The Influence of General and Local Muscle Fatigue on Kinematics and Plantar Pressure Distribution during Running: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Protocol Registration
2.2. Inclusion and Exclusion Criteria
2.3. Search Strategy
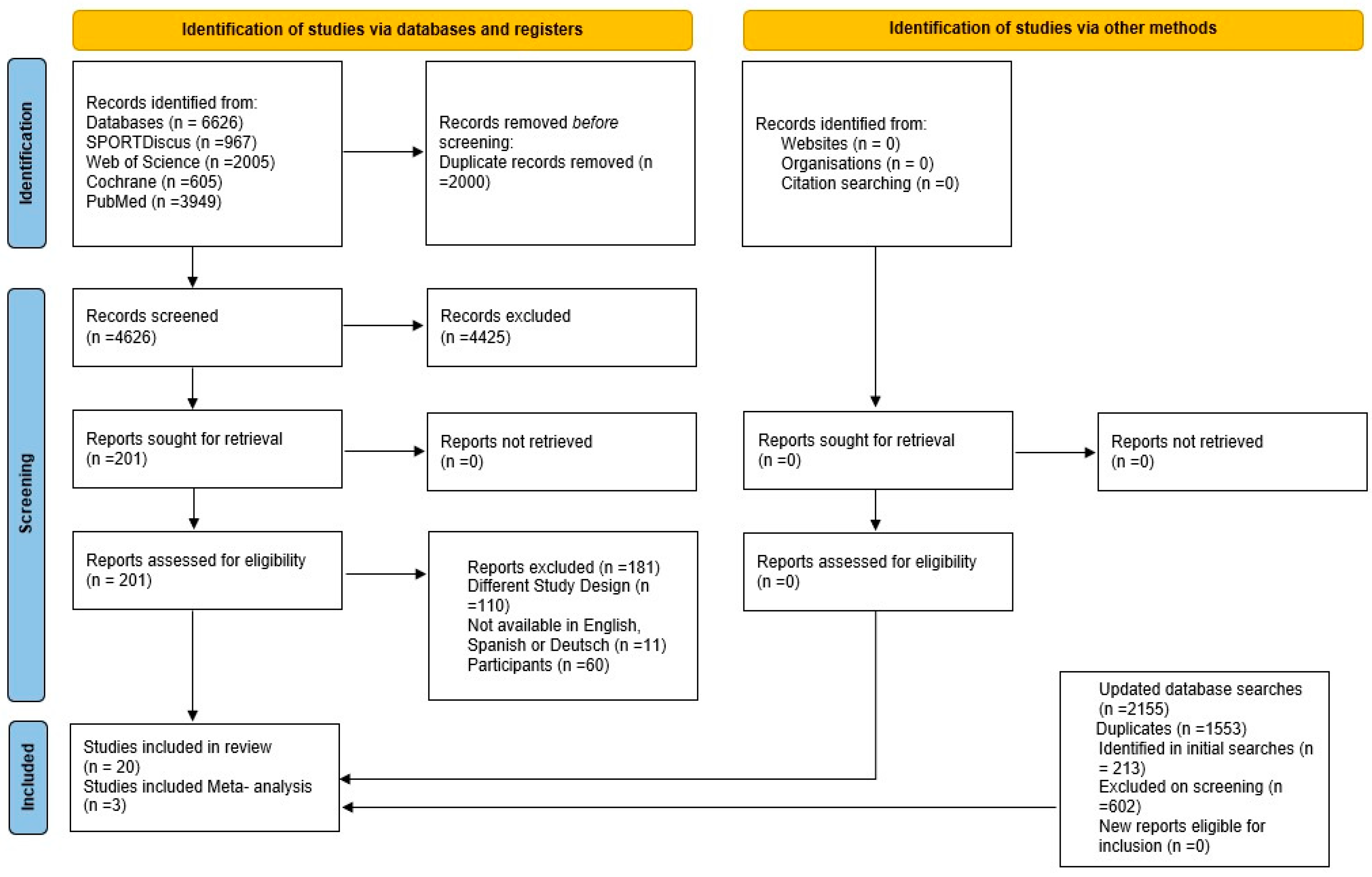
2.4. Study Selection
2.5. Data Extraction
2.6. Quality Assessment
2.7. Statistical Analysis
3. Results
3.1. Baseline and Summary of Included Studies
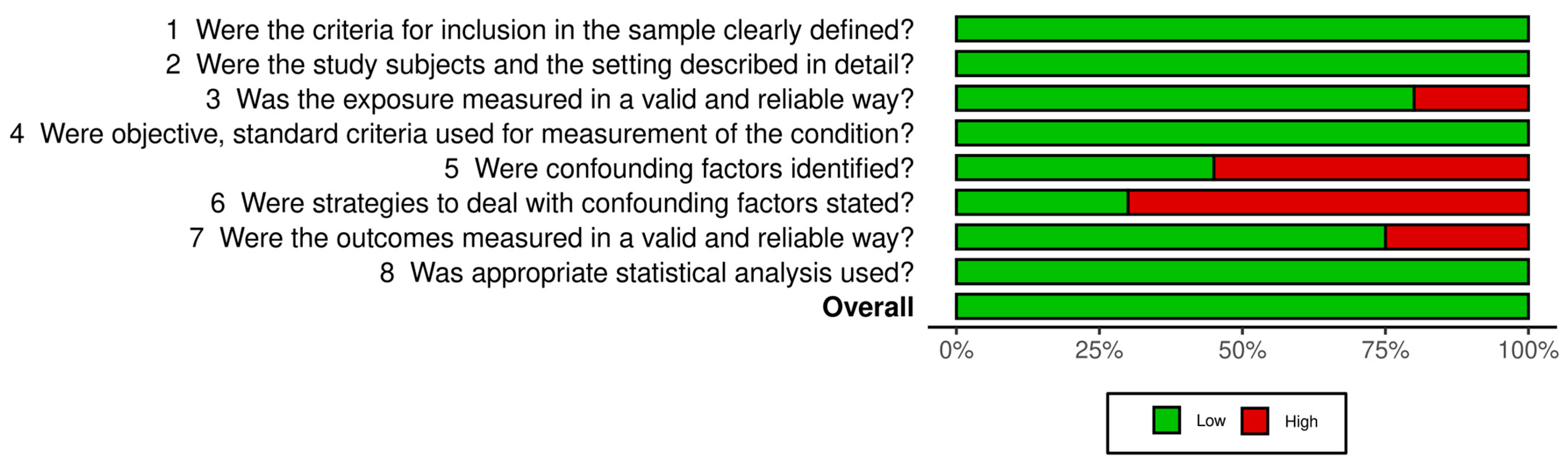
3.2. Risk of Bias
3.3. Systematic Literature Review
3.3.1. Fatiguing Protocol
3.3.2. Changes in Running Gait or Stride Characteristics with Fatigue
3.3.3. Plantar Pressure
3.3.4. Kinematic Variable
- -
- Contact Time
- -
- Step Length
- -
- Stride Frequency
- -
- Flight Time
3.3.5. Kinetic Variable
3.3.6. Accelerometer Load
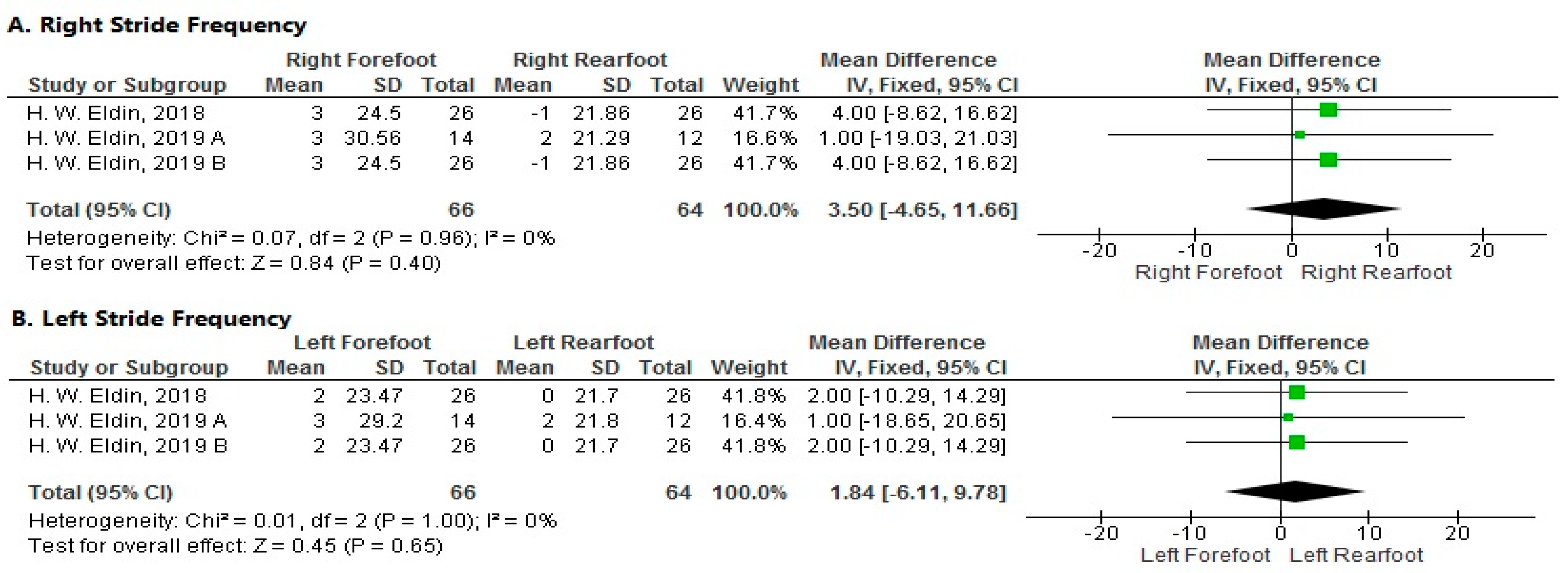
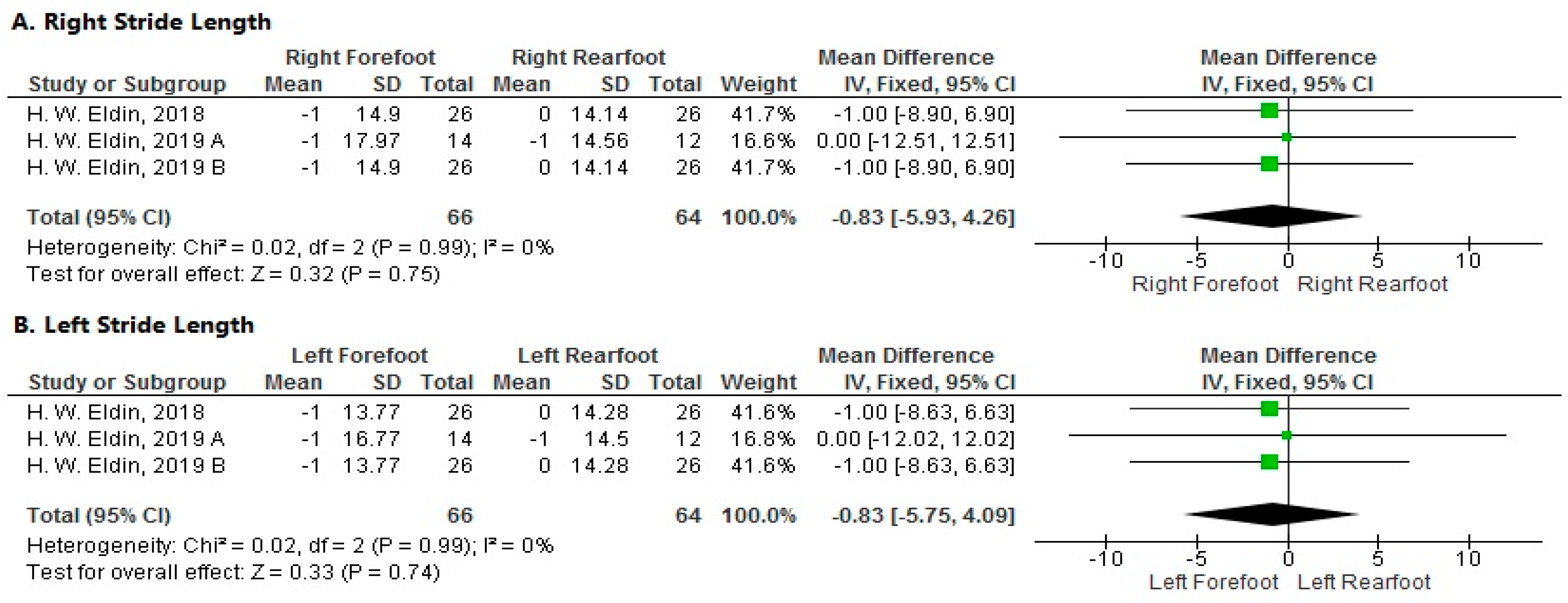
3.3.7. Meta-Analysis
- Stride Frequency
- 1.
- Right Side
- 2.
- Left Side
- Stride Length
- 1.
- Right Side
- 2.
- Left Side
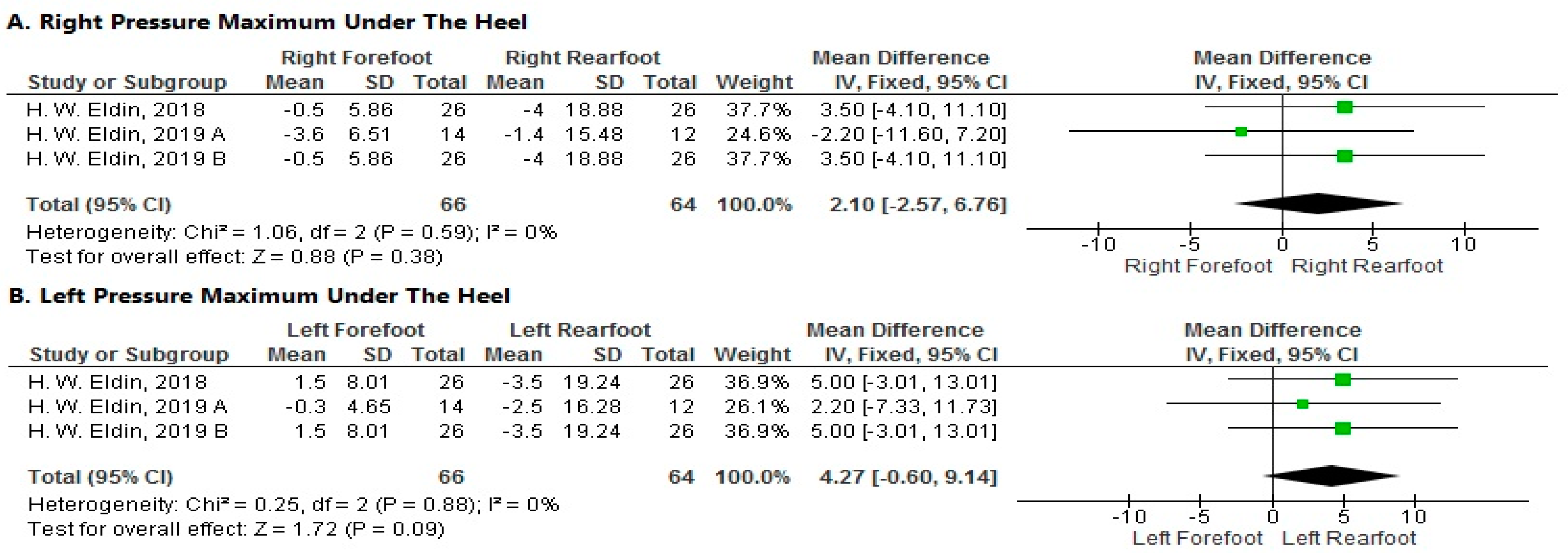
- Pressure Maximum under the Heel
- 1.
- Right Side
- 2.
- Left Side
- Pressure Maximum under the Metatarsus.
- 1.
- Right Side
- 2.
- Left Side
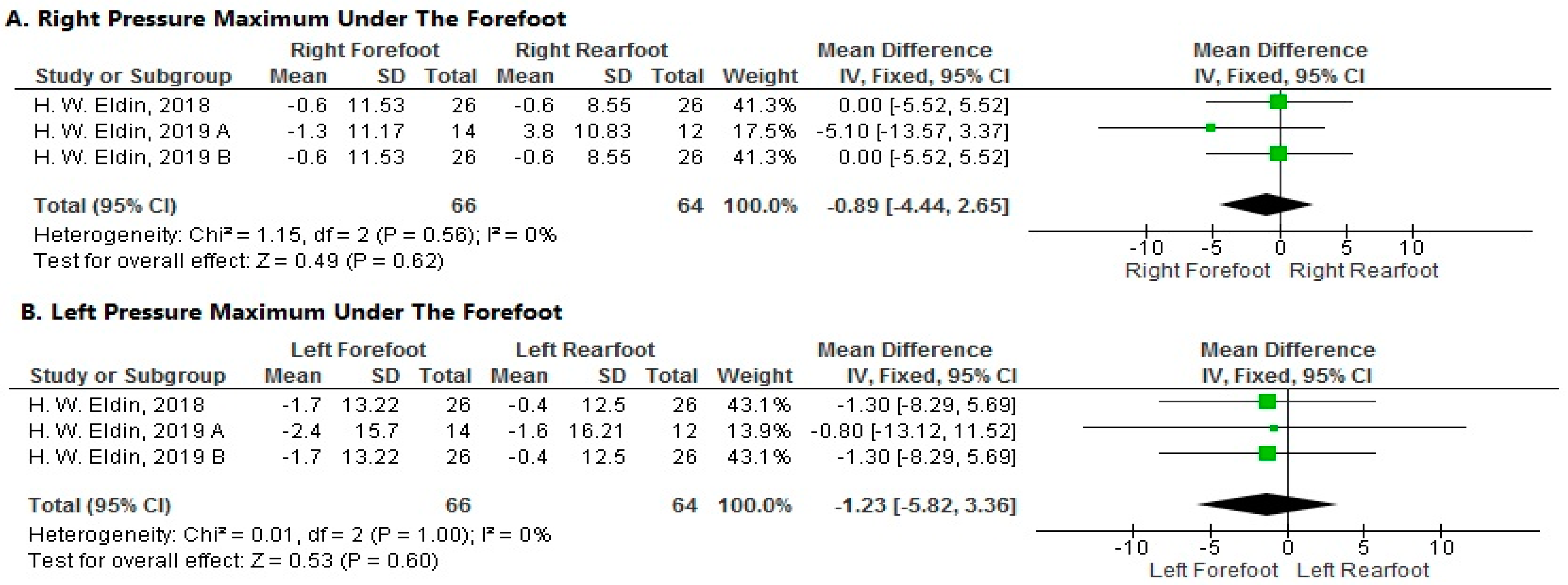
- Pressure Maximum under the Forefoot
- 1.
- Right Side
- 2.
- Left Side
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hreljac, A. Etiology, prevention, and early intervention of overuse injuries in runners: A biomechanical perspective. Phys. Med. Reha. Clin. 2005, 16, 651–667. [Google Scholar] [CrossRef]
- García-Pérez, J.A.; Pérez-Soriano, P.; Llana, S.; Martínez-Nova, A.; Sánchez-Zuriaga, D. Effect of overground vs treadmill running on plantar pressure: Influence of fatigue. Gait Posture 2013, 38, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Fredericson, M.; Misra, A.K. Epidemiology and aetiology of marathon running injuries. Sport Med. 2007, 37, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Van Gent, R.N.; Siem, D.; van Middelkoop, M.; Van Os, A.G.; Bierma-Zeinstra, S.M.A.; Koes, B.W. Incidence and determinants of lower extremity running injuries in long distance runners: A systematic review. Br. J. Sport Med. 2007, 41, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Hamill, J.; Gruber, A.H.; Derrick, T.R. Lower extremity joint stiffness characteristics during running with different footfall patterns. Eur. J. Sport Sci. 2013, 14, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Kernozek, T.W.; Vannatta, C.N.; Gheidi, N.; Kraus, S.; Aminaka, N. Plantar loading changes with alterations in foot strike patterns during a single session in habitual rear foot strike female runners. Phys. Ther. Sport 2016, 18, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Landreneau, L.L.; Watts, K.; Heitzman, J.E.; Childers, W.L. Lower limb muscle activity during forefoot and rearfoot strike running techniques. Inter. J. Sport Phys. Ther. 2014, 9, 888. [Google Scholar]
- Milner, C.E.; Ferber, R.; Pollard, C.D.; Hamill, J.; Davis, I.S. Biomechanical factors associated with tibial stress fracture in female runners. Med. Sci. Sport Exerc. 2006, 38, 323. [Google Scholar] [CrossRef]
- Bennell, K.; Brukner, P. Preventing and managing stress fractures in athletes. Phy. Ther. Sport 2005, 6, 171–180. [Google Scholar] [CrossRef]
- Zadpoor, A.A.; Nikooyan, A.A. The effects of lower-extremity muscle fatigue on the vertical ground reaction force: A meta-analysis. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2012, 226, 579–588. [Google Scholar] [CrossRef]
- Girard, O.; Millet, G.; Slawinski, J.; Racinais, S.; Micallef, J.P. Changes in leg-spring behavior during a 5000 m self-paced run in differently trained athletes. Sci. Sport 2010, 25, 99–102. [Google Scholar] [CrossRef]
- Nummela, A.; Rusko, H.; Mero, A. EMG activities and ground reaction forces during fatigued and nonfatigued sprinting. Med. Sci. Sport Exerc. 1994, 26, 605–609. [Google Scholar] [CrossRef]
- Rabita, G.; Slawinski, J.; Girard, O.; Bignet, F.; Hausswirth, C. Spring-mass behavior during exhaustive run at constant velocity in elite triathletes. Med. Sci. Sport Exerc. 2011, 43, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, J.A.; Cook, S.D.; Leinhardt, T.M. The measurement of shock waves following heel strike while running. J. Biomech. 1985, 18, 415–422. [Google Scholar] [CrossRef]
- Gerlach, K.E.; White, S.C.; Burton, H.W.; Dorn, J.M.; Leddy, J.J.; Horvath, P.J. Kinetic changes with fatigue and relationship to injury in female runners. Med. Sci. Sport. Exerc. 2005, 37, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Slawinski, J.; Heubert, R.; Quievre, J. Changes in spring-mass model parameters and energy cost during track running to exhaustion. J. Strength Cond. Res. 2008, 22, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Christina, K.A.; White, S.C.; Gilchrist, L.A. Effect of localized muscle fatigue on vertical ground reaction forces and ankle joint motion during running. Hum. Mov. Sci. 2001, 20, 257–276. [Google Scholar] [CrossRef]
- Morin, J.B.; Samozino, P.; Millet, G.Y. Changes in running kinematics, kinetics, and spring-mass behavior over a 24-h run. Med. Sci. Sport Exerc. 2011, 43, 829–836. [Google Scholar] [CrossRef]
- Morin, J.B.; Tomazin, K.; Edouard, P.; Millet, G.Y. Changes in running mechanics and spring-mass behavior induced by a mountain ultra-marathon race. J. Biomech. 2011, 44, 1104–1107. [Google Scholar] [CrossRef]
- Quammen, D.; Cortes, N.; Van Lunen, B.L.; Lucci, S.; Ringleb, S.I.; Onate, J. Two different fatigue protocols and lower extremity motion patterns during a stop-jump task. J. Athl. Train. 2012, 47, 32. [Google Scholar] [CrossRef]
- Bisiaux, M.; Moretto, P. The effects of fatigue on plantar pressure distribution in walking. Gait Posture 2008, 28, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Willson, J.D.; Kernozek, T.W. Plantar loading and cadence alterations with fatigue. Med. Sci. Sport Exerc. 1999, 31, 1828–1833. [Google Scholar] [CrossRef] [PubMed]
- Willems, T.M.; De Ridder, R.; Roosen, P. The effect of a long-distance run on plantar pressure distribution during running. Gait Posture 2012, 35, 405–409. [Google Scholar] [CrossRef]
- Olivier, P.E. Effect of invertor/evertor and plantar-/dorsiflexor fatigue on plantar pressure distribution. S. Afr. J. Res. Sport Phys. Educ. Recreat. 2013, 35, 143–152. [Google Scholar]
- Alfuth, M.; Rosenbaum, D. Long distance running and acute effects on plantar foot sensitivity and plantar foot loading. Neurosci. Lett. 2011, 503, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Weist, R.; Eils, E.; Rosenbaum, D. The influence of muscle fatigue on electromyogram and plantar pressure patterns as an explanation for the incidence of metatarsal stress fractures. Am. J. Sport Med. 2004, 32, 1893–1898. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, M.O.; Saragiotto, B.T.; Yamato, T.P.; Lopes, A.D. Is the rearfoot pattern the most frequently foot strike pattern among recreational shod distance runners. Phys. Ther. Sport. 2015, 16, 29–33. [Google Scholar] [CrossRef]
- Jewell, C.; Boyer, K.A.; Hamill, J. Do footfall patterns in forefoot runners change over an exhaustive run? J. Sport Sci. 2017, 35, 74–80. [Google Scholar] [CrossRef]
- Pajek, M.B.; Hedbávný, P.; Kalichová, M.; Čuk, I. The Asymmetry of lower Limb load in Balance Beam Routines. Sci. Gymnast. J. 2016, 8, 5–13. [Google Scholar]
- Hunter, I.; Smith, G.A. Preferred and optimal stride frequency, stiffness and economy: Changes with fatigue during a 1-h highintensity run. Eur. J. Appl. Physiol. 2007, 100, 653–661. [Google Scholar] [CrossRef]
- Riley, P.O.; Dicharry, J.; Franz, J.; Croce, U.D.; Wilder, R.P.; Kerrigan, D.C. A kinematics and kinetic comparison of overground and treadmill running. Med. Sci. Sport Exerc. 2008, 40, 1093. [Google Scholar] [CrossRef] [PubMed]
- Schache, A.G.; Blanch, P.D.; Rath, D.A.; Wrigley, T.V.; Starr, R.; Bennell, K.L. A comparison of overground and treadmill running for measuring the three-dimensional kinematics of the lumbo-pelvic-hip complex. Clin. Biomech. 2001, 16, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Wank, V.; Frick, U.; Schmidtbleicher, D. Kinematics and electromyography of lower limb muscles in overground and treadmill running. Int. J. Sport Med. 1998, 19, 455–461. [Google Scholar] [CrossRef]
- Hong, Y.; Wang, L.; Li, J.X.; Zhou, J.H. Comparison of plantar loads during treadmill and overground running. J. Sci. Med. Sport 2012, 15, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Fellin, R.E.; Manal, K.; Davis, I.S. Comparison of lower extremity kinematic curves during overground and treadmill running. J. Appl. Biomech. 2010, 26, 407. [Google Scholar] [CrossRef] [PubMed]
- Kellis, E.; Liassou, C. The effect of selective muscle fatigue on sagittal lower limb kinematics and muscle activity during level running. J. Orthop. Sport Phys. Ther. 2009, 39, 210–220. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Finni, T.; Kyrolainen, H.; Avela, J.; Komi, P.V. Maximal but not submaximal performance is reduced by constant-speed 10-km run. J. Sport Med. Phys. Fit. 2003, 43, 411–417. [Google Scholar]
- Saldanha, A.; Nordlund Ekblom, M.M.; Thorstensson, A. Central fatigue affects plantar flexor strength after prolonged running. Scan. J. Med. Sci. Sport 2008, 18, 383–388. [Google Scholar] [CrossRef]
- Reber, L.; Perry, J.; Pink, M. Muscular control of the ankle in running. Am. J. Sport Med. 1993, 21, 805–810. [Google Scholar] [CrossRef]
- Ross, E.Z.; Middleton, N.; Shave, R.; George, K.; Nowicky, A. Corticomotor excitability contributes to neuromuscular fatigue following marathon running in man. Exp. Physiol. 2007, 92, 417–426. [Google Scholar] [CrossRef]
- Mizrahi, J.; Verbitsky, O.; Isakov, E.; Daily, D. Effect of fatigue on leg kinematics and impact acceleration in long distance running. Hum. Mov. Sci. 2000, 19, 139–151. [Google Scholar] [CrossRef]
- Bruggemann, G.P. Influence of fatigue on lower extremity function. In Proceedings of the 14 International Symposium on Biomechanics in Sports 1996, Funchal, Portugal, 25–26 June 1996; Volume 1. [Google Scholar]
- Derrick, T.R.; Dereu, D.; Mclean, S.P. Impacts and kinematic adjustments during an exhaustive run. Med. Sci. Sport Exerc. 2002, 34, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma, G. Preferred reporting items for systematic reviews and meta-analyses: The Prisma statement. PLoS Med. 2009, 151, 264–269. [Google Scholar]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Chapter 7: Systematic reviews of etiology and risk. In Joanna Briggs Institute Reviewer’s Manual; Aromataris, E., Munn, Z., Eds.; JBI: Miami, FL, USA, 2017. [Google Scholar] [CrossRef]
- Mc Guinness, L.A.; Higgins, J.P.T. Risk-of-bias Visualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.; Shokraneh, F.; Steinhausen, K.; Adams, C.E. Introducing Raptor: RevMan parsing tool for reviewers. Syst. Rev. 2019, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.D.; Mascarinas, A.; Hespanhol, L. Are alterations in running biomechanics associated with running injuries? A systematic review with meta-analysis. Braz. J. Phys. Ther. 2023, 27, 100538. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Hazzaa Walaa Eldin, A.; Mattes, K. Influence of Foot Strike Pattern and Local Fatigue of Plantar Flexors and Dorsiflexors on Plantar Pressure during Running. Ger. J. Sport Med. 2018, 69, 19–26. [Google Scholar] [CrossRef]
- Hazzaa Walaa Eldin, A.; Mattes, K. The effect of local muscle fatigue and foot strike pattern during barefoot running at different speeds. Ger. J. Sport Med. 2019, 70, S175–S182. [Google Scholar] [CrossRef]
- Hazzaa Walaa Eldin, A.; Mattes, K. Effect of local muscle fatigue and difference foot strike pattern on plantar pressure during barefoot running on treadmill. Biol. Exerc. 2019, 15, 87–102. [Google Scholar]
- Mattes, K.; Hazzaa Walaa Eldin, A.; Manzer, S.; Schaffert, N. Influence of local fatigue of the plantar flexors and dorsiflexors on plantar pressure during running at three running speeds. J. Sport Hum. Perform. 2016, 4, 1–13. [Google Scholar]
- Ament, W.; Verkerke, G.J.; Kernell, D. Change of required foot impact position during running to exhaustion on a treadmill. Inter. J. Sport Med. 2003, 24, 473–480. [Google Scholar]
- Brocherie, F.; Millet, G.P.; Morin, J.B.; Girard, O. Mechanical alterations to repeated treadmill sprints in normobaric hypoxia. Med. Sci. Sport Exerc. 2016, 48, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, J.A.; Pérez-Soriano, P.; Llana Belloch, S.; Lucas-Cuevas, Á.G.; Sánchez-Zuriaga, D. Effects of treadmill running and fatigue on impact acceleration in distance running. Sports Biomech. 2014, 13, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Hanley, B.; Mohan, A. Gait parameter changes during 10000 metre treadmill running. In Proceedings of the 24 International Symposium on Biomechanics in Sports 2006, Salzburg, Austria, 14–18 July 2006; pp. 518–521. [Google Scholar]
- Hanley, B.; Mohan, A.K. Changes in gait during constant pace treadmill running. J. Strength Cond. Res. 2014, 28, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Boyer, E.R.; Ward, E.D.; Derrick, T.R. Medial longitudinal arch mechanics before and after a 45-min run. J. Am. Podiatr. Med. Assoc. 2014, 104, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Abt, J.P.; Sell, T.C.; Chu, Y.; Lovalekar, M.; Burdett, R.G.; Lephart, S.M. Running kinematics and shock absorption do not change after brief exhaustive running. J. Strength Cond. Res. 2011, 25, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.M.; Zifchock, R.A.; Hillstrom, H.J. The effects of limb dominance and fatigue on running biomechanics. Gait Posture 2014, 39, 915–919. [Google Scholar] [CrossRef]
- Dutto, D.J.; Smith, G.A. Changes in spring-mass characteristics during treadmill running to exhaustion. Med. Sci. Sports Exerc. 2002, 34, 1324–1331. [Google Scholar] [CrossRef]
- Mizrahi, J.; Voloshin, A.; Russek, D.; Verbitski, O.; Isakov, E. The influence of fatigue on EMG and impact acceleration in running. Basic Appl. Myol. BAM 1997, 7, 111–118. [Google Scholar]
- Bercovitz, T.; Herman, A.; Solomonow-Avnon, D.; Wolf, A.; Kodesh, E. Plantar pressure modifications in experienced runners following an exhaustive run. Sport Biomech. 2022, 21, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- García-Pinillos, F.; Cartón-Llorente, A.; Jaén-Carrillo, D.; Delgado-Floody, P.; Carrasco-Alarcón, V.; Martínez, C.; Roche-Seruendo, L.E. Does fatigue alter step characteristics and stiffness during running? Gait Posture 2020, 76, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Dickhuth, H.H.; Mayer, F.; Grau, S.; Baur, H.; Hirschmüller, A.; Horstmann, T.; Gollhofer, A. Verletzungen und Beschwerden im Laufsport. Dtsch. Arztebl. 2001, 98, 1254–1259. [Google Scholar]
- Nagel, A.; Fernholz, F.; Kibele, C.; Rosenbaum, D. Long distance running increases plantar pressures beneath the metatarsal heads: A barefoot walking investigation of 200 marathon runners. Gait Posture 2008, 27, 152–155. [Google Scholar] [CrossRef]
- Pohl, M.B.; Rabbito, M.; Ferber, R. The role of tibialis posterior fatigue on foot kinematics during walking. J. Foot Ankle Res. 2010, 3, 6. [Google Scholar] [CrossRef]
- Kitaoka, H.B.; Luo, Z.P.; An, K.N. Effect of posterior tibial tendon on the arch of the foot during simulated weight bearing: Biomechanical analysis. Foot Ankle Int. 1997, 18, 43–46. [Google Scholar] [CrossRef]
- Place, N.; Lepers, R.; Deley, G.; Millet, G.Y. Time course of neuromuscular alterations during a prolonged running exercise. Med. Sci. Sport Exerc. 2004, 36, 1347–1356. [Google Scholar] [CrossRef]
- Nigg, B.M.; Cole, G.K.; Brüggemann, G.P. Impact forces during heel-toe running. J. Appl. Biomech. 1995, 11, 432. [Google Scholar] [CrossRef]
- White, S.C.; Yack, H.J.; Tucker, C.A.; Lin, H.Y. Comparison of vertical ground reaction forces during overground and treadmill walking. Med. Sci. Sport Exerc. 1998, 30, 1537–1542. [Google Scholar] [CrossRef]


| Criterion | Description |
|---|---|
| Type of participant | Healthy adult runners (all competitive levels, all sexes). |
| Type of comparison | Effect analysis (fatigue by jumping, fatigue by running, and local muscle fatigue) or regression analysis (running biomechanics). |
| Type of outcome measure | Plantar pressure distribution, running biomechanics (kinematics, kinetics, and EMG outcomes), temporal-spatial data |
| Type of Study | cross-sectional studies will be included |
| Publication status | Peer-reviewed journal publication |
| Publication date | The included studies were not restricted to a specific date of publication. |
| Language of publication | English or German language |
| Study ID | Country | Study Design | Sample Size | Inclusion Criteria | Aim | Outcomes | Conclusion | |
|---|---|---|---|---|---|---|---|---|
| Primary Outcome | Secondary Outcome | |||||||
| Garcı’a-Pe´rez et al. [2]. | Spain | Cross-Sectional Study | 27 (10 females, 17 males) | Fatigue During Running | To examine the impact of treadmill versus overground conditions on plantar pressures before and after experiencing fatigue. | On both types of surfaces, fatigue (S2) resulted in a decrease in stride frequency (2.78%) and a reduction in plantar pressure (PP) on the lateral heel and hallux (15.96% and 16.35%, respectively). Fatigue (S1) also increased the relative load on the medial arch (9.53%). | No notable interaction was observed between the two factors under investigation, namely surface type and fatigue. | The influence of surface type, irrespective of fatigue levels, should be considered when interpreting findings from studies incorporating treadmill use in their experimental designs or recommending physical exercise on a treadmill. |
| Bisiaux et al. [21]. | France | Cross-Sectional Study | 11 males | 30-Min After Intensive Running | To evaluate alterations in plantar pressure caused by fatigue. | Significant reductions in pressure peaks and relative impulses were noted under the heel and midfoot, accompanied by a noteworthy increase in pressure peaks and relative impulses under the forefoot 30 min post-run. | Following a 30-min rest, there was a significant alteration in loading at both the heel and forefoot compared to pre-test conditions, while variability, step length, and frequency remained consistent. | The study illustrates short- and long-term variations in plantar pressure caused by fatigue resulting from an intensive 30-min run; this contrasts with prior studies that indicated minimal changes in ground reaction force. |
| Willson et al. [22]. | USA | Cross-Sectional Study | 19 (11 females, eight males) | Fatigue During Running | To pinpoint alterations in foot loading patterns associated with fatigue during running | The reduction in step time was accompanied by significantly lower plantar pressure values under the heel. | A discernible shift towards heightened medial forefoot loading was detected when subjects ran under fatigued conditions (α < 0.05). | The subjects exhibited an alteration in running technique and plantar surface loading characteristics in response to fatigue, characterized by an augmented cadence, reduced heel loading, and heightened medial forefoot loading. |
| Derrick et al. [43]. | USA | Cross-Sectional Study | Ten males | Fatigue During Running | To investigate the kinematic adaptations runners make during an exhaustive run and their impact on shock absorption and attenuation. | The knee exhibited a notable increase in flexion at heel impact (initial: 164.9 ± 2.3°; final: 160.5 ± 2.9°; p < 0.05). | The rearfoot angle displayed increased inversion at impact (initial: 12.2 ± 1.6°; final: 13.6 ± 1.9°; p < 0.05). | The heightened peak impact accelerations at the leg were not regarded as an augmented injury risk due to the reduced effective mass. However, the modified kinematics may have led to heightened metabolic costs during the latter stages of the exhaustive run. |
| Hazzaa et al. [50]. | Germany | Cross-Sectional Study | 52 males | Local Muscle Fatigue | The study explored how the foot strike pattern and local muscle fatigue of the plantar flexors and dorsiflexors impact plantar pressure distribution during barefoot treadmill running at three distinct speeds. | Plantar pressure distribution across the foot varied based on the foot strike pattern and local muscle fatigue. | The maximum pressure experienced in the exposed regions of the foot decreased after fatigue. | Injury prevention strategies should encompass variations in foot strike patterns to alleviate pressure on the heel and forefoot areas; this is particularly relevant for forefoot strikers. |
| Hazzaa et al. [51]. | Germany | Cross-Sectional Study | 26 males | Local Muscle Fatigue | The study examined how foot strike patterns and local muscle fatigue of the plantar and dorsiflexors influence plantar pressure distribution and selected kinematic characteristics during barefoot treadmill running at three different speeds. | Following fatigue, the maximum pressure experienced in the exposed foot regions decreased, specifically under the forefoot in the case of a forefoot strike and under the heel for a rearfoot strike. | The two groups of runners exhibited variations in foot angle at foot strike, with forefoot runners displaying higher values. | The increased foot angle observed in forefoot runners enhances shock absorption, potentially reducing the risk of injury. |
| Hazzaa et al. [52]. | Germany | Cross-Sectional Study | 52 males | Local Muscle Fatigue | The aim was to evaluate plantar pressure deviations attributed to fatigue and the specific foot strike pattern. | Both groups exhibited significant disparities in plantar pressure distribution at baseline and after experiencing fatigue. | In both running groups, as running speed increased, there was an associated rise in stride length, stride frequency, and maximum plantar pressure under the midfoot and forefoot. | To prevent injuries, it is recommended to strengthen the plantar muscles of forefoot strikers through targeted strength training. |
| Mattes et al. [53]. | Germany | Cross-Sectional Study | 30 males | Local Muscle Fatigue | Considering leg strength asymmetries, the study investigated how a standardized fatigue protocol affects plantar pressure distribution in rearfoot strike runners. | The observed reductions in pressure values suggest a potential protective strategy to mitigate injuries during muscle fatigue. | Leg asymmetry was present, and the fatigue protocol had a more pronounced effect on dorsiflexor performance, likely because plantar flexors, benefiting from their larger muscle mass, exhibited higher fatigue resistance. | The observed decrease in pressure values could suggest a potential protective mechanism aimed at mitigating injuries during muscle fatigue. |
| Bercovitz et al. [64]. | Israel | Cross-Sectional Study | Nine males | Fatigue During Running | The objective was to evaluate changes in plantar pressure and center of pressure (COP) trajectory after a 30-min run at sub-maximal speed in experienced long-distance runners. | Substantial alterations in the plantar pressure map were identified post-run, characterized by heightened impulses in the first metatarsal head (9.92%, p < 0.001) and hallux areas (16.19%, p < 0.001), along with reduced impulses in the fourth and fifth metatarsal heads (4.95%, p < 0.05). | The center of pressure (COP) curve demonstrated a significant medial shift (p < 0.01). The plantar pressure map and COP trajectory changed after a 30-min exhaustive run. | These alterations might signify heightened stress on joints and tissues when individuals are fatigued, potentially increasing the risk of overload injuries. |
| Boyer et al. [59]. | USA | Cross-Sectional Study | 30 (15 females, 15 males) | Fatigue During Running | The study aimed to assess alterations in the arch mechanics of healthy runners before and after a 45-min run at a comfortable pace. The hypothesis was that there would be increased arch deformation and reduced arch stiffness following the run. | The study found no statistically significant differences in the mean (95% confidence interval) values for navicular displacement (5.6 mm [4.7–6.4 mm]), arch lengthening (3.2 mm [2.6–3.9 mm]), change in arch height index (0.015 [0.012–0.018]), or arch rigidity index (0.95 [0.94–0.96]) following the 45-min run. | The multivariate analyses of variance did not yield statistically significant differences, with all p-values (p) being greater than or equal to 0.065. | Given the absence of statistically significant changes in arch deformation or rigidity, it can be concluded that the structures of a healthy and intact medial longitudinal arch can adapt to cyclical loading, such as enduring a 45-min run, without undergoing compromise. |
| Abt et al. [60]. | USA | Cross-Sectional Study | 12 (six females, six males) | Fatigue During Running | The aim was to investigate whether an exhaustive running about at physiologically determined high-intensity levels causes changes in running kinematics, impacts accelerations, and alters shock attenuating capabilities. | No significant differences were observed for this study’s kinematic or acceleration variables. | While the results of this study did not align with the initial hypotheses, it is essential to note that the impact of running fatigue on kinematics and accelerations remains inconclusive. Further research and exploration are warranted to understand this relationship better. | Future research should investigate fatigue-induced alterations in running kinematics and accelerations to pinpoint the threshold at which these changes manifest. |
| Ament et al. [54]. | Netherland | Cross-Sectional Study | 13 males | Fatigue Running on Treadmill to Exhaustion | The objective was to ascertain the speed and accuracy with which individuals could use visual cues to adapt to the impact position of their feet. | This involved plotting the average adjustment of foot impact position for a single subject. The plot displayed the relative longitudinal foot impact position on the treadmill plate (measured in centimeters) against step number, averaging all the relevant target-line switching cycles throughout a full test run. | The findings revealed prolonged multi-step motor adjustment processes during treadmill running, highlighting the sensitivity of foot placement to the task’s duration as individuals neared exhaustion. | |
| Brocherie et al. [55]. | SWITZERLAND | Cross-Sectional Study | 13 males | Fatigue Running on a Treadmill | Strong evidence indicates greater performance decrements during repeated sprinting in hypoxic (low oxygen) conditions than in normal (normoxic) conditions. | The study found that the sprint decrement score, which indicates the decline in sprint performance, was comparable across different conditions. The pooled values for the sprint decrement score showed no significant difference, with an average of 11.4 ± 7.9% (p = 0.49). | The study found that fatigue-induced changes in standard shoes (SH) differed from those observed in minimalist shoes (SL) and maximalist shoes (MH). Specifically, fatigue in SH resulted in more pronounced alterations in repeated sprint ability, including kinetics, kinematics, and spring-mass characteristics, compared to SL and MH. These alterations were characterized by a reduced ability to effectively apply forward-oriented ground reaction force, maintain vertical stiffness, and sustain stride frequency during repeated sprints in SH. In contrast, SL and MH showed either no or minimal changes in these aspects following fatigue. | In the study, it was observed that impairments in repeated sprint ability and the associated kinetics, kinematics, and spring-mass characteristics were more pronounced in SH (standard shoes) compared to SL (minimalist shoes) and MH (maximalist shoes). The differences were notable, particularly in SH, where fatigue during repeated sprints reduced the ability to effectively apply forward-oriented ground reaction force, maintain vertical stiffness, and sustain stride frequency. On the other hand, SL and MH showed either no or minimal differences in these aspects. |
| Brown et al. [61]. | USA | Cross-Sectional Study | 20 females | Fatigue During Running | To establish whether lower extremity limb dominance influences overground running mechanics. | There were no significant differences between the kinematic or kinetic patterns of the dominant and non-dominant lower extremities during fresh-state overground running. | Fatigue was not shown to interact with limb dominance. | Limb dominance did not affect kinematic or kinetic side-to-side differences. Therefore, physical therapists can continue to use a resolution of lower extremity symmetry as a goal of therapy without having to account for limb dominance. |
| Dutto et al. [62]. | USA | Cross-Sectional Study | 15 (4 females, 11 males) | Fatigue Running on Treadmill to Exhaustion | The objective was to investigate if leg stiffness characteristics undergo alterations during a treadmill run to voluntary exhaustion. | The group analysis demonstrated a significant decrease (p = 0.01) in both vertical (from 23.9 to 23.1 kn·m−1) and leg (from 9.3 to 9.0 kn·m−1) stiffness over the course of the run. | Ten of the 15 runners experienced statistically significant changes in their stride rate. | The observed changes in stride rate are likely a result of alterations in the stiffness characteristics of the leg during a run to fatigue. |
| García-Pe´rez, et al. [56]. | Spain | Cross-Sectional Study | 20 (9 females, 11 males) | Fatigue Running on a Treadmill | To examine the impact acceleration and shock attenuation before and after fatigue during treadmill running. | Treadmill running reduced both head and tibial peak impact accelerations and impact rates, yet no significant distinction was noted in shock attenuation between the two surfaces. | A significant interaction was observed between the surface (treadmill and overground) and fatigue state (pre-fatigue and post-fatigue). | The impact of treadmill running and its interaction with other variables should be considered when interpreting the outcomes of studies utilizing treadmill-based experimental protocols and designing exercise prescriptions. |
| García-Pinillos et al. [65]. | Chile | Cross-Sectional Study | 22 males | Fatigue During Running | To investigate how a running protocol inducing fatigue affects spatiotemporal gait parameters, step variability, vertical (Kvert), and leg stiffness (Kleg) during treadmill running. | Pairwise comparisons, specifically between non-fatigued and fatigued conditions, revealed significant alterations in temporal parameters (i.e., CT and FT) with p-values of 0.001 and less than 0.001, respectively. | In the fatigued condition, Kleg demonstrated a significant reduction (p < 0.001), whereas Kvert remained unchanged (p = 0.602). | The findings suggest that a 60-min trial run induces adaptations in spatiotemporal gait characteristics and stiffness due to fatigue among trained endurance runners. |
| Hanley et al. [57]. | United Kingdom | Cross-Sectional Study | 13 males | Fatigue During Running | The objective was to quantify the impact of fatigue on gait parameters during running. | Significant variations were noted in maximum force, impulse, and contact time (p < 0.01). | Dependent t-tests revealed a notable difference in the knee angle at take-off (p < 0.01). The kinetics and temporal aspects alterations were noticeable as early as the 3000 m mark. | Athletes are encouraged to maintain a consistent race pace to mitigate the impact of fatigue. |
| Hanley et al. [58]. | United Kingdom | Cross-Sectional Study | 15 males | Fatigue Running on treadmill | The aim was to assess alterations in gait parameters during a 10-km treadmill run. | Before reaching the halfway point, there was an increase in step length and a decrease in cadence. | In the latter stages, notable reductions were observed in impulse and maximum force. Concurrently, contact time decreased, and flight time consistently increased. However, the majority of gait variables remained relatively stable. | Running at a constant pace on a treadmill can be beneficial for maintaining a specific distance and speed while keeping a consistent technique unaffected by variables like gradient or fatigue. |
| Mizrahi et al. [63]. | USA | Cross-Sectional Study | 22 males | Fatigue During Running | The study investigated how fatigue impacts the human musculoskeletal system’s ability to absorb shock waves generated during heel strikes. Additionally, the research sought to establish correlations between changes in the electromyography (EMG) signal and other fatigue measures. | The study observed a consistent rise in acceleration signal amplitude when experiencing general fatigue. | No correlation was observed between EMG activity and fatigue in the time or frequency domains. | In conclusion, fatigue appears to diminish the ability of the human musculoskeletal system to absorb the shock acceleration resulting from heel strikes effectively. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazzaa, W.A.; Hottenrott, L.; Kamal, M.A.; Mattes, K. The Influence of General and Local Muscle Fatigue on Kinematics and Plantar Pressure Distribution during Running: A Systematic Review and Meta-Analysis. Sports 2023, 11, 241. https://doi.org/10.3390/sports11120241
Hazzaa WA, Hottenrott L, Kamal MA, Mattes K. The Influence of General and Local Muscle Fatigue on Kinematics and Plantar Pressure Distribution during Running: A Systematic Review and Meta-Analysis. Sports. 2023; 11(12):241. https://doi.org/10.3390/sports11120241
Chicago/Turabian StyleHazzaa, Walaaeldin Aly, Laura Hottenrott, Manar Ahmed Kamal, and Klaus Mattes. 2023. "The Influence of General and Local Muscle Fatigue on Kinematics and Plantar Pressure Distribution during Running: A Systematic Review and Meta-Analysis" Sports 11, no. 12: 241. https://doi.org/10.3390/sports11120241
APA StyleHazzaa, W. A., Hottenrott, L., Kamal, M. A., & Mattes, K. (2023). The Influence of General and Local Muscle Fatigue on Kinematics and Plantar Pressure Distribution during Running: A Systematic Review and Meta-Analysis. Sports, 11(12), 241. https://doi.org/10.3390/sports11120241







