Does Grappling Combat Sports Experience Influence Exercise Tolerance of Handgrip Muscles in the Severe-Intensity Domain?
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
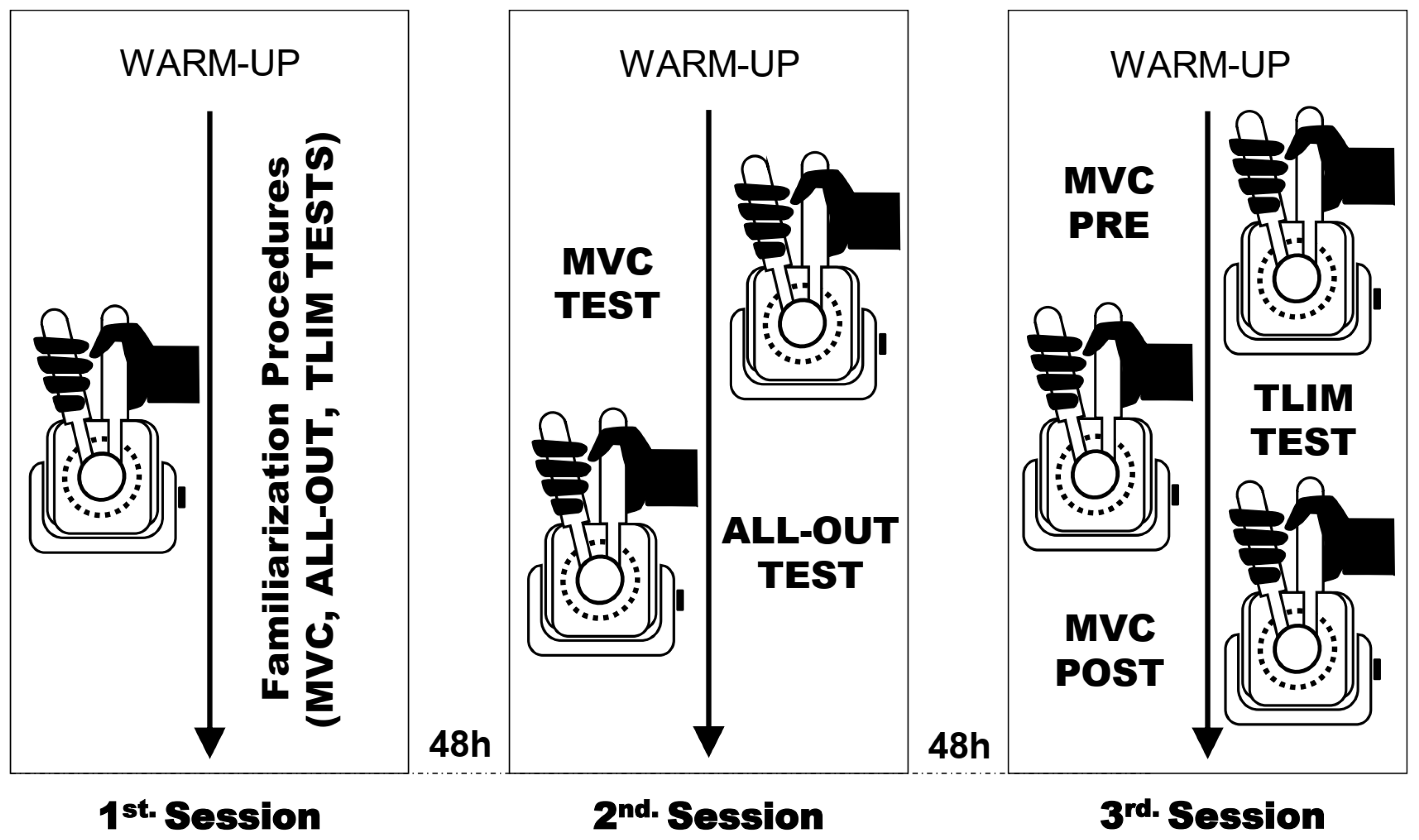
2.2. Experimental Procedures
2.3. Dynamometer Settings
2.4. Maximal Voluntary Contractions
2.5. CT and W′ Determination
2.6. Exercise Tolerance
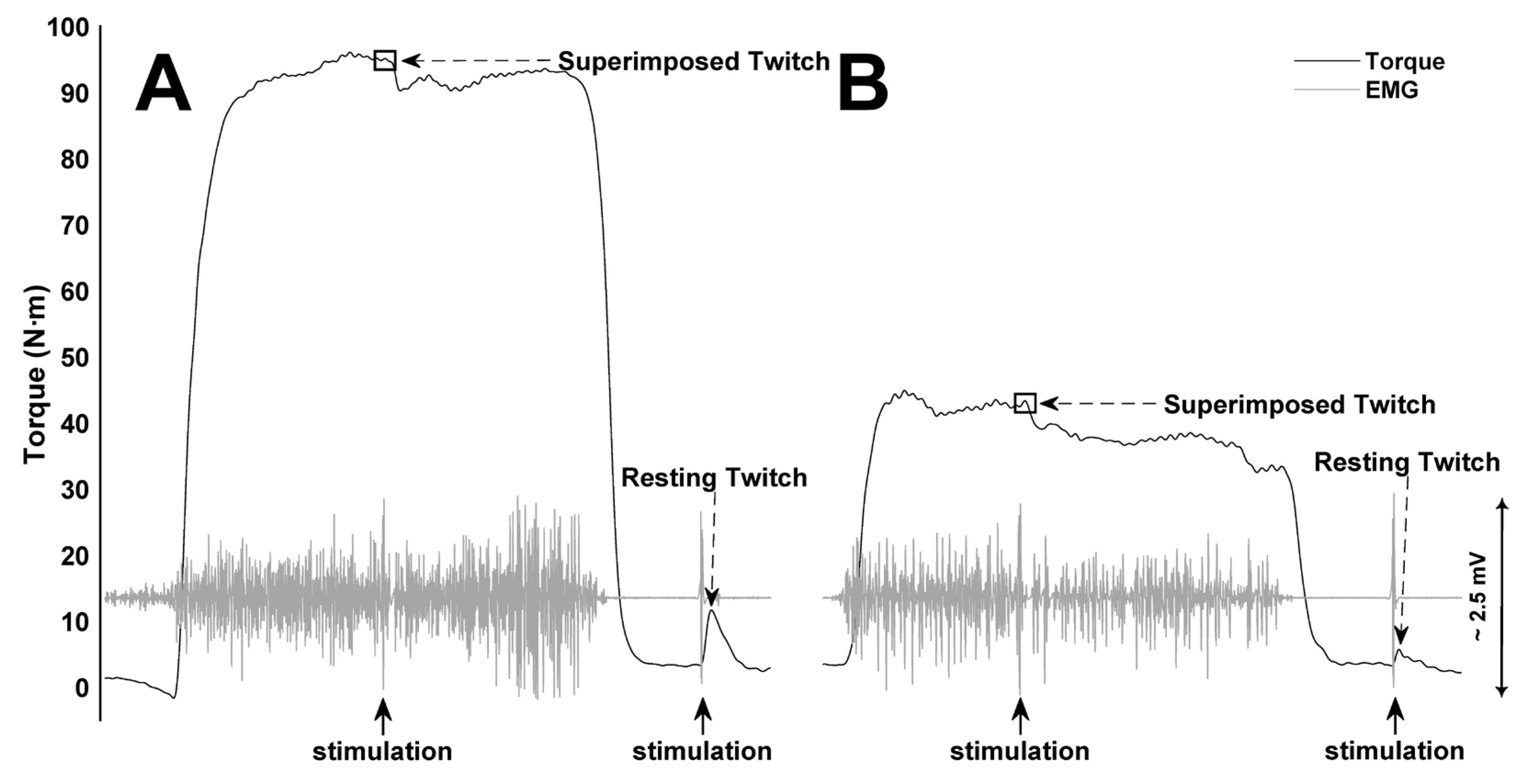
2.7. Neuromuscular Fatigue Assessment
2.8. Electromyography
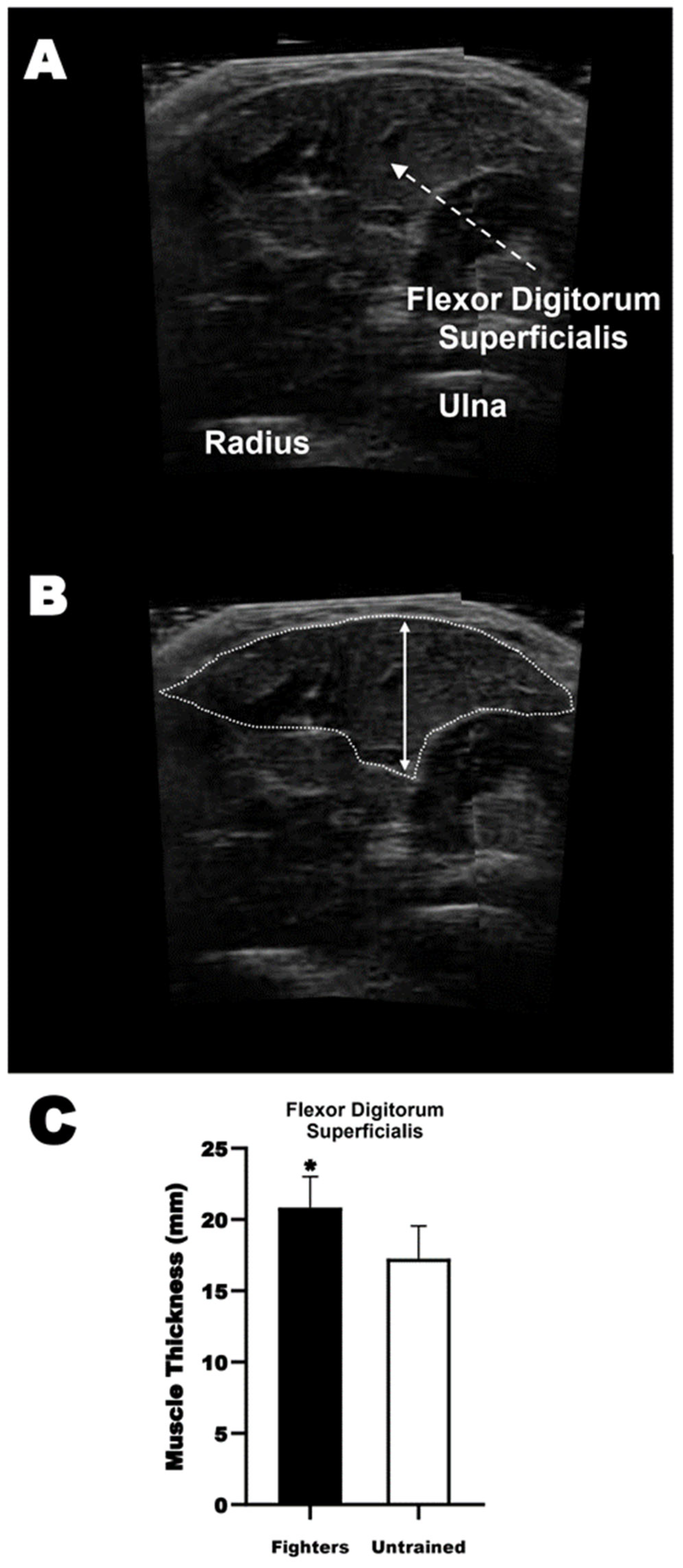
2.9. Ultrasonography
2.10. Torque Data Acquisition and Analysis
2.11. Statistical Analyses
3. Results
3.1. Anthropometrical Data
3.2. Neuromuscular Function
3.3. CT, W′, and Exercise Tolerance
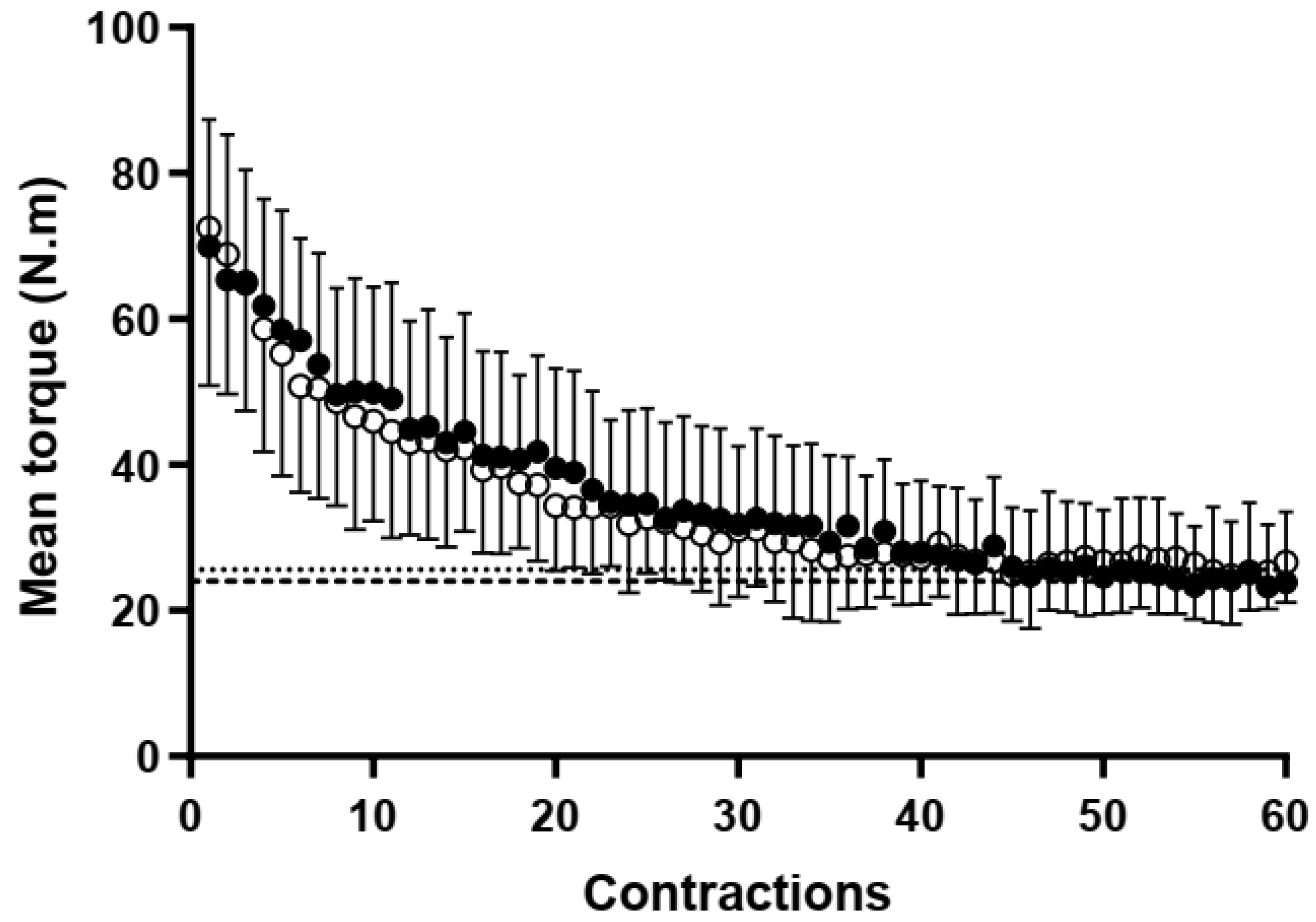
3.4. Neuromuscular Fatigue during Handgrip Severe-Intensity Contractions
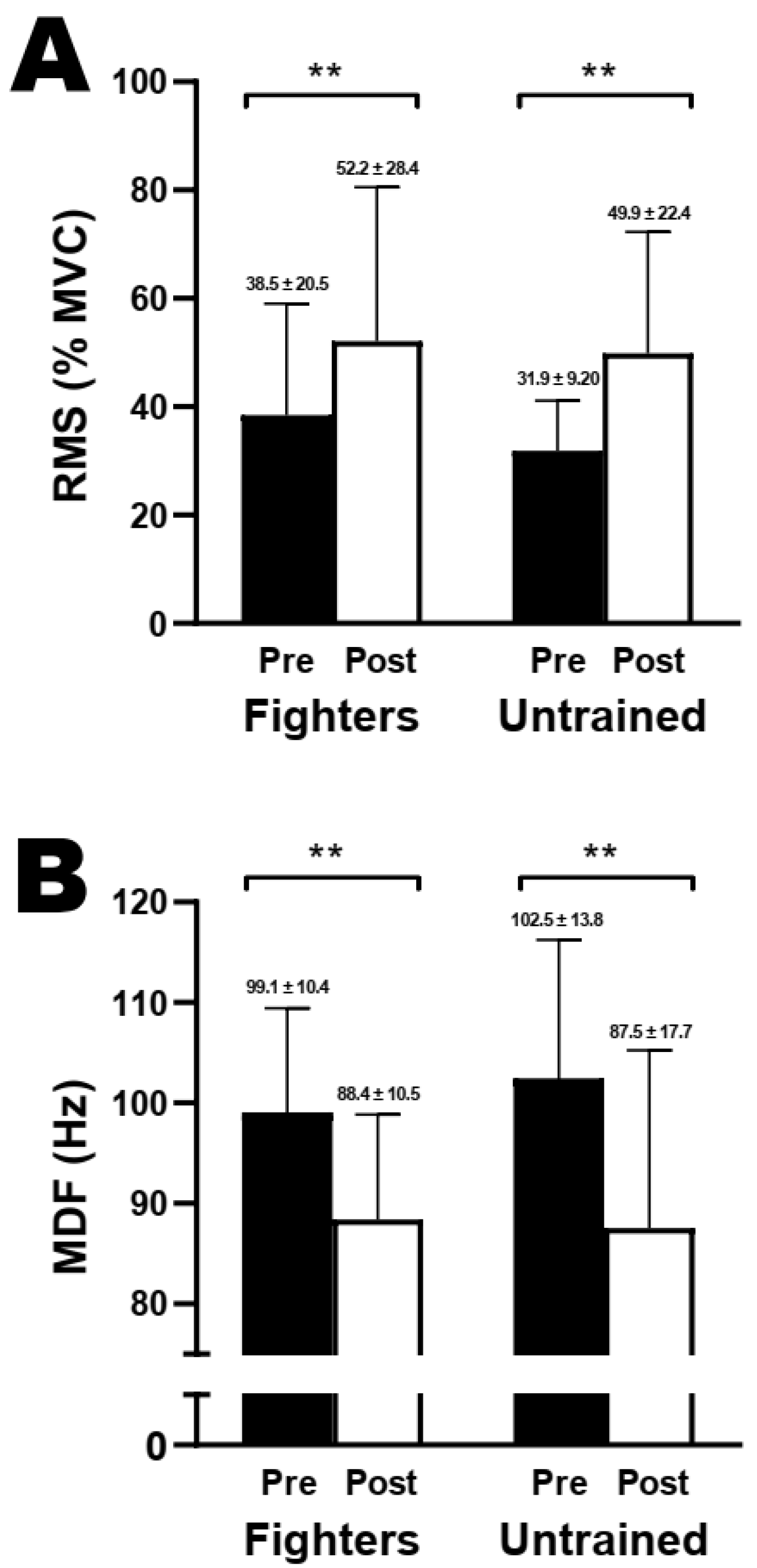
3.5. Electromyography
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franchini, E.; Del Vecchio, F.B.; Matsushigue, K.A.; Artioli, G.G. Physiological profiles of elite judo athletes. Sports Med. 2011, 41, 147–166. [Google Scholar] [CrossRef]
- Kashiwagura, D.B.; Franchini, E. The grip dispute (kumi-kata) in judo: A scoping review. Rev. Artes Marciales Asiát. 2022, 17, 1–18. [Google Scholar] [CrossRef]
- Burnley, M. Estimation of critical torque using intermittent isometric maximal voluntary contractions of the quadriceps in humans. J. Appl. Physiol. 2009, 106, 975–983. [Google Scholar] [CrossRef]
- Denadai, B.S.; Greco, C.C. Methodological issues influence determination of critical force during intermittent exercise: Time to task failure vs. contraction time. J. Physiol. 2019, 597, 5985–5986. [Google Scholar] [CrossRef]
- Abdalla, L.H.P.; Denadai, B.S.; Bassan, N.M.; Greco, C.C. Exercise tolerance during muscle contractions below and above the critical torque in different muscle groups. Appl. Physiol. Nutr. Metab. 2018, 43, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Wilkerson, D.P.; DiMenna, F.; Fulford, J.; Poole, D.C. Muscle metabolic responses to exercise above below the “critical power” assessed using 31P-MRS. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2008, 294, R585–R593. [Google Scholar] [CrossRef] [PubMed]
- Poole, D.C.; Burnley, M.; Vanhatalo, A.; Rossiter, H.B.; Jones, A.M. Critical Power: An Important Fatigue Threshold in Exercise Physiology. Med. Sci. Sports Exerc. 2016, 48, 2320–2334. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Vanhatalo, A.; Burnley, M.; Morton, R.H.; Poole, D.C. Critical power: Implications for determination of O2max and exercise tolerance. Med. Sci. Sports Exerc. 2010, 42, 1876–1890. [Google Scholar] [CrossRef] [PubMed]
- Broxterman, R.M.; Ade, C.J.; Wilcox, S.L.; Schlup, S.J.; Craig, J.C.; Barstow, T.J. Influence of duty cycle on the power-duration relationship: Observations and potential mechanisms. Respir. Physiol. Neurobiol. 2014, 192, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Broxterman, R.M.; Ade, C.J.; Craig, J.C.; Wilcox, S.L.; Schlup, S.J.; Barstow, T.J. Influence of blood flow occlusion on muscle oxygenation characteristics and the parameters of the power-duration relationship. J. Appl. Physiol. 2015, 118, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Ament, W.; Verkerke, G.J. Exercise and fatigue. Sports Med. 2009, 39, 389–422. [Google Scholar] [CrossRef]
- Burnley, M.; Vanhatalo, A.; Jones, A.M. Distinct profiles of neuromuscular fatigue during muscle contractions below and above the critical torque in humans. J. Appl. Physiol. 2012, 113, 215–223. [Google Scholar] [CrossRef]
- Detanico, D.; Dellagrana, R.A.; Athayde, M.S.D.S.; Kons, R.L.; Góes, A. Effect of a Brazilian Jiu-jitsu-simulated tournament on strength parameters and perceptual responses. Sports Biomech. 2017, 16, 115–126. [Google Scholar] [CrossRef]
- Kons, R.L.; Pupo, J.D.; Ache-Dias, J.; Garcia, T.; da Silva, R.R.; Katicips, L.F.G.; Detanico, D. Effect of official judo matches on handgrip strength and perceptual responses. J. Exerc. Rehabil. 2018, 14, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Andreato, L.V.; Julio, U.F.; Gonçalves Panissa, V.L.; Del Conti Esteves, J.V.; Hardt, F.; Franzói de Moraes, S.M.; Oliveira de Souza, C.; Franchini, E. Brazilian Jiu-Jitsu Simulated Competition Part II: Physical Performance, Time-Motion, Technical-Tactical Analyses, and Perceptual Responses. J. Strength Cond. Res. 2015, 29, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- de Menezes Bassan, N.; Denadai, B.S.; de Lima, L.C.R.; Caritá, R.A.C.; Abdalla, L.H.P.; Greco, C.C. Effects of resistance training on impulse above end-test torque and muscle fatigue. Exp. Physiol. 2019, 104, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Broxterman, R.M.; Craig, J.C.; Smith, J.R.; Wilcox, S.L.; Jia, C.; Warren, S.; Barstow, T.J. Influence of blood flow occlusion on the development of peripheral and central fatigue during small muscle mass handgrip exercise. J. Physiol. 2015, 593, 4043–4054. [Google Scholar] [CrossRef] [PubMed]
- Hammer, S.M.; Alexander, A.M.; Didier, K.D.; Barstow, T.J. Influence of blood flow occlusion on muscular recruitment and fatigue during maximal-effort small muscle-mass exercise. J. Physiol. 2020, 598, 4293–4306. [Google Scholar] [CrossRef] [PubMed]
- Millet, G.Y.; Martin, V.; Martin, A.; Vergès, S. Electrical stimulation for testing neuromuscular function: From sport to pathology. Eur. J. Appl. Physiol. 2011, 111, 2489–2500. [Google Scholar] [CrossRef]
- Gandevia, S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [CrossRef]
- Criswell, E.; Cram, J.R. Cram’s Introduction to Surface Electromyography, 2nd ed.; Jones & Bartlett Learning: Burlington, MA, USA, 2011. [Google Scholar]
- Winter, D.A. Biomechanics and Motor Control of Human Movement, 4th ed.; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Merletti, R.; Rainoldi, A.; Farina, D. Myoelectric manifestations of muscle fatigue. In Electromyography. Physiology, Engineering and Non Invasive Applications; Merletti, R., Parker, P.A., Eds.; Wiley: Hoboken, NJ, USA, 2005; pp. 233–258. [Google Scholar]
- Abe, T.; Kondo, M.; Kawakami, Y.; Fukunaga, T. Prediction equations for body composition of Japanese adults by B-mode ultrasound. Am. J. Hum. Biol. 1994, 6, 161–170. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988. [Google Scholar]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Corrêa da Silva, B.V.; Junior, M.M.; de Moura Simim, M.A.; Franchini, E.; da Mota, G.R. Performance in kimono grip strength tests among Brazilian Jiu-Jitsu practitioners from different levels. J. Combat Sports Martial Arts 2014, 5, 11–15. [Google Scholar] [CrossRef]
- Berg, O.K.; Nyberg, S.K.; Windedal, T.M.; Wang, E. Maximal strength training-induced improvements in forearm work efficiency are associated with reduced blood flow. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H853–H862. [Google Scholar] [CrossRef] [PubMed]
- Coffey, V.G.; Hawley, J.A. The molecular bases of training adaptation. Sports Med. 2007, 37, 737–763. [Google Scholar] [CrossRef] [PubMed]
- Kemi, O.J.; Rognmo, O.; Amundsen, B.H.; Stordahl, S.; Richardson, R.S.; Helgerud, J.; Hoff, J. One-arm maximal strength training improves work economy and endurance capacity but not skeletal muscle blood flow. J. Sports Sci. 2011, 29, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Boushel, R.; Ara, I.; Gnaiger, E.; Helge, J.W.; González-Alonso, J.; Munck-Andersen, T.; Sondergaard, H.; Damsgaard, R.; van Hall, G.; Saltin, B.; et al. Low-intensity training increases peak arm VO2 by enhancing both convective and diffusive O2 delivery. Acta Physiol. 2014, 211, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Egan, B.; Zierath, J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef]
- Goulding, R.P.; Marwood, S. Interaction of Factors Determining Critical Power. Sports Med. 2023, 53, 595–613. [Google Scholar] [CrossRef] [PubMed]
- Greco, C.C.; Caritá, R.A.; Dekerle, J.; Denadai, B.S. Effect of aerobic training status on both maximal lactate steady state and critical power. Appl. Physiol. Nutr. Metab. 2012, 37, 736–743. [Google Scholar] [CrossRef]
- Mitchell, E.A.; Martin, N.R.W.; Bailey, S.J.; Ferguson, R.A. Critical power is positively related to skeletal muscle capillarity and type I muscle fibers in endurance-trained individuals. J. Appl. Physiol. 2018, 125, 737–745. [Google Scholar] [CrossRef]
- Vanhatalo, A.; Black, M.I.; DiMenna, F.J.; Blackwell, J.R.; Schmidt, J.F.; Thompson, C.; Wylie, L.J.; Mohr, M.; Bangsbo, J.; Krustrup, P.; et al. The mechanistic bases of the power-time relationship: Muscle metabolic responses and relationships to muscle fibre type. J. Physiol. 2016, 594, 4407–4423. [Google Scholar] [CrossRef] [PubMed]
- Franchini, E.; Artioli, G.G.; Brito, C.J. Judo combat: Time-motion analysis and physiology. Int. J. Perform. Anal. Sport 2013, 13, 624–641. [Google Scholar] [CrossRef]
- Andreato, L.V.; Lara, F.J.D.; Andrade, A.; Branco, B.H.M. Physical and Physiological Profiles of Brazilian Jiu-Jitsu Athletes: A Systematic Review. Sports Med. Open 2017, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Miura, A.; Endo, M.; Sato, H.; Barstow, T.J.; Fukuba, Y. Relationship between the curvature constant parameter of the power-duration curve and muscle cross-sectional area of the thigh for cycle ergometry in humans. Eur. J. Appl. Physiol. 2002, 87, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Byrd, M.T.; Switalla, J.R.; Eastman, J.E.; Wallace, B.J.; Clasey, J.L.; Bergstrom, H.C. Contributions of Body-Composition Characteristics to Critical Power and Anaerobic Work Capacity. Int. J. Sports Physiol. Perform. 2018, 13, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Murgatroyd, S.R.; Wylde, L.A. The power-duration relationship of high-intensity exercise: From mathematical parameters to physiological mechanisms. J. Physiol. 2011, 589, 2443–2445. [Google Scholar] [CrossRef] [PubMed]
- Miura, A.; Kino, F.; Kajitani, S.; Sato, H.; Sato, H.; Fukuba, Y. The effect of oral creatine supplementation on the curvature constant parameter of the power-duration curve for cycle ergometry in humans. Jpn. J. Physiol. 1999, 49, 169–174. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miura, A.; Sato, H.; Sato, H.; Hipp, B.J.W.; Fukuba, Y. The effect of glycogen depletion on the curvature constant parameter of the power-duration curve for cycle ergometry. Ergonomics 2000, 43, 133–141. [Google Scholar] [CrossRef]
- Broxterman, R.M.; Layec, G.; Hureau, T.J.; Amann, M.; Richardson, R.S. Skeletal muscle bioenergetics during all-out exercise: Mechanistic insight into the oxygen uptake slow component and neuromuscular fatigue. J. Appl. Physiol. 2017, 122, 1208–1217. [Google Scholar] [CrossRef]
| Fighters (n = 11) | Untrained (n = 12) | |
|---|---|---|
| CT (N·m) | 24.0 ± 8.49 | 25.6 ± 5.84 |
| CT (%MVC) | 29.9 ± 8.67 | 33.5 ± 7.60 |
| W′ (N·m·s) | 2238.8 ± 581.2 | 1670.4 ± 680.6 * |
| Fighters (n = 11) | Untrained (n = 12) | ||||||
|---|---|---|---|---|---|---|---|
| Pre | Post | % Changes in Task Failure | Pre | Post | % Changes in Task Failure | p Value | |
| MVC (N·m) | 83.2 ± 19.2 | 35.4 ± 9.45 ** | −56.4 ± 11.2 | 77.8 ± 19.5 | 40.4 ± 9.23 ** | −47.3 ± 9.36 | 0.065 |
| Voluntary Activation (%) | 96.5 ± 3.51 | 84.9 ± 6.96 ** | −11.9 ± 7.76 | 95.6 ± 4,66 | 87.9 ± 6.71 ** | −7.97 ± 5.11 | 0.140 |
| Resting Twitch (N·m) | 11.3 ± 2.32 | 7.78 ± 2.10 ** | −30.3 ± 18.1 | 12.0 ± 1.96 | 8.52 ± 1.60 ** | −28.0 ± 14.3 | 0.806 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Junior, R.C.; Barreto, R.V.; Oliveira, A.S.; Greco, C.C. Does Grappling Combat Sports Experience Influence Exercise Tolerance of Handgrip Muscles in the Severe-Intensity Domain? Sports 2024, 12, 66. https://doi.org/10.3390/sports12030066
Junior RC, Barreto RV, Oliveira AS, Greco CC. Does Grappling Combat Sports Experience Influence Exercise Tolerance of Handgrip Muscles in the Severe-Intensity Domain? Sports. 2024; 12(3):66. https://doi.org/10.3390/sports12030066
Chicago/Turabian StyleJunior, Rubens Correa, Renan Vieira Barreto, Anderson Souza Oliveira, and Camila Coelho Greco. 2024. "Does Grappling Combat Sports Experience Influence Exercise Tolerance of Handgrip Muscles in the Severe-Intensity Domain?" Sports 12, no. 3: 66. https://doi.org/10.3390/sports12030066
APA StyleJunior, R. C., Barreto, R. V., Oliveira, A. S., & Greco, C. C. (2024). Does Grappling Combat Sports Experience Influence Exercise Tolerance of Handgrip Muscles in the Severe-Intensity Domain? Sports, 12(3), 66. https://doi.org/10.3390/sports12030066





