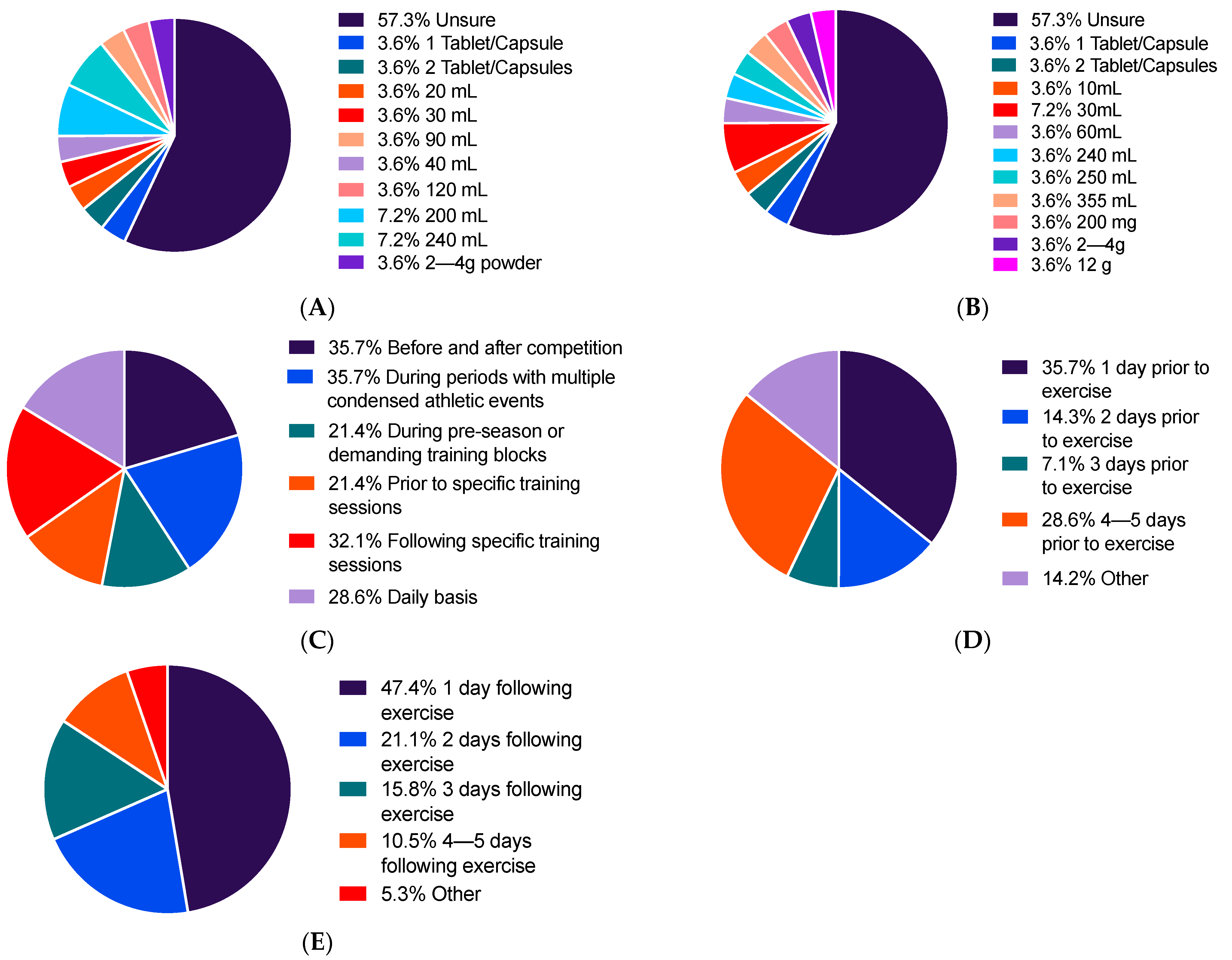
3.2. Use of Tart Cherry Supplements
3.3. Attitudes towards Tart Cherry Supplements
3.4. Sub-Group Analysis: Elite vs. Sub-Elite
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Connolly, D.A.J.; McGhee, D.E.; Steele, J.R. Efficacy of a tart cherry juice blend in preventing the symptoms of muscle damage. Br. J. Sports Med. 2006, 40, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Howatson, G.; McHugh, M.P.; Hill, J.A.; Brouner, J.; Jewell, A.P.; Van Someren, K.A.; Shave, R.E.; Howatson, S.A. Influence of tart cherry juice on indices of recovery following marathon running. Scand. J. Med. Sci. Sports 2009, 20, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Bowtell, J.L.; Sumners, D.P.; Dyer, A.; Fox, P.; Mileva, K.N. Montmorency Cherry Juice Reduces Muscle Damage Caused by Intensive Strength Exercise. Med. Sci. Sports Exerc. 2011, 43, 1544–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, P.G.; Walshe, I.H.; Davison, G.W.; Stevenson, E.J.; Howatson, G. Recovery facilitation with Montmorency cherries following high-intensity, metabolically challenging exercise. Appl. Physiol. Nutr. Metab. 2015, 40, 414–423. [Google Scholar] [CrossRef] [Green Version]
- Bell, P.G.; Stevenson, E.; Davison, G.W.; Howatson, G. The Effects of Montmorency Tart Cherry Concentrate Supplementation on Recovery Following Prolonged, Intermittent Exercise. Nutrients 2016, 8, 441. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.A.; Stevenson, E.J.; Howatson, G. Montmorency tart cherry (Prunus cerasus L.) supplementation accelerates recovery from exercise-induced muscle damage in females. Eur. J. Sport Sci. 2018, 19, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, R.; Hill, J.A. The Efficacy of Tart Cherry Juice in Aiding Recovery after Intermittent Exercise. Int. J. Sports Physiol. Perform. 2020, 15, 368–374. [Google Scholar] [CrossRef] [Green Version]
- Keane, K.M.; Bailey, S.J.; Vanhatalo, A.; Jones, A.M.; Howatson, G. Effects of montmorency tart cherry (L. Prunus Cerasus) consumption on nitric oxide biomarkers and ex-ercise performance. Scand. J. Med. Sci. Sports 2018, 28, 1746–1756. [Google Scholar] [CrossRef]
- Morgan, P.T.; Barton, M.J.; Bowtell, J.L. Montmorency cherry supplementation improves 15-km cycling time-trial performance. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 119, 675–684. [Google Scholar] [CrossRef] [Green Version]
- Howatson, G.; Bell, P.G.; Tallent, J.; Middleton, B.; McHugh, M.P.; Ellis, J. Effect of tart cherry juice (Prunus cerasus) on melatonin levels and enhanced sleep quality. Eur. J. Nutr. 2012, 51, 909–916. [Google Scholar] [CrossRef]
- Pigeon, W.R.; Carr, M.; Gorman, C.; Perlis, M.L. Effects of a Tart Cherry Juice Beverage on the Sleep of Older Adults with Insomnia: A Pilot Study. J. Med. Food 2010, 13, 579–583. [Google Scholar] [CrossRef] [Green Version]
- Losso, J.N.; Finley, J.W.; Karki, N.; Liu, A.G.; Prudente, A.; Tipton, R.; Yu, Y.; Greenway, F.L. Pilot Study of the Tart Cherry Juice for the Treatment of Insomnia and Investigation of Mechanisms. Am. J. Ther. 2018, 25, e194–e201. [Google Scholar] [CrossRef]
- Gao, L.; Mazza, G. Characterization, Quantitation, and Distribution of Anthocyanins and Colorless Phenolics in Sweet Cherries. J. Agric. Food Chem. 1995, 43, 343–346. [Google Scholar] [CrossRef]
- Duthie, G.G.; Gardner, P.T.; Kyle, J.A.M. Plant polyphenols: Are they the new magic bullet? Proc. Nutr. Soc. 2003, 62, 599–603. [Google Scholar] [CrossRef] [Green Version]
- Lattanzio, V.; Lattanzino, V.M.T.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Phytochem. Adv. Res. 2006, 661, 23–67. [Google Scholar]
- Pandey, K.B.; Mishra, N.; Rizvi, S.I. Protective Role of Myricetin on Markers of Oxidative Stress in Human Erythrocytes Subjected to Oxidative Stress. Nat. Prod. Commun. 2009, 4, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Arts, I.C.W.; Hollman, P.C.H. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Kelley, D.S.; Adkins, Y.; Laugero, K.D. A Review of the Health Benefits of Cherries. Nutrients 2018, 10, 368. [Google Scholar] [CrossRef] [Green Version]
- Bowtell, J.; Kelly, V. Fruit-Derived Polyphenol Supplementation for Athlete Recovery and Performance. Sports Med. 2019, 49, 3–23. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Nair, M.G.; Strasburg, G.M.; Chang, Y.-C.; Booren, A.M.; Gray, J.I.; DeWitt, D.L. Antioxidant and Antiinflammatory Activities of Anthocyanins and Their Aglycon, Cyanidin, from Tart Cherries. J. Nat. Prod. 1999, 62, 294–296. [Google Scholar] [CrossRef]
- Wang, H.; Nair, M.G.; Strasburg, G.M.; Booren, A.M.; Gray, J.I. Novel antioxidant compounds from tart cherries (Prunus cerasus). J. Nat. Prod. 1999, 62, 86–88. [Google Scholar] [CrossRef]
- Seeram, N.P. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine 2001, 8, 362–369. [Google Scholar] [CrossRef]
- Hou, D.X.; Yanagita, T.; Uto, T.; Masuzaki, S.; Fujii, M. Anthocyanidins inhibit cyclooxygenase-2 expression in LPS-evoked macrophages: Structure-activity rela-tionship and molecular mechanisms involved. Biochem. Pharmacol. 2005, 70, 417–425. [Google Scholar] [CrossRef]
- Masferrer, J.L.; Zweifel, B.S.; Manning, P.T.; Hauser, S.D.; Leahy, K.M.; Smith, W.G.; Isakson, P.C.; Seibert, K. Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc. Natl. Acad. Sci. USA 1994, 91, 3228–3232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bondesen, B.A.; Mills, S.T.; Kegley, K.M.; Pavlath, G.K. The COX-2 pathway is essential during early stages of skeletal muscle regeneration. Am. J. Physiol. Physiol. 2004, 287, C475–C483. [Google Scholar] [CrossRef]
- Burkhardt, S.; Tan, D.X.; Manchester, L.C.; Hardeland, R.; Reiter, R.J. Detection and quantification of the antioxidant melatonin in Montmorency and Balaton tart cherries (Prunus cerasus). J. Agric. Food Chem. 2001, 49, 4898–4902. [Google Scholar] [CrossRef]
- Hughes, R.J.; Sack, R.L.; Lewy, A.J. The role of melatonin and circadian phase in age-related sleep-maintenance insom-nia: Assessment in a clinical trial of melatonin replacement. Sleep 1998, 21, 52–68. [Google Scholar]
- Claustrat, B.; Leston, J. Melatonin: Physiological effects in humans. Neurochirurgie 2015, 61, 77–84. [Google Scholar] [CrossRef]
- Garthe, I.; Maughan, R.J. Athletes and Supplements: Prevalence and Perspectives. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 126–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundgot-Borgen, J.; Berglund, B.; Torstveit, M.K. Nutritional supplements in Norwegian elite athletes-impact of international ranking and advisors. Scand. J. Med. Sci. Sports 2003, 13, 138–144. [Google Scholar] [CrossRef]
- Dascombe, B.J.; Karunaratna, M.; Cartoon, J.; Fergie, B.; Goodman, C. Nutritional supplementation habits and perceptions of elite athletes within a state-based sporting insti-tute. J. Sci. Med. Sport 2010, 13, 274–280. [Google Scholar] [CrossRef]
- Kelly, V.G.; Leveritt, M.D.; Brennan, C.T.; Slater, G.J.; Jenkins, D.G. Prevalence, knowledge and attitudes relating to β-alanine use among professional footballers. J. Sci. Med. Sport 2017, 20, 12–16. [Google Scholar] [CrossRef] [Green Version]
- Sabou, V.; Wangdi, J.T.; O’Leary, M.F.; Kelly, V.G.; Bowtell, J.L. Use, Practices and Attitudes of Sports Nutrition and Strength and Conditioning Practitioners towards Tart Cherry Supplementation. Sports 2020, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Slater, G.; Tan, B.; Teh, K.C. Dietary Supplementation Practices of Singaporean Athletes. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Desbrow, B.; Leveritt, M. Well-Trained Endurance Athletes’ Knowledge, Insight, and Experience of Caffeine Use. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 328–339. [Google Scholar] [CrossRef]
- Lamb, K.L.; Ranchordas, M.K.; Johnson, E.; Denning, J.; Downing, F.; Lynn, A. No Effect of Tart Cherry Juice or Pomegranate Juice on Recovery from Exercise-Induced Muscle Damage in Non-Resistance Trained Men. Nutrients 2019, 11, 1593. [Google Scholar] [CrossRef] [Green Version]
- Hillman, A.R.; Taylor, B.C.; Thompkins, D. The effects of tart cherry juice with whey protein on the signs and symptoms of exercise-induced muscle damage following plyometric exercise. J. Funct. Foods 2017, 29, 185–192. [Google Scholar] [CrossRef]
- Levers, K.; Dalton, R.; Galvan, E.; Goodenough, C.; O’Connor, A.; Simbo, S.; Barringer, N.; Mertens-Talcott, S.U.; Rasmussen, C.; Greenwood, M.; et al. Effects of powdered Montmorency tart cherry supplementation on an acute bout of intense lower body strength exercise in resistance trained males. J. Int. Soc. Sports Nutr. 2015, 12, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Abbott, W.; Brashill, C.; Brett, A.; Clifford, T. Tart Cherry Juice: No Effect on Muscle Function Loss or Muscle Soreness in Professional Soccer Players after a Match. Int. J. Sports Physiol. Perform. 2020, 15, 249–254. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.-C.; Domenech, E.; Romagnoli, M.; Arduini, A.; Borras, C.; Pallardo, F.V.; Sastre, J.; Viña, J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am. J. Clin. Nutr. 2008, 87, 142–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Cabrera, M.C.; Salvador-Pascual, A.; Cabo, H.; Ferrando, B.; Viña, J. Redox modulation of mitochondriogenesis in exercise. Does antioxidant supplementation blunt the benefits of exercise training? Free Radic. Biol. Med. 2015, 86, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Viña, J.; Ji, L.L. Role of Redox Signaling and Inflammation in Skeletal Muscle Adaptations to Training. Antioxidants 2016, 5, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjørnsen, T.; Salvesen, S.; Berntsen, S.; Hetlelid, K.J.; Stea, T.H.; Lohne-Seiler, H.; Rohde, G.; Haraldstad, K.; Raastad, T.; Køpp, U.; et al. Vitamin C and E supplementation blunts increases in total lean body mass in elderly men after strength training. Scand. J. Med. Sci. Sports 2015, 26, 755–763. [Google Scholar] [CrossRef]
- Yfanti, C.; Åkerström, T.; Nielsen, S.; Nielsen, A.R.; Mounier, R.; Mortensen, O.H.; Lykkesfeldt, J.; Rose, A.J.; Fischer, C.P.; Pedersen, B.K. Antioxidant Supplementation Does Not Alter Endurance Training Adaptation. Med. Sci. Sports Exerc. 2010, 42, 1388–1395. [Google Scholar] [CrossRef]
- Merry, T.L.; Ristow, M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J. Physiol. 2016, 594, 5135–5147. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Sanchez, I.; Taub, P.R.; Ciaraldi, T.P.; Nogueira, L.; Coe, T.; Perkins, G.A.; Hogan, M.C.; Maisel, A.S.; Henry, R.R.; Ceballos, G.; et al. (-)-Epicatechin rich cocoa mediated modulation of oxidative stress regulators in skeletal muscle of heart failure and type 2 diabetes patients. Int. J. Cardiol. 2013, 168, 3982–3990. [Google Scholar] [CrossRef] [Green Version]
- Beals, K.; Allison, K.F.; Darnell, M.; Lovalekar, M.; Baker, R.; Nieman, D.C.; Vodovotz, Y.; Lephart, S.M. The effects of a tart cherry beverage on reducing exercise-induced muscle soreness. Isokinet. Exerc. Sci. 2017, 25, 53–63. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Medina-Remón, A.; Pérez-Jiménez, J.; Neveu, V.; Knaze, V.; Slimani, N.; Scalbert, A. Effects of food processing on polyphenol contents: A systematic analysis using Phenol-Explorer data. Mol. Nutr. Food Res. 2014, 59, 160–170. [Google Scholar] [CrossRef]
- Jacob, R.A.; Spinozzi, G.M.; Simon, V.A.; Kelley, D.S.; Prior, R.L.; Hess-Pierce, B.; Kader, A.A. Consumption of Cherries Lowers Plasma Urate in Healthy Women. J. Nutr. 2003, 133, 1826–1829. [Google Scholar] [CrossRef]
- Kelley, D.S.; Adkins, Y.; Reddy, A.; Woodhouse, L.R.; Mackey, B.E.; Erickson, K.L.; Jr, C.J.B.; Schmidt, B.; Zhang, L.; Hodges, J.S.; et al. Sweet Bing Cherries Lower Circulating Concentrations of Markers for Chronic Inflammatory Diseases in Healthy Humans. J. Nutr. 2013, 143, 340–344. [Google Scholar] [CrossRef]
- Santos, H.O.; Genario, R.; Gomes, G.K.; Schoenfeld, B.J. Cherry intake as a dietary strategy in sport and diseases: A review of clinical applicability and mecha-nisms of action. Crit. Rev. Food Sci. Nutr. 2021, 61, 417–430. [Google Scholar] [CrossRef]
- Ferretti, G.; Bacchetti, T.; Belleggia, A.; Neri, D. Cherry Antioxidants: From Farm to Table. Molecules 2010, 15, 6993–7005. [Google Scholar] [CrossRef]
- Kuehl, K.S. Cherry Juice Targets Antioxidant Potential and Pain Relief. Acute Top. Sport Nutr. 2012, 59, 86–93. [Google Scholar]
- Schumacher, H.; Pullman-Mooar, S.; Gupta, S.; Dinnella, J.; Kim, R.; McHugh, M. Randomized double-blind crossover study of the efficacy of a tart cherry juice blend in treatment of osteoarthritis (OA) of the knee. Osteoarthr. Cartil. 2013, 21, 1035–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kent, K.; Charlton, K.E.; Jenner, A.; Roodenrys, S. Acute reduction in blood pressure following consumption of anthocyanin-rich cherry juice may be dose-interval dependant: A pilot cross-over study. Int. J. Food Sci. Nutr. 2015, 67, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, L.; Hill, J.A.; Jehnali, A.; Dunbar, J.; Brouner, J.; Mahugn, M.P.; Howatson, G. Influence of a montmorency cherry juice blend on indices of exercise-induced stress and upper respirato-ry tract symptoms following marathon running--a pilot investigation. J. Int. Soc. Sports Nutr. 2015, 12, 22. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-O.; Heo, H.J.; Kim, Y.J.; Yang, H.S.; Lee, C.Y. Sweet and Sour Cherry Phenolics and Their Protective Effects on Neuronal Cells. J. Agric. Food Chem. 2005, 53, 9921–9927. [Google Scholar] [CrossRef] [PubMed]
- Kent, K.; Charlton, K.; Roodenrys, S.; Batterham, M.; Potter, J.; Traynor, V.; Gilbert, H.; Morgan, O.; Richards, R. Consumption of anthocyanin-rich cherry juice for 12 weeks improves memory and cognition in older adults with mild-to-moderate dementia. Eur. J. Nutr. 2015, 56, 333–341. [Google Scholar] [CrossRef] [Green Version]
- Heikkinen, A.; Alaranta, A.; Helenius, I.; Vasankari, T. Dietary Supplementation Habits and Perceptions of Supplement Use Among Elite Finnish Athletes. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Nieper, A. Nutritional supplement practices in UK junior national track and field athletes. Br. J. Sports Med. 2005, 39, 645–649. [Google Scholar] [CrossRef] [Green Version]
- Wiens, K.; Erdman, K.A.; Stadnyk, M.; Parnell, J.A. Dietary Supplement Usage, Motivation, and Education in Young Canadian Athletes. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 613–622. [Google Scholar] [CrossRef]
- Baylis, A.; Cameron-Smith, D.; Burke, L.M. Inadvertent doping through supplement use by athletes: Assessment and manage-ment of the risk in Australia. Int. J. Sport Nutr. Exerc. Metab. 2001, 11, 365–383. [Google Scholar] [CrossRef]
- Maughan, R.J.; Depiesse, F.; Geyer, H. The use of dietary supplements by athletes. J. Sports Sci. 2007, 25, S103–S113. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kang, S.; Jung, H.; Chun, Y.; Trilk, J.; Jung, S.H. Dietary Supplementation Patterns of Korean Olympic Athletes Participating in the Beijing 2008 Summer Olym-pic Games. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Lun, V.; Erdman, K.A.; Fung, T.S.; Reimer, R.A. Dietary supplementation practices in Canadian high-performance athletes. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 31–37. [Google Scholar] [CrossRef] [PubMed]
| Type of Training | Aerobic Conditioning | Resistance Training | Speed Training | Skills Training | Mobility Exercises | - | - | - |
|---|---|---|---|---|---|---|---|---|
| 96.3% | 90.0% | 66.3% | 51.3% | 1.3% | - | - | - | |
| Current Competitive Level | International | National | Professional | Semi-Professional | State/Regional | County | Club | Recreational |
| 5.0% | 33.8% | 2.5% | 2.5% | 6.3% | 1.3% | 27.5% | 20.0% | |
| Time at Current Competitive Level | <1 year | 1–2 years | 2–5 years | 5–10 years | ≥10 years | - | - | - |
| 12.5% | 21.3% | 28.8% | 21.3% | 15.0% | - | - | - | |
| Highest Competitive Level | International | National | Professional | Semi-Professional | State/Regional | County | Club | Recreational |
| 20.0% | 30.0% | 2.5% | 2.5% | 12.5% | 2.5% | 16.3% | 12.5% | |
| Competition Frequency | Weekly or More | Monthly or More | 6–11 Times per Year | 2–5 Times Per Year | Do Not Compete | - | - | - |
| 27.5% | 28.8% | 13.8% | 23.8% | 6.3% | - | - | - |
| Side Effect | Severity of Side Effect | ||||
|---|---|---|---|---|---|
| No Effect | Unsure | Small Effect | Noticeable Effect | Severe Effect | |
| Gastrointestinal distress/Diarrhoea | 77.8% | 5.6% | 11.1% | 5.6% | 0.0% |
| Weight Loss | 83.3% | 11.1% | 0.0% | 5.6% | 0.0% |
| Increased Appetite | 83.3% | 11.1% | 0.0% | 5.6% | 0.0% |
| Decreased Appetite | 94.4% | 5.6% | 0.0% | 0.0% | 0.0% |
| Increased thirst/fluid consumption | 77.8% | 11.1% | 5.6% | 0.0% | 5.6% |
| Bruising under the skin | 83.3% | 11.1% | 5.6% | 0.0% | 0.0% |
| Dizziness | 94.4% | 5.6% | 0.0% | 2.0% | 0.0% |
| Joint Pain | 88.9% | 11.1% | 0.0% | 0.0% | 0.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wangdi, J.T.; Sabou, V.; O’Leary, M.F.; Kelly, V.G.; Bowtell, J.L. Use, Practices and Attitudes of Elite and Sub-Elite Athletes towards Tart Cherry Supplementation. Sports 2021, 9, 49. https://doi.org/10.3390/sports9040049
Wangdi JT, Sabou V, O’Leary MF, Kelly VG, Bowtell JL. Use, Practices and Attitudes of Elite and Sub-Elite Athletes towards Tart Cherry Supplementation. Sports. 2021; 9(4):49. https://doi.org/10.3390/sports9040049
Chicago/Turabian StyleWangdi, Jimmy T., Vlad Sabou, Mary F. O’Leary, Vincent G. Kelly, and Joanna L. Bowtell. 2021. "Use, Practices and Attitudes of Elite and Sub-Elite Athletes towards Tart Cherry Supplementation" Sports 9, no. 4: 49. https://doi.org/10.3390/sports9040049







