Effect of a Single Bout of Acute Aerobic Exercise at Moderate-to-Vigorous Intensities on Motor Learning, Retention and Transfer
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
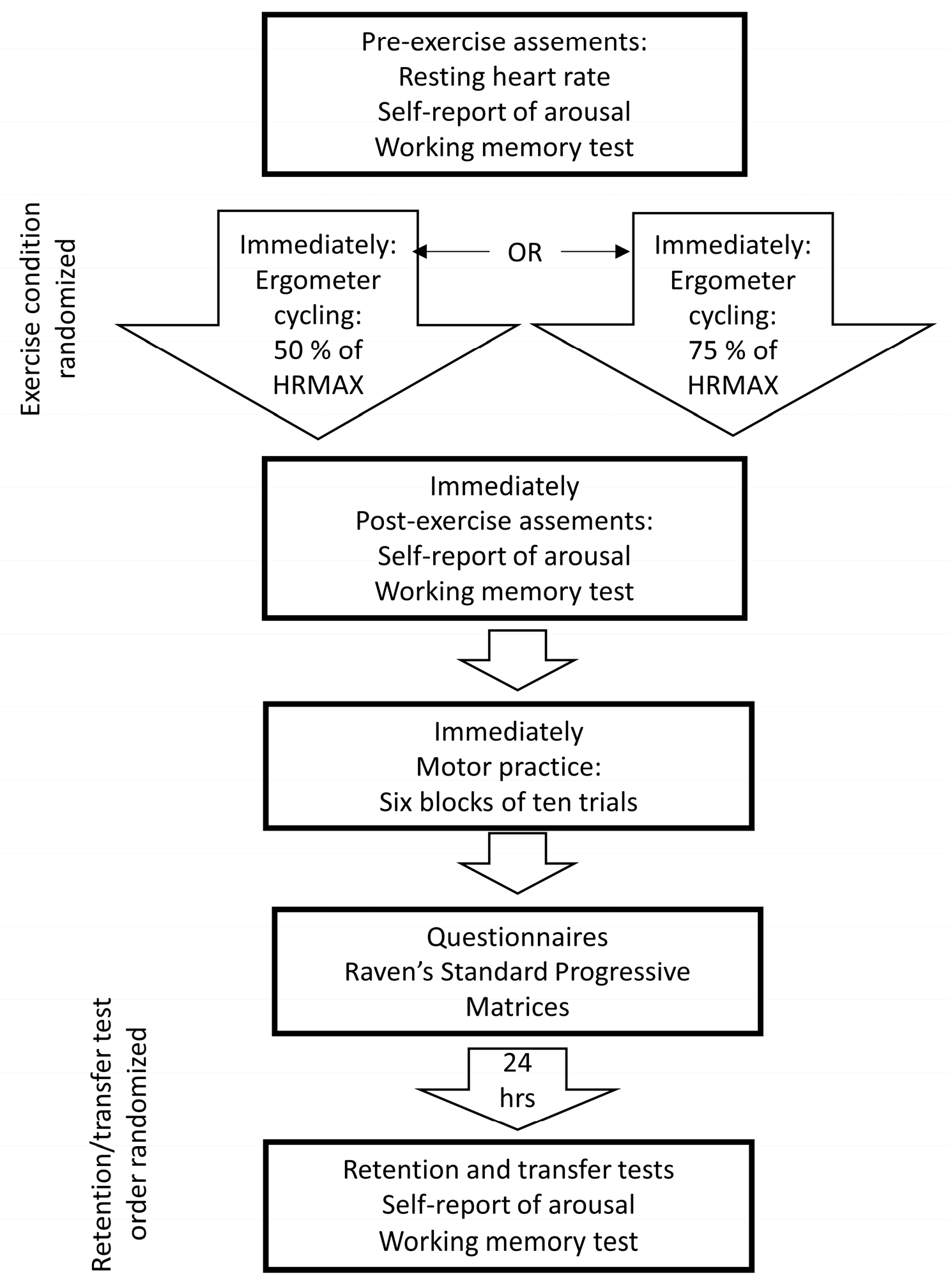
2.2. Procedures
2.3. Questionnaire
2.4. Cognitive Assessments
2.5. Self-Reported Arousal
2.6. Aerobic Exercise
2.7. Golf Putting Task
2.8. Statistical Analysis
3. Results
3.1. Motor practice
3.2. Motor Transfer and Retention
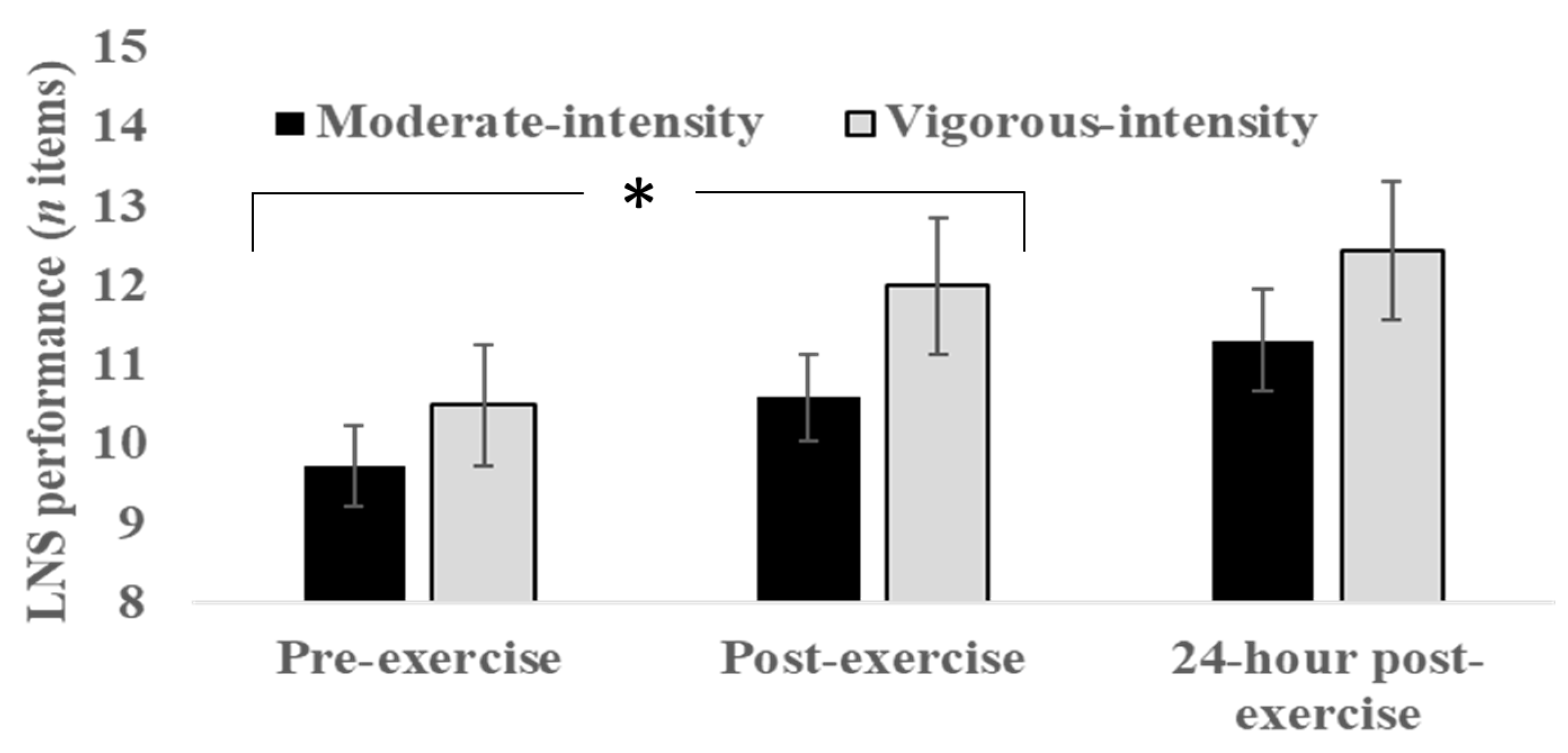
3.3. Working Memory
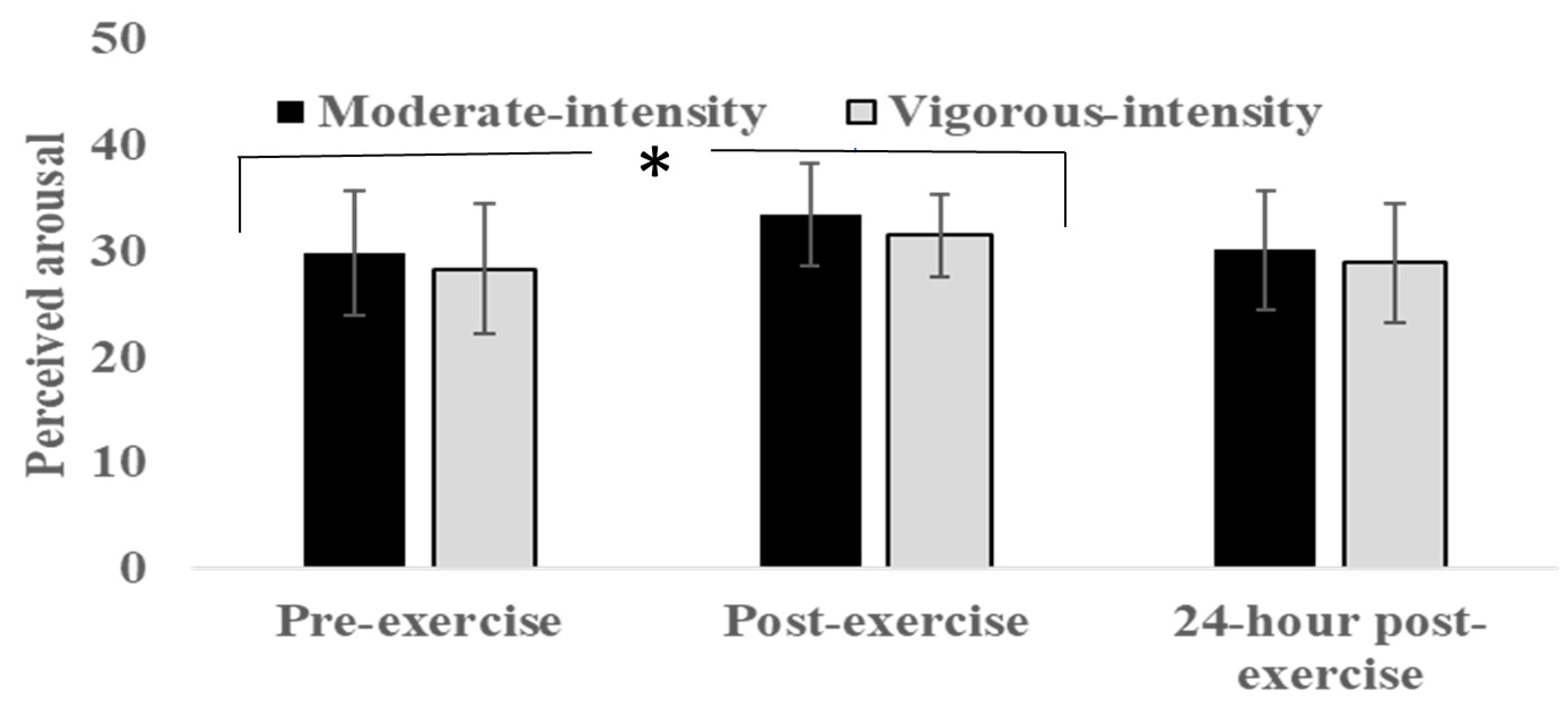
3.4. Self-Reported Arousal
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cotman, C.W.; Berchtold, N.C. Exercise: A behavioral intervention to enhance brain health and plasticity. TRENDS Neurosci. 2002, 25, 295–301. [Google Scholar] [CrossRef]
- Roig, M.; Nordbrandt, S.; Geertsen, S.S.; Nielsen, J.B. The effects of cardiovascular exercise on human memory: A review with meta-analysis. Neurosci. Biobehav. Rev. 2013, 37, 1645–1666. [Google Scholar] [CrossRef] [PubMed]
- McMorris, T.; Sproule, J.; Turner, A.; Hale, B.J. Acute, intermediate intensity exercise, and speed and accuracy in working memory tasks: A meta-analytical comparison of effects. Physiol. Behav. 2011, 102, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Taubert, M.; Villringer, A.; Lehmann, N. Endurance exercise as an “endogenous” neuro-enhancement strategy to facilitate motor learning. Front. Hum. Neurosci. 2015, 9, 692. [Google Scholar] [CrossRef]
- Roig, M.; Thomas, R.; Mang, C.S.; Snow, N.J.; Ostadan, F.; Boyd, L.A.; Lundbye-Jensen, J. Time-dependent effects of cardiovascular exercise on memory. Exerc. Sport Sci. Rev. 2016, 44, 81–88. [Google Scholar] [CrossRef]
- Skriver, K.; Roig, M.; Lundbye-Jensen, J.; Pingel, J.; Helge, J.W.; Kiens, B.; Nielsen, J.B. Acute exercise improves motor memory: Exploring potential biomarkers. Neurobiol. Learn. Mem. 2014, 116, 46–58. [Google Scholar] [CrossRef]
- Phillips, C.; Baktir, M.A.; Srivatsan, M.; Salehi, A. Neuroprotective effects of physical activity on the brain: A closer look at trophic factor signaling. Front. Cell Neurosci. 2014, 8, 170. [Google Scholar] [CrossRef]
- Lucas, S.J.E.; Cotter, J.D.; Brassard, P.; Bailey, D.M. High-intensity interval exercise and cerebrovascular health: Curiosity, cause, and consequence. J. Cereb. Blood Flow Metab. 2015, 35, 902–911. [Google Scholar] [CrossRef]
- Singh, A.M.; Staines, W.R. The effects of acute aerobic exercise on the primary motor cortex. J. Mot. Behav. 2015, 47, 328–339. [Google Scholar] [CrossRef]
- Coco, M.; Perciavalle, V.; Cavallari, P.; Perciavalle, V. Effects of an Exhaustive Exercise on Motor Skill Learning and on the Excitability of Primary Motor Cortex and Supplementary Motor Area. Medicine (Baltimore) 2016, 95, e2978. [Google Scholar] [CrossRef]
- Singh, A.M.; Neva, J.L.; Staines, W.R. Aerobic exercise enhances neural correlates of motor skill learning. Behav. Brain Res. 2016, 301, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Tomporowski, P.D.; Ellis, N.R. Effects of exercise on cognitive processes: A review. Psychol. Bull. 1986, 99, 338–346. [Google Scholar] [CrossRef]
- Pescatello, L.S.; Ross, A.; Riebe, D.; Paul, D.; Thompson (Eds.) ACSM’s Guidelines for Exercise Testing and Prescription, 9th ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2014. [Google Scholar]
- Audiffren, M. Acute Exercise and Physiological Functions: A Cognitive-Energetic Approach. Chapter 1 in Exercise and Cognitive Function; Mcmorris, T., Tomporowski, P.D., Audiffren, M., Eds.; John Wiley & Sons: Oxford, UK, 2009. [Google Scholar]
- Cooper, C.J. Anatomical and physiological mechanisms of arousal with specific reference to the effects of exercise. Ergonomics 1973, 16, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Kahneman, D. Attention and Effort; Prentice-Hall Inc.: Englewood Cliffs, NJ, USA, 1973. [Google Scholar]
- Sanders, A.F. Towards a model of stress and performance. Acta Psychol. 1983, 53, 61–97. [Google Scholar] [CrossRef]
- Yerkes, R.M.; Dodson, J.D. The relation of strength of stimulus to rapidity of habit-formation. J. Comp. Neurol. Psychol. 1908, 18, 459–482. [Google Scholar] [CrossRef]
- McMorris, T.; Hale, B.J. Differential effects of differing intensities of acute exercise on speed and accuracy of cognition: A meta-analytical investigation. Brain Cogn. 2012, 80, 338–351. [Google Scholar] [CrossRef] [PubMed]
- McMorris, T. Exercise and Cognitive Function: A Neuroendocrinological Explanation. Chapter 2 in Exercise and Cognitive Function; Mcmorris, T., Tomporowski, P.D., Audiffren, M., Eds.; John Wiley & Sons: Oxford, UK, 2009. [Google Scholar]
- McMorris, T. Developing the catecholamines hypothesis for the acute exercise-cognition interaction in humans: Lessons from animal studies. Physiol. Behav. 2016, 165, 291–299. [Google Scholar] [CrossRef]
- McMorris, T.; Turner, A.; Hale, B.J.; Sproule, J. Beyond the catecholamines hypothesis for an acute exercise–cognition interaction: A neurochemical perspective. In Exercise-Cognition Interaction: Neuroscience Perspectives; McMorris, T., Ed.; Elsevier Academic Press: Cambridge, MA, USA, 2016; pp. 65–103. [Google Scholar]
- Taylor, J.L.; Gandevia, S.C. A comparison of central aspects of fatigue in submaximal and maximal voluntary contractions. J. Appl. Physiol. 2008, 104, 542–550. [Google Scholar] [CrossRef]
- Allen, D.G. Skeletal muscle function: Role of ionic changes in fatigue, damage and disease. Clin. Exp. Pharmacol. Physiol. 2004, 31, 485–493. [Google Scholar] [CrossRef]
- Debold, E.P. Recent insights into the molecular basis of neuromuscular fatigue. Med. Sci. Sports Exerc. 2012, 44, 1440–1452. [Google Scholar] [CrossRef]
- McMorris, T.; Hale, B.J.; Corbett, J.; Robertson, K.; Hodgson, C.I. Does acute exercise affect the performance of whole-body, psychomotor skills in an inverted-U fashion? A meta-analytic investigation. Physiol. Behav. 2015, 141, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Perini, R.; Bortoletto, M.; Capogrosso, M.; Fertonani, A.; Miniussi, C. Acute effects of aerobic exercise promote learning. Sci. Rep. 2016, 6, 25440. [Google Scholar] [CrossRef] [PubMed]
- Chartrand, G.; Kaneva, P.; Kolozsvari, N.; Li, C.; Petrucci, A.M.; Mutter, A.F.; Vassiliou, M.C. The effects of acute aerobic exercise on the acquisition and retention of laparoscopic skills. Surg. Endosc. 2015, 29, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Statton, M.A.; Encarnacion, M.; Celnik, P.; Bastian, A.J. A Single Bout of Moderate Aerobic Exercise Improves Motor Skill Acquisition. PLoS ONE 2015, 10, e0141393. [Google Scholar] [CrossRef] [PubMed]
- Snow, N.J.; Mang, C.S.; Roig, M.; McDonnell, M.N.; Campbell, K.L.; Boyd, L.A. The Effect of an Acute Bout of Moderate-Intensity Aerobic Exercise on Motor Learning of a Continuous Tracking Task. PLoS ONE 2016, 11, e0150039. [Google Scholar] [CrossRef]
- Mang, C.S.; Snow, N.J.; Wadden, K.P.; Campbell, K.L.; Boyd, L.A. High-Intensity Aerobic Exercise Enhances Motor Memory Retrieval. Med. Sci. Sports Exerc. 2016, 48, 2477–2486. [Google Scholar] [CrossRef]
- Roig, M.; Skriver, K.; Lundbye-Jensen, J.; Kiens, B.; Nielsen, J.B. A single bout of exercise improves motor memory. PLoS ONE 2012, 7, e44594. [Google Scholar] [CrossRef]
- Thomas, R.; Johnsen, L.K.; Geertsen, S.S.; Christiansen, L.; Ritz, C.; Roig, M.; Lundbye-Jensen, J. Acute exercise and motor memory consolidation: The role of exercise intensity. PLoS ONE 2016, 11, e0159589. [Google Scholar] [CrossRef]
- Chisholm, D.M.; Collis, L.L.; Kulak, W.; Davenport, G.N. Physical activity readiness. Br. Col. Med. J. 1975, 17, 375–378. [Google Scholar]
- Randomizer. Available online: https://www.randomizer.org/. (accessed on 28 January 2020).
- Kurtze, N.; Rangul, V.; Hustvedt, B.E. Reliability and validity of the international physical activity questionnaire in the nord-trondelag health study (hunt) population of men. BMC Med. Res. Methodol. 2008, 8, 63. [Google Scholar] [CrossRef]
- Lee, P.H.; Macfarlane, D.J.; Lam, T.H.; Stewart, S.M. Validity of the international physical activity questionnaire short form (ipaq-sf): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, D.E.; Norman, G.J.; Wagner, N.; Patrick, K.; Calfas, K.J.; Sallis, J.F. Reliability and validity of the Sedentary Behavior Questionnaire (SBQ) for adults. J. Phys. Act. Health 2010, 7, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.C. Standard Progressive Matrices; Oxford Psychologists Press: Oxford, UK, 2003. [Google Scholar]
- Deary, I.J.; Penke, L.; Johnson, W. The neuroscience of human intelligence differences. Nat. Rev. Neurosci. 2010, 11, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, D. Wechsler Adult Intelligence Scale–Fourth Edition (WAIS–IV); Pearson Assessments: San Antonio, TA, USA, 2008. [Google Scholar]
- Haut, M.W.; Kuwabara, H.; Leach, S.; Arias, R.G. Neural activation during performance of number-letter sequencing. Appl. Neuropsychol. 2000, 7, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.A.; Deuser, W.E.; DeNeve, K. Hot temperatures, hostile affect, hostile cognition, and arousal: Tests of a general model of affective aggression. Personal. Soc. Psychol. Bull. 1995, 21, 434–448. [Google Scholar] [CrossRef]
- Anderson, C.A.; Anderson, K.B.; Deuser, W.E. Examining an affective aggression framework: Weapon and temperature effects on aggressive thoughts, affect, and attitudes. Personal. Soc. Psychol. Bull. 1996, 22, 366–376. [Google Scholar] [CrossRef]
- Karvonen, M.J. The effects of training on heart rate: A longitudinal study. Ann. Med. Exp. Biol. Fenn. 1957, 35, 307–315. [Google Scholar]
- Nes, B.M.; Janszky, I.; Wisløff, U.; Støylen, A.; Karlsen, T. Age-predicted maximal heart rate in healthy subjects: The HUNT fitness study. Scand. J. Med. Sci. Sports 2013, 23, 697–704. [Google Scholar] [CrossRef]
- Al Haddad, H.; Laursen, P.B.; Chollet, D.; Ahmaidi, S.; Buchheit, M. Reliability of resting and post exercise heart rate measures. Int. J. Sports Med. 2011, 32, 598–605. [Google Scholar] [CrossRef]
- Norskgolf. Available online: http://www.norskgolf.no/artikler/nyheter/golfskolen-grunnleggende-putting. (accessed on 28 January 2020).
- Frank, C.; Land, W.M.; Schack, T. Perceptual-Cognitive Changes During Motor Learning: The Influence of Mental and Physical Practice on Mental Representation, Gaze Behavior, and Performance of a Complex Action. Front. Psychol. 2016, 6, 1981. [Google Scholar] [CrossRef]
- van de Pol, P.K.; Kavussanu, M.; Ring, C. The effects of training and competition on achievement goals, motivational responses, and performance in a golf-putting task. J. Sport Exerc. Psychol. 2012, 34, 787–807. [Google Scholar] [CrossRef] [PubMed]
- Lewthwaite, R.; Chiviacowsky, S.; Drews, R.; Wulf, G. Choose to move: The motivational impact of autonomy support on motor learning. Psychon. Bull. Rev. 2015, 22, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Kinovea. Available online: https://www.kinovea.org/ (accessed on 28 January 2020).
- Cooke, A.; Kavussanu, M.; McIntyre, D.; Ring, C. Psychological, muscular, and kinematic factors mediate performance under pressure. Psychophysiology 2010, 47, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Taylor & Francis Inc.: New York, NY, USA, 1988. [Google Scholar]
- Ferrer-Uris, B.; Busquets, A.; Lopez-Alonso, V.; Fernandez-del-Olmo, M.; Angulo-Barroso, R. Enhancing consolidation of a rotational visuomotor adaptation task through acute exercise. PLoS ONE 2017, 12, e0175296. [Google Scholar] [CrossRef]
- Stavrinos, E.L.; Coxon, J.P. High-intensity interval exercise promotes motor cortex disinhibition and early motor skill consolidation. J. Cogn. Neurosci. 2017, 29, 593–604. [Google Scholar] [CrossRef]
- Dal Maso, F.; Desormeau, B.; Boudrias, M.H.; Roig, M. Acute cardiovascular exercise promotes functional changes in cortico-motor networks during the early stages of motor memory consolidation. NeuroImage 2018, 174, 380–392. [Google Scholar] [CrossRef]
- Charalambous, C.C.; French, M.A.; Morton, S.M.; Reisman, D.S. A single high-intensity exercise bout during early consolidation does not influence retention or relearning of sensorimotor locomotor long-term memories. Exp. Brain Res. 2019, 237, 2799–2810. [Google Scholar] [CrossRef]
- Neva, J.L.; Ma, J.A.; Orsholits, D.; Boisgontier, M.P.; Boyd, L.A. The effects of acute exercise on visuomotor adaptation, learning, and inter-limb transfer. Exp. Brain Res. 2019, 237, 1109–1127. [Google Scholar] [CrossRef]
- Chang, Y.K.; Labban, J.D.; Gapin, J.I.; Etnier, J.L. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012, 1453, 87–101. [Google Scholar] [CrossRef]
- Winter, B.; Breitenstein, C.; Mooren, F.C.; Voelker, K.; Fobker, M.; Lechtermann, A.; Knecht, S. High impact running improves learning. Neurobiol. Learn. Mem. 2007, 87, 597–609. [Google Scholar] [CrossRef]
- Lorås, H.; Haga, M.; Sigmundsson, H. Effect of different exercise modes at high intensity on immediate learning and arousal. Int. J. Sport Exerc. Psychol. 2018, 1–13. [Google Scholar] [CrossRef]
- Newell, K.M.; Liu, Y.T.; Mayer-Kress, G. Time scales, difficulty/skill duality, and the dynamics of motor learning. In Progress in Motor Control; Springer: Boston, MA, USA, 2009; pp. 457–476. [Google Scholar]
- Newell, K.M.; Slifkin, A.B. The nature of movement variability. In Motor Behavior and Human Skill: A Multidisciplinary Perspective; Piek, Ed.; Human Kinetics: Champaign, IL, USA, 1998; pp. 143–160. [Google Scholar]
- Aune, T.K.; Aune, M.A.; Ingvaldsen, R.P.; Vereijken, B. Transfer of Motor Learning Is More Pronounced in Proximal Compared to Distal Effectors in Upper Extremities. Front. Physiol. 2017, 8, 1530. [Google Scholar] [CrossRef] [PubMed]
- Hillman, C.H.; Erickson, K.I.; Kramer, A.F. Be smart, exercise your heart: Exercise effects on brain and cognition. Nat. Rev. Neurosci. 2008, 9, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Kantak, S.S.; Winstein, C.J. Learning–performance distinction and memory processes for motor skills: A focused review and perspective. Behav. Brain Res. 2012, 228, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Stranda, H.; Haga, M.; Sigmundsson, H.; Lorås, H. The Effect of Aerobic Exercise on Speed and Accuracy Task Components in Motor Learning. Sports 2019, 7, 54. [Google Scholar] [CrossRef] [PubMed]

| Variable | Moderate intensity (n = 22) | Vigorous intensity (n = 18) | p1 | |
| Male/Female (n) | 12/10 | 8/10 | 0.52 2 | |
| Age (years) | 23.77 (1.88) | 23.83 (2.15) | 0.93 | |
| BMI (weight/height2) | 23.08 (2.42) | 23.49 (2.88) | 0.63 | |
| Letter Number Sequencing | 9.73 (2.43) | 10.50 (3.26) | 0.39 | |
| Ravens progressive matrices | 51.67 (3.73) | 50.72 (3.29) | 0.40 | |
| Resting heart rate (beats/min) | 72.78 (10.89) | 73.33 (8.48) | 0.86 | |
| Leisure time PA (Hours/week) | Walking | 2.34 (2.32) | 1.75 (1.33) | 0.36 |
| Moderate | 1.94 (3.10) | 1.63 (1.96) | 0.72 | |
| Vigorous | 2.25 (1.86) | 2.91 (2.78) | 0.40 | |
| Total sedentary hours/week | Weekdays | 42.74 (16.32) | 44.09 (21.62) | 0.82 |
| Weekend | 13.64 (5.48) | 14.64 (6.01) | 0.61 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorås, H.; Haga, M.; Sigmundsson, H. Effect of a Single Bout of Acute Aerobic Exercise at Moderate-to-Vigorous Intensities on Motor Learning, Retention and Transfer. Sports 2020, 8, 15. https://doi.org/10.3390/sports8020015
Lorås H, Haga M, Sigmundsson H. Effect of a Single Bout of Acute Aerobic Exercise at Moderate-to-Vigorous Intensities on Motor Learning, Retention and Transfer. Sports. 2020; 8(2):15. https://doi.org/10.3390/sports8020015
Chicago/Turabian StyleLorås, Håvard, Monika Haga, and Hermundur Sigmundsson. 2020. "Effect of a Single Bout of Acute Aerobic Exercise at Moderate-to-Vigorous Intensities on Motor Learning, Retention and Transfer" Sports 8, no. 2: 15. https://doi.org/10.3390/sports8020015
APA StyleLorås, H., Haga, M., & Sigmundsson, H. (2020). Effect of a Single Bout of Acute Aerobic Exercise at Moderate-to-Vigorous Intensities on Motor Learning, Retention and Transfer. Sports, 8(2), 15. https://doi.org/10.3390/sports8020015




