Spatial Distribution of Forensically Significant Blow Flies in Subfamily Luciliinae (Diptera: Calliphoridae), Chiang Mai Province, Northern Thailand: Observations and Modeling Using GIS
Abstract
1. Introduction
2. Materials and Methods
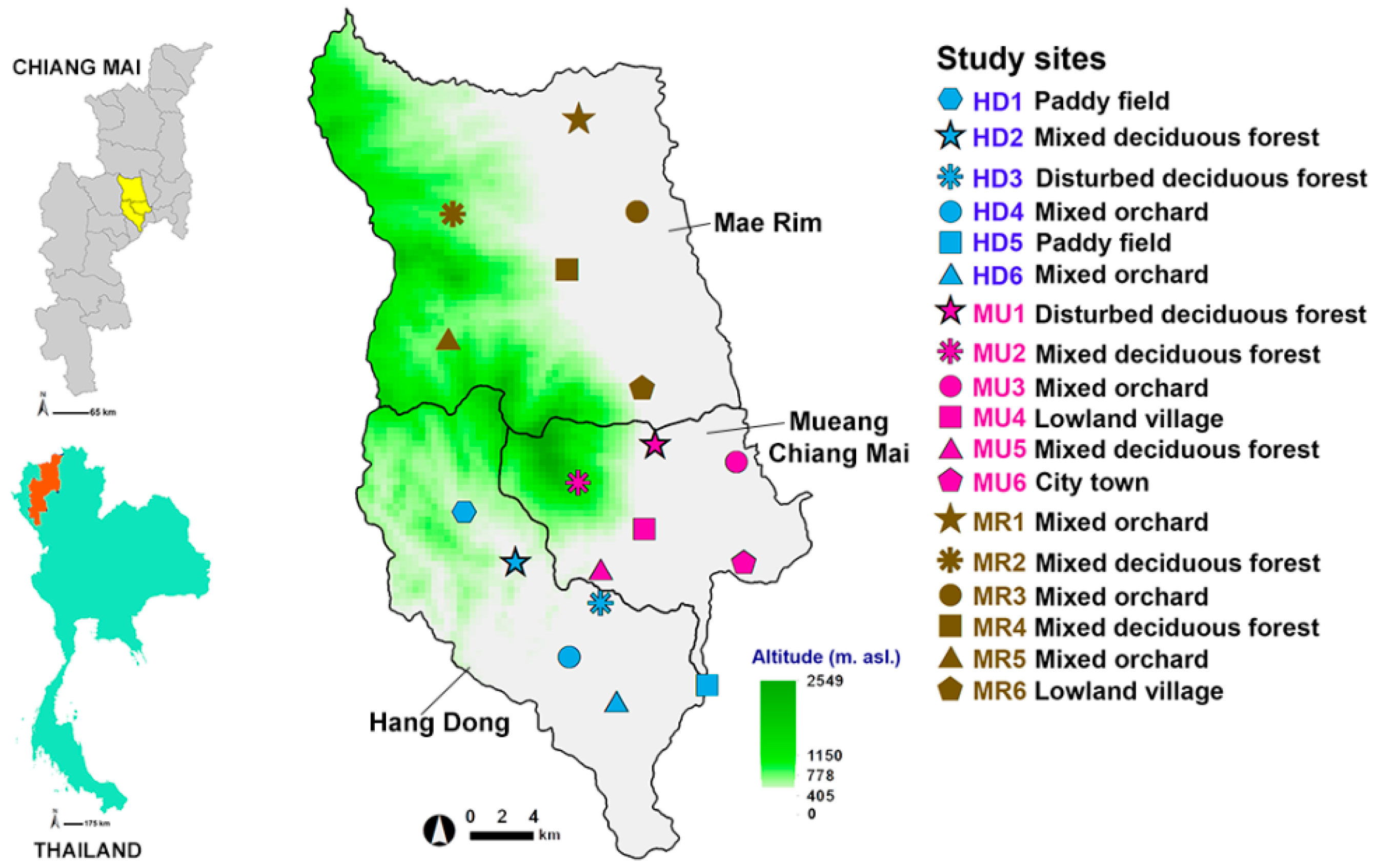
2.1. Study Areas
2.2. Adult Flies Collection
2.3. Statistical Analysis
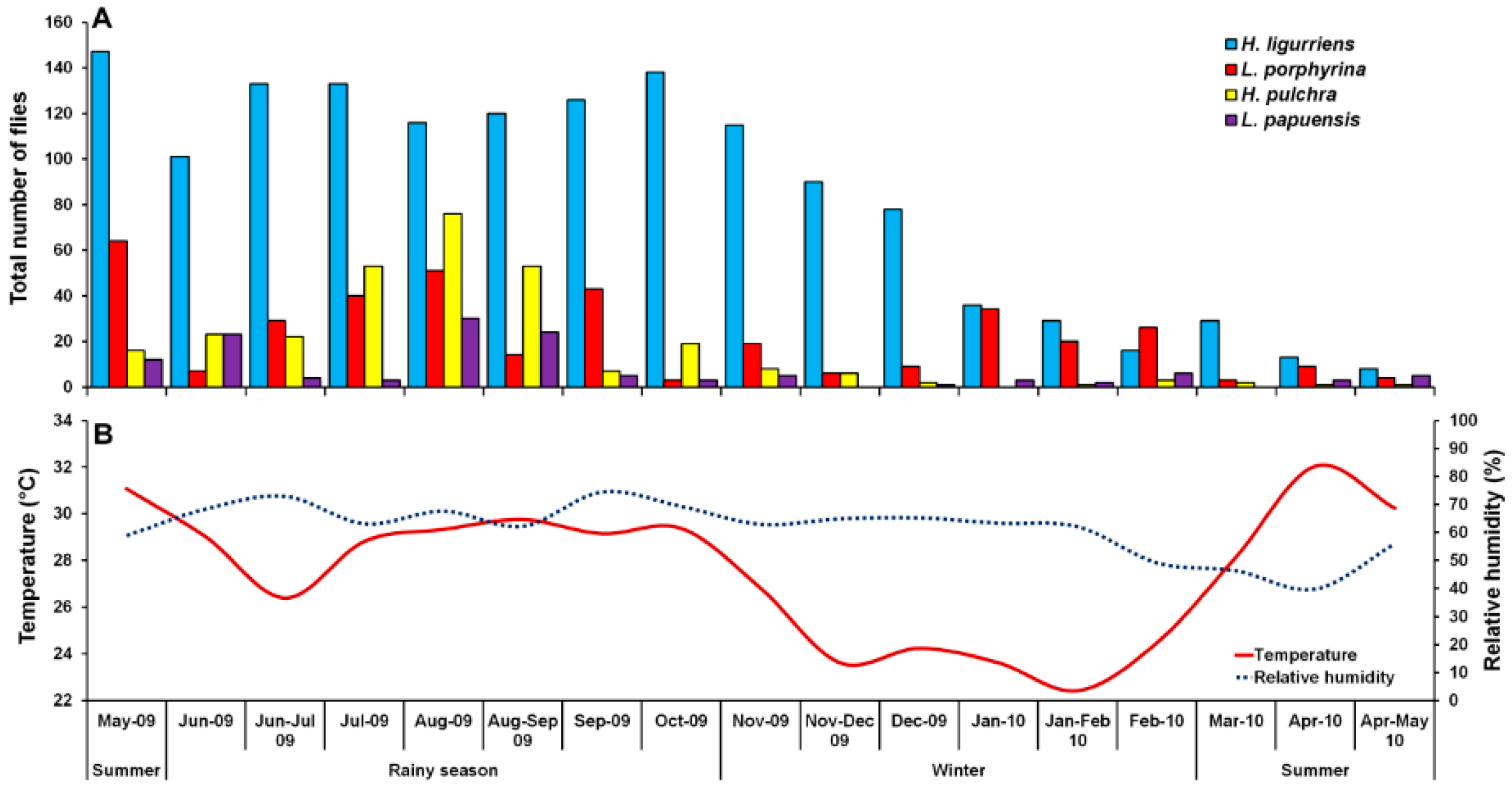
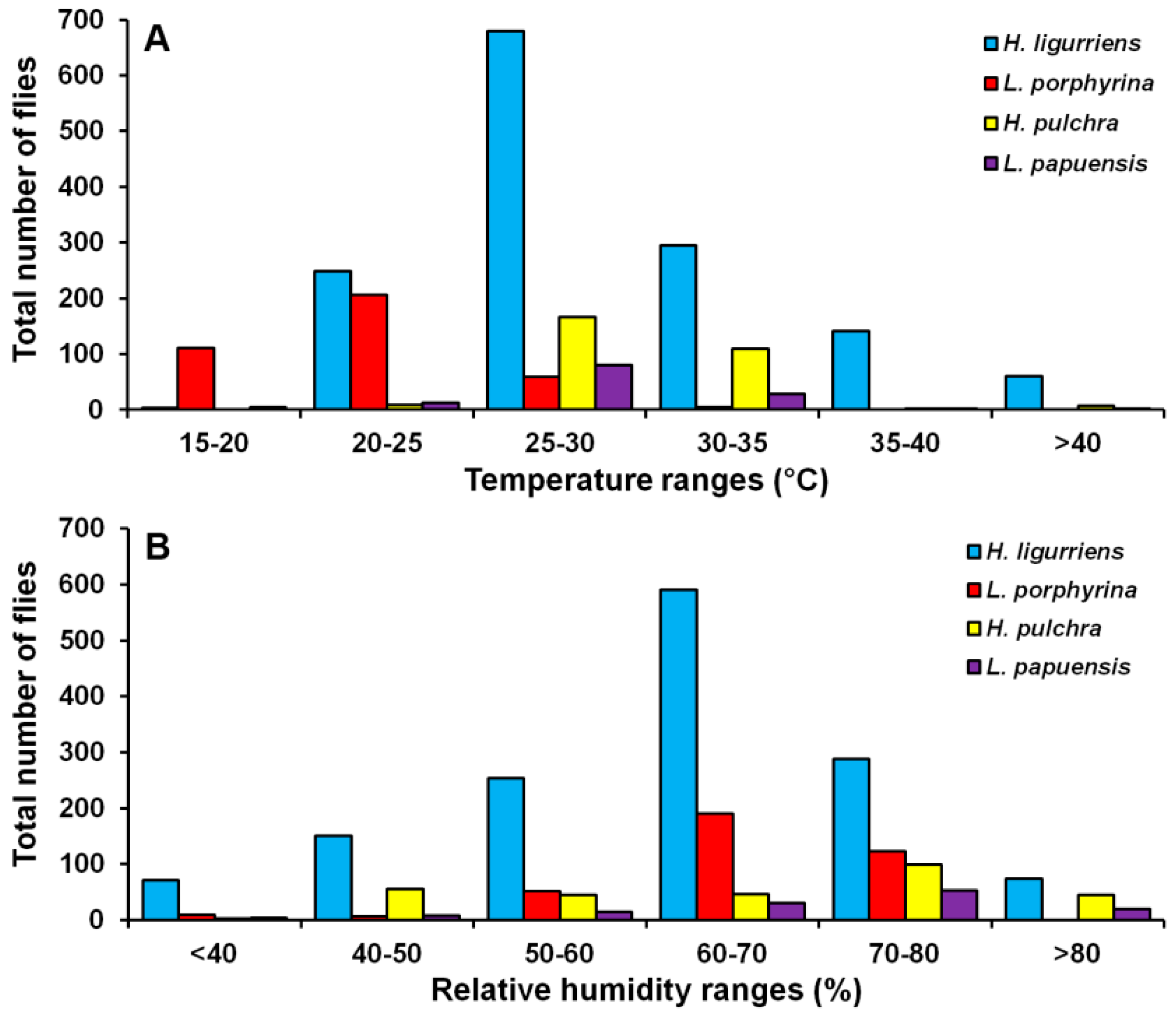
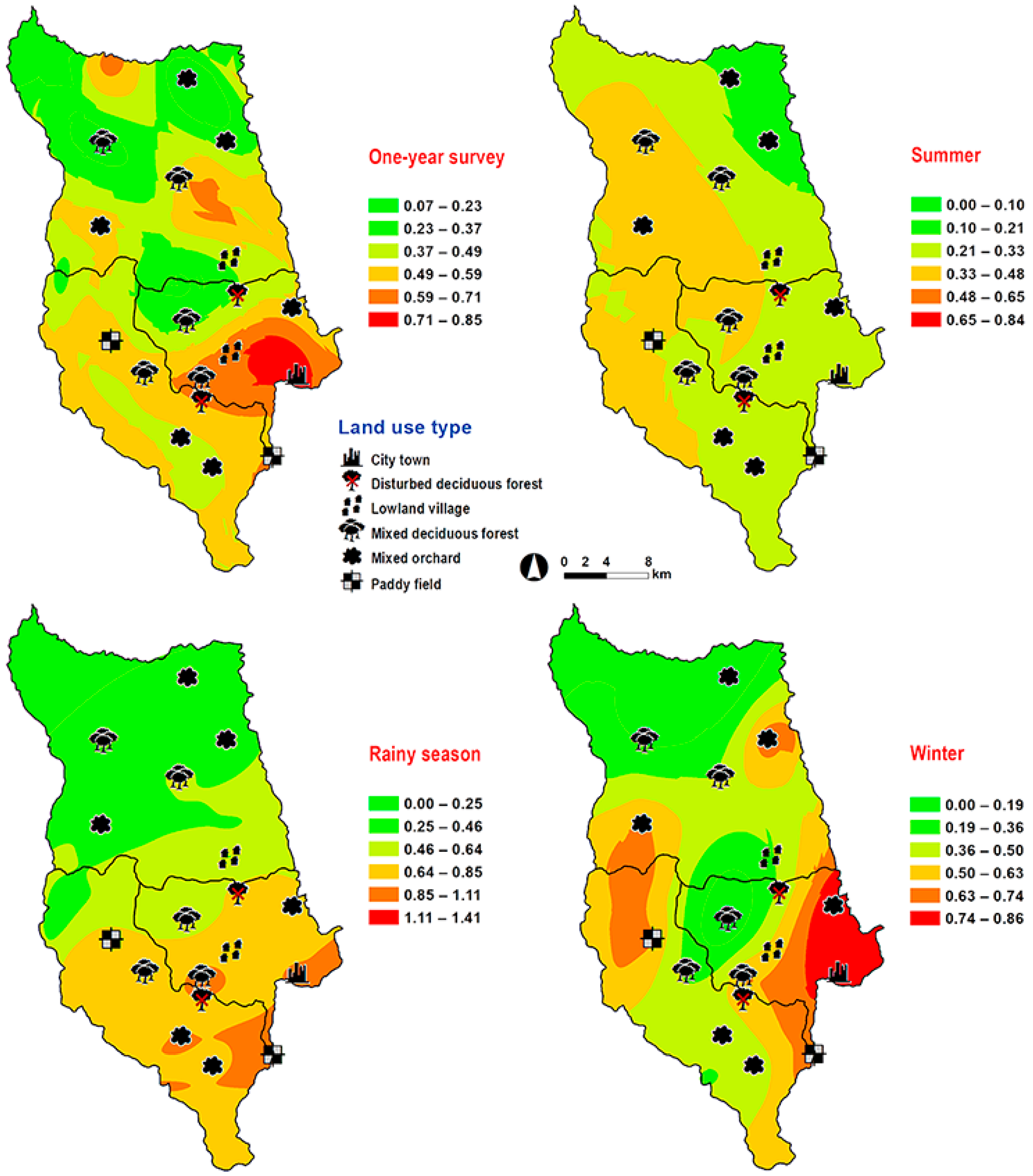
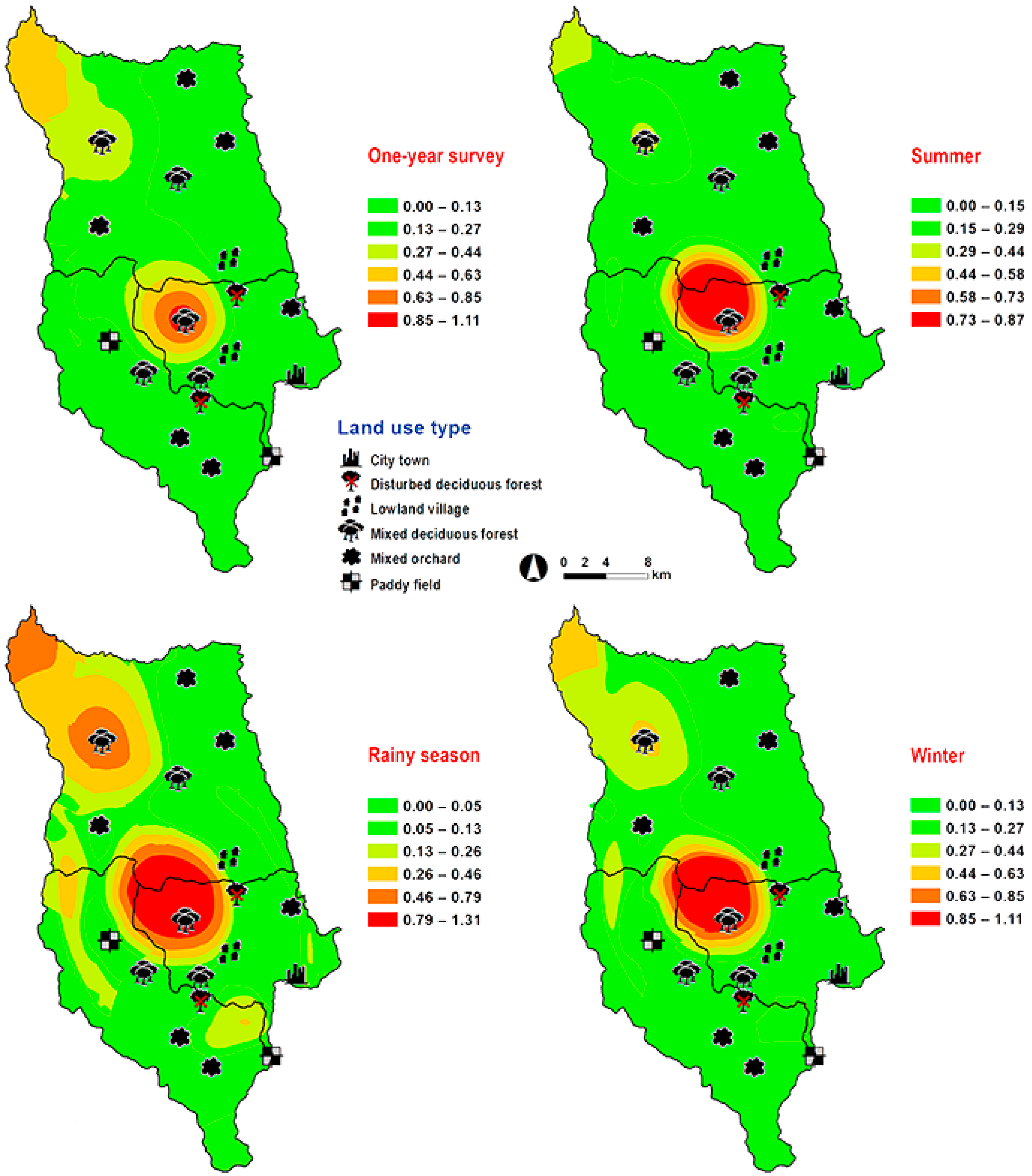
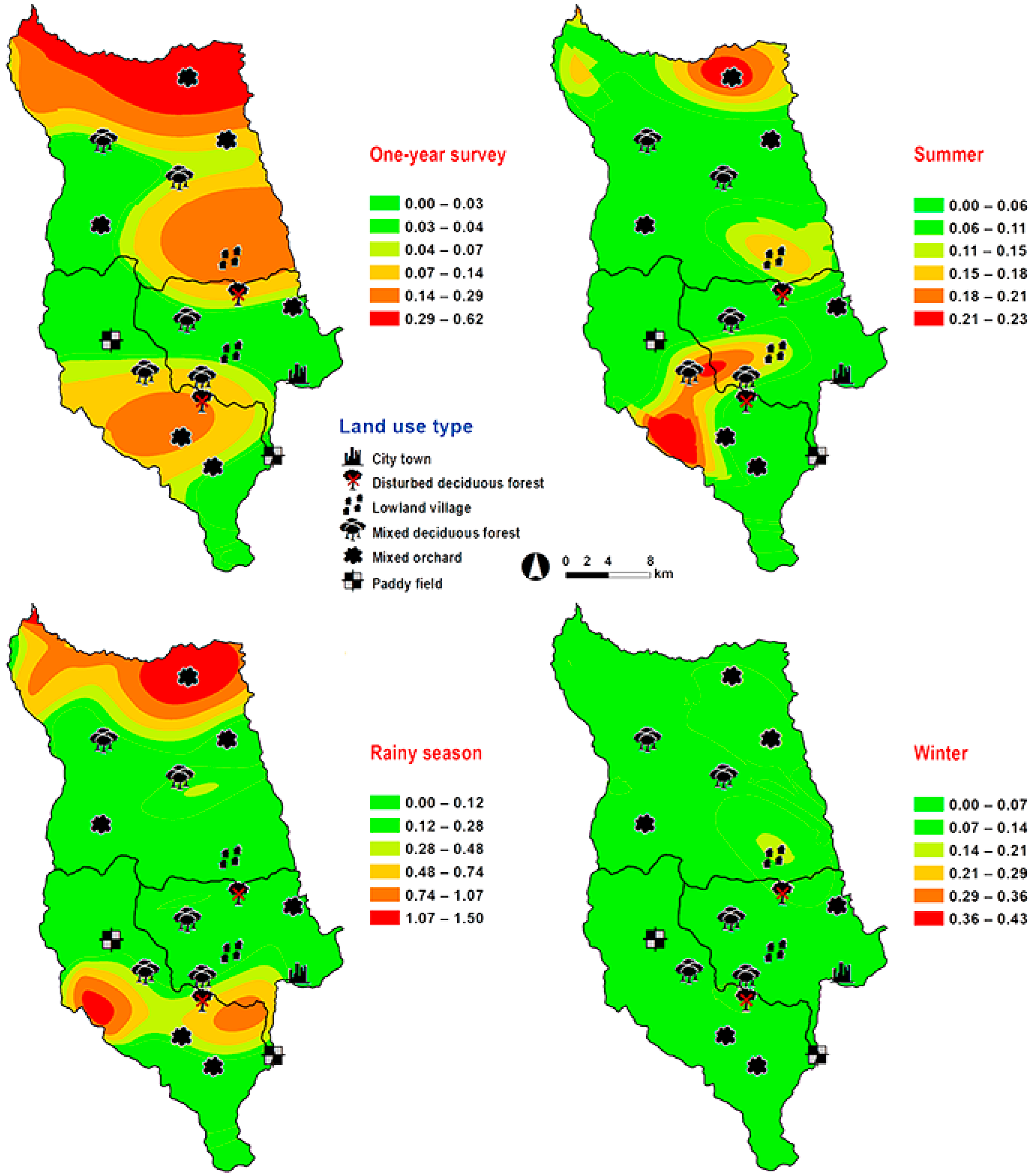
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tomberlin, J.K.; Crippen, T.L.; Tarone, A.M.; Chaudhury, M.F.B.; Singh, B.; Cammack, J.A.; Meisel, R.P. A review of bacterial interactions with blow flies (Diptera: Calliphoridae) of Medical, Veterinary, and Forensic importance. Ann. Entomol. Soc. Am. 2017, 110, 19–36. [Google Scholar] [CrossRef]
- Catts, E.P.; Goff, M.L. Forensic entomology in criminal investigations. Annu. Rev. Entomol. 1992, 37, 253–272. [Google Scholar] [CrossRef] [PubMed]
- Tomberlin, J.K.; Mohr, R.; Benbow, M.E.; Tarone, A.M.; VanLaerhoven, S. A roadmap for bridging basic and applied research in forensic entomology. Annu. Rev. Entomol. 2011, 56, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Sukontason, K.; Narongchai, P.; Kanchai, C.; Vichairat, K.; Sribanditmongkol, P.; Bhoopat, T.; Kurahashi, H.; Chockjamsai, M.; Piangjai, S.; Bunchu, N.; et al. Forensic entomology cases in Thailand: A review of cases from 2000 to 2006. Parasitol. Res. 2007, 101, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Amendt, J.; Richards, C.S.; Campobasso, C.P.; Zehner, R.; Hall, M.J. Forensic entomology: Applications and limitations. Forensic Sci. Med. Pathol. 2011, 7, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Benecke, M. Six Forensic entomology cases: Description and commentary. J. Forensic Sci. 1998, 43, 797–805. [Google Scholar] [CrossRef]
- Silahuddin, S.A.; Latif, B.; Kurahashi, H.; Heo, C.C. The importance of habitat in the ecology of decomposition on rabbit carcasses in Malaysia: Implications in forensic entomology. J. Med. Entomol. 2015, 52, 9–23. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, M.Y.; Jiang, X.Y.; Wang, J.F.; Li, L.L.; Yin, X.J.; Wang, M.; Lai, Y.; Tao, L.Y. Insect succession on remains of human and animals in Shenzhen, China. Forensic Sci. Int. 2017, 271, 75–86. [Google Scholar] [CrossRef]
- Bernhardt, V.; Balint, M.; Verhoff, M.A.; Amendt, J. Species diversity and tissue specific dispersal of necrophagous Diptera on human bodies. Forensic Sci. Med. Pathol. 2018, 14, 76–84. [Google Scholar] [CrossRef]
- Vanin, S.; Tasinato, P.; Ducolin, G.; Terranova, C.; Zancaner, S.; Montisci, M.; Ferrara, S.D.; Turchetto, M. Use of Lucilia species for forensic investigations in Southern Europe. Forensic Sci. Int. 2008, 177, 37–41. [Google Scholar] [CrossRef]
- Farrell, J.F.; Whittington, A.E.; Zalucki, M.P. A review of necrophagous insects colonising human and animal cadavers in South-East Queensland, Australia. Forensic Sci. Int. 2015, 257, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, R.; Nazni, W.A.; Tan, T.C.; Lee, H.L.; Azirun, M.S. Review of forensically important entomological specimens collected from human cadavers in Malaysia (2005–2010). J. Forensic Leg. Med. 2013, 20, 480–482. [Google Scholar] [CrossRef] [PubMed]
- Kumara, T.K.; Disney, R.H.; Abu Hassan, A.; Flores, M.; Hwa, T.S.; Mohamed, Z.; CheSalmah, M.R.; Bhupinder, S. Occurrence of oriental flies associated with indoor and outdoor human remains in the tropical climate of North Malaysia. J. Vector Ecol. 2012, 37, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Firdaus, M.S.; Marwi, M.A.; Syamsa, R.A.; Zuha, R.M.; Ikhwan, Z.; Omar, B. Morphological descriptions of second and third instar larvae of Hypopygiopsis violacea Macquart (Diptera: Calliphoridae), a forensically important fly in Malaysia. Trop. Biomed. 2010, 27, 134–137. [Google Scholar] [PubMed]
- Bunchu, N. Blow fly (Diptera: Calliphoridae) in Thailand: Distribution, morphological identification and medical importance appraisals. Int. J. Parasitol. Res. 2012, 4, 57–64. [Google Scholar] [CrossRef]
- Monum, T.; Sukontason, K.L.; Sribanditmongkol, P.; Sukontason, K.; Samerjai, C.; Limsopatham, K.; Suwannayod, S.; Klong-klaew, T.; Wannasan, A. Forensically important blow flies Chrysomya pinguis, C. villeneuvi, and Lucilia porphyrina (Diptera: Calliphoridae) in a case of human remains in Thailand. Korean. J. Parasitol. 2017, 55, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Kurahashi, H.; Chowanadisai, L. Blow flies (Insecta: Diptera: Calliphoridae) from Indochina. Spec. Div. 2001, 6, 185–242. [Google Scholar] [CrossRef]
- James, T.M. New species are records of Australasian Calliphorinae, with special reference to the fauna of New Guinea. Pac. Insects 1971, 13, 1–12. [Google Scholar]
- Nandi, B.C. Blow flies (Diptera: Calliphoridae) of West Bengal, India with a note on their biodiversity. Rec. Zool. Surv. India 2002, 100, 117–129. [Google Scholar]
- Tumrasvin, W.; Kurahashi, H.; Kano, R. Studies on medically important flies in Thailand VII. Report on 42 species of Calliphorid flies, including the taxonomic keys (Diptera: Calliphoridae). Bull. Tokyo Med. Dent. Univ. 1979, 26, 243–272. [Google Scholar] [PubMed]
- Tumrasvin, W.; Kurahashi, H.; Kano, R. Studies on medically important flies in Thailand II. Record of four species of Lucilia Robineau-Desvoidy (Diptera: Calliphoridae). Bull. Tokyo Med. Dent. Univ. 1977, 24, 1–8. [Google Scholar] [PubMed]
- Klong-klaew, T.; Sontigun, N.; Sanit, S.; Samerjai, C.; Sukontason, K.; Kurahashi, H.; Koehler, P.G.; Pereira, R.M.; Limsopatham, K.; Suwannayod, S.; et al. Field evaluation of a semi-automatic funnel trap targeted the medically important non-biting flies. Acta Trop. 2017, 176, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Amendt, J.; Campobasso, C.P.; Gaudry, E.; Reiter, C.; LeBlanc, H.N.; Hall, M.J. Best practice in forensic entomology—standards and guidelines. Int. J. Legal Med. 2007, 121, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Amendt, J.; Krettek, R.; Zehner, R. Forensic entomology. Naturwissenschaften 2004, 91, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Klong-klaew, T.; Sukontason, K.; Sribanditmongkol, P.; Moophayak, K.; Sanit, S.; Sukontason, K.L. Observations on morphology of immature Lucilia porphyrina (Diptera: Calliphoridae), a fly species of forensic importance. Parasitol. Res. 2012, 111, 1965–1975. [Google Scholar] [CrossRef] [PubMed]
- Sanit, S.; Sukontason, K.; Kurahashi, H.; Tomberlin, J.K.; Wannasan, A.; Kraisittipanit, R.; Sukontason, K.L. Morphology of immature stages of blow fly, Lucilia sinensis Aubertin (Diptera: Calliphoridae), a potential species of forensic importance. Acta Trop. 2017, 176, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Sontigun, N.; Sanit, S.; Wannasan, A.; Sukontason, K.; Amendt, J.; Yasanga, T.; Sukontason, K.L. Ultrastructure of male genitalia of blow flies (Diptera: Calliphoridae) of forensic importance. Acta Trop. 2018, 179, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Bunchu, N.; Thaipakdee, C.; Vitta, A.; Sanit, S.; Sukontason, K.; Sukontason, K.L. Morphology and developmental rate of the blow fly, Hemipyrellia ligurriens (Diptera: Calliphoridae): Forensic entomology applications. J. Parasitol. Res. 2012, 2012, 371243. [Google Scholar] [CrossRef]
- Sanit, S.; Sukontason, K.; Klong-klaew, T.; Tomberlin, J.K.; Limsopatham, K.; Samerjai, C.; Sontigun, N.; Sukontason, K.L. Ontogenensis and developmental rate of the blow fly, Hypopygiopsis tumrasvini Kurahashi (Diptera: Calliphoridae). Trop. Biomed. 2014, 31, 760–768. [Google Scholar]
- Sukontason, K.; Sukontason, K.L.; Piangjai, S.; Tippanun, J.; Lertthamnongtham, S.; Vogtsberger, R.C.; Olson, J.K. Survey of forensically-relevant fly species in Chiang Mai, northern Thailand. J. Vector Ecol. 2003, 28, 135–138. [Google Scholar]
- Sukontason, K.L.; Samerjai, C.; Sanit, S.; Klong-klaew, T.; Limsopatham, K.; Sontigun, N.; Suwannayod, S.; Kurahashi, H.; Bunchu, N.; Chaiwong, T.; et al. Survey of forensically important fly species in northern Thailand. Southeast Asian. J. Trop. Med. Public Health. 2018, 49, 580–589. [Google Scholar]
- Moophayak, K.; Klong-klaew, T.; Sukontason, K.; Kurahashi, H.; Tomberlin, J.K.; Sukontason, K.L. Species composition of carrion blow flies in northern Thailand: Altitude appraisal. Rev. Inst. Med. Trop. São Paulo 2014, 56, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Ngoen-klan, R.; Moophayak, K.; Klong-klaew, T.; Irvine, K.N.; Sukontason, K.L.; Prangkio, C.; Somboon, P.; Sukontason, K. Do climatic and physical factors affect populations of the blow fly Chrysomya megacephala and house fly Musca domestica? Parasitol. Res. 2011, 109, 1279–1292. [Google Scholar] [CrossRef] [PubMed]
- Klong-klaew, T.; Sukontason, K.; Ngoen-klan, R.; Moophayak, K.; Irvine, K.N.; Kurahashi, H.; Prangkio, C.; Sanit, S.; Sukontason, K.L. Impact of abiotic factor changes in blowfly, Achoetandrus rufifacies (Diptera: Calliphoridae), in northern Thailand. Parasitol. Res. 2014, 113, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Klong-klaew, T.; Ngoen-klan, R.; Moophayak, K.; Sukontason, K.; Irvine, K.N.; Tomberlin, J.K.; Chareonviriyaphap, T.; Kurahashi, H.; Sukontason, K.L. Predicting geographic distribution of forensically significant blow flies of subfamily Chrysomyinae (Diptera: Calliphoridae) in northern Thailand. Insects 2018, 9, 106. [Google Scholar] [CrossRef]
- Rogers, D.J.; Williams, B.G. Monitoring trypanosomiasis in space and time. Parasitology 1993, 106, S77–S92. [Google Scholar] [CrossRef]
- Bunchu, N.; Sukontason, K.L.; Olson, J.K.; Kurahashi, H.; Sukontason, K. Behavioral responses of Chrysomya megacephala to natural products. Parasitol. Res. 2008, 102, 419–429. [Google Scholar] [CrossRef]
- Hwang, C.; Turner, B.D. Spatial and temporal variability of necrophagous Diptera from urban to rural areas. Med. Vet. Entomol. 2005, 19, 379–391. [Google Scholar] [CrossRef]
- Monfared, S.; Khorshidian, K.; Monfared, A.; Mohammadi, Z.; Khodaparast, R. Prediction of bees (Apoidea, Hymenoptera) frequency and distribution using best linear spatial prediction techniques (kriging and cokriging). Entomofauna 2013, 34, 81–104. [Google Scholar]
- Jacob, B.G.; Burkett-Cadena, N.D.; Luvall, J.C.; Parcak, S.H.; McClure, C.J.W.; Estep, L.K.; Hill, G.E.; Cupp, E.W.; Novak, R.J.; Unnasch, T.R. Developing GIS-based eastern equine encephalitis vector-host models in Tuskegee, Alabama. Int. J. Health Geogr. 2010, 9, 12. [Google Scholar] [CrossRef]
- Zabala, J.; Díaz, B.; Saloña-Bordas, M.I. Seasonal blowfly distribution and abundance in fragmented landscapes. Is it useful in forensic inference about where a corpse has been decaying? PLoS ONE. 2014, 9, e99668. [Google Scholar] [CrossRef] [PubMed]
- Kurahashi, H.; Banu, Q. Notes on the Bangladesh calliphorid flies of medical importance (Insecta: Diptera). Jpn. J. Sanit. Zool. 1989, 40, 97–111. [Google Scholar] [CrossRef]
- Bunchu, N.; Sukontason, K.; Sanit, S.; Chidburee, P.; Kurahashi, H.; Sukontason, K.L. Occurrence of blow fly species (Diptera: Calliphoridae) in Phitsanulok Province, northern Thailand. Trop. Biomed. 2012, 29, 532–543. [Google Scholar] [PubMed]
- Suenaga, O.; Kurahashi, H. Life cycle of an oriental blow fly, Lucilia porphyrina (Walker) in Nagasaki, Western Japan. Trop. Med. 1995, 37, 99–107. [Google Scholar]
- Lertthamnongtham, S.; Sukontason, K.L.; Sukontason, K.; Piangjai, S.; Choochote, W.; Vogtsberger, R.C.; Olson, J.K. Seasonal fluctuations in populations of the two most forensically important fly species in northern Thailand. Ann. Trop. Med. Parasitol. 2003, 97, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Weidner, L.M.; Jennings, D.E.; Tomberlin, J.K.; Hamilton, G.C. Seasonal and geographic variation in biodiversity of forensically important blow flies (Diptera: Calliphoridae) in New Jersey, USA. J. Med. Entomol. 2015, 52, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Q.; Lyu, Z.; Li, X.B.; Li, K.; Yao, L.; Wan, L.H. Technical note: Development of Hemipyrellia ligurriens (Wiedemann) (Diptera: Calliphoridae) at constant temperatures: Applications in estimating postmortem interval. Forensic Sci. Int. 2015, 253, 48–54. [Google Scholar] [CrossRef]
- Sontigun, N.; Sukontason, K.L.; Klong-klaew, T.; Sanit, S.; Samerjai, C.; Somboon, P.; Thanapornpoonpong, S.N.; Amendt, J.; Sukontason, K. Bionomics of the oriental latrine fly Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae): Temporal fluctuation and reproductive potential. Parasit. Vectors 2018, 11, 415. [Google Scholar] [CrossRef]
- Klong-klaew, T.; Sontigun, N.; Sanit, S.; Samerjai, C.; Sukontason, K.; Koehler, P.G.; Pereira, R.M.; Chareonviriyaphap, T.; Kurahashi, H.; Sukontason, K.L. Daily and seasonal prevalence of the blow fly Chrysomya rufifacies (Diptera: Calliphoridae) as revealed by semi-automatic trap collections in suburban Chiang Mai Province, northern Thailand. Fla. Entomol. 2018, 101, 1–6. [Google Scholar]

| Land Use Types | ||||||
|---|---|---|---|---|---|---|
| Mixed Deciduous Forest | Disturbed Mixed Deciduous Forest | Mixed Orchard | Paddy Field | Lowland Village | City/Town | |
| Climatic factor recorded * | ||||||
| Temperature (°C) | 26.5 (16.7–39.8) | 28.4 (21.0–39.3) | 28.4 (19.7–45.2) | 27.5 (19.6–40.8) | 28.3 (18.7–35.8) | 25.8 (18.8–30.7) |
| Relative humidity (%) | 67.0 (35–87) | 61.5 (35–80) | 59.0 (25–89) | 63.5 (39–83) | 65.0 (36–83) | 64.0 (40–78) |
| Light intensity (Lux) | 26,550 (193–107,500) | 59,403 (2320–359,600) | 47,200 (1000–118,500) | 36,300 (6700–112,000) | 38,550 (2900–95,700) | 22,000 (442–94,000) |
| Number of adult flies collected ** | ||||||
| Hemipyrellia ligurriens (Wiedemann) | 4.70 ± 1.00 a | 4.53 ± 1.13 a | 4.16 ± 0.73 a | 5.71 ± 0.95 a | 4.32 ± 1.13 a | 7.87 ± 2.18 a |
| Hemipyrellia pulchra (Wiedemann) | 0.18 ± 0.06 ab | 0.62 ± 0.22 a | 2.25 ± 0.92 a | 0 b | 0.88 ± 0.29 a | 0 b |
| Lucilia porphyrina (Walker) | 4.43 ± 1.23 a | 0 b | 0.09 ± 0.05 b | 0 b | 0 b | 0 b |
| Lucilia papuensis Macquart | 1.21 ± 0.38 a | 0.012 ± 0.06 b | 0.05 ± 0.03 b | 0.09 ± 0.09 b | 0.41 ± 0.15 ab | 0.07 ± 0.07 b |
| Lucilia cuprina (Wiedemann) | 0.07 ± 0.04 | 0.03 ± 0.03 | 0.32 ± 0.09 | 0.06 ± 0.06 | 0.06 ± 0.06 | 1.60 ± 0.64 |
| Hypopygiopsis tumrasvini Kurahashi | 0.23 ± 0.11 | 0 | 0.02 ± 0.02 | 0.03 ± 0.03 | 0.03 ± 0.03 | 0 |
| Lucilia sinensis Aubertin | 0.05 ± 0.03 | 0 | 0 | 0 | 0.06 ± 0.06 | 0 |
| Hypopygiopsis infumata (Bigot) | 0.02 ± 0.02 | 0 | 0 | 0 | 0.06 ± 0.06 | 0 |
| Species | Factors | Regression Coefficient (B) | SE | 95% CI | p Value |
|---|---|---|---|---|---|
| H. ligurriens | Temperature | 0.103 | 0.0225 | 0.059, 0.147 | 0.0001 |
| RH | 0.035 | 0.0092 | 0.017, 0.054 | 0.0001 | |
| Light intensity | −0.0000002 | 0.0000032 | −0.000006, 0.000006 | 0.954 | |
| H. pulchra | Temperature | 0.325 | 0.0655 | 0.197, 0.453 | 0.0001 |
| RH | 0.077 | 0.0217 | 0.035, 0.120 | 0.0001 | |
| Light intensity | −0.000006 | 0.00001 | −0.000025, 0.000014 | 0.0578 | |
| L. porphyrina | Temperature | −0.263 | 0.0325 | −0.326, −0.199 | 0.0001 |
| RH | −0.003 | 0.0102 | −0.023, 0.017 | 0.741 | |
| Light intensity | −0.000034 | 0.000005 | −0.000044, −0.000025 | 0.0001 | |
| L. papuensis | Temperature | 0.126 | 0.0107 | 0.001, 0.043 | 0.022 |
| RH | 0.033 | 0.0036 | 0.001, 0.015 | 0.048 | |
| Light intensity | −0.00003 | 0.00001 | −0.00005, −0.00001 | 0.003 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klong-klaew, T.; Ngoen-klan, R.; Moophayak, K.; Sukontason, K.; Irvine, K.N.; Tomberlin, J.K.; Kurahashi, H.; Chareonviriyaphap, T.; Somboon, P.; Sukontason, K.L. Spatial Distribution of Forensically Significant Blow Flies in Subfamily Luciliinae (Diptera: Calliphoridae), Chiang Mai Province, Northern Thailand: Observations and Modeling Using GIS. Insects 2018, 9, 181. https://doi.org/10.3390/insects9040181
Klong-klaew T, Ngoen-klan R, Moophayak K, Sukontason K, Irvine KN, Tomberlin JK, Kurahashi H, Chareonviriyaphap T, Somboon P, Sukontason KL. Spatial Distribution of Forensically Significant Blow Flies in Subfamily Luciliinae (Diptera: Calliphoridae), Chiang Mai Province, Northern Thailand: Observations and Modeling Using GIS. Insects. 2018; 9(4):181. https://doi.org/10.3390/insects9040181
Chicago/Turabian StyleKlong-klaew, Tunwadee, Ratchadawan Ngoen-klan, Kittikhun Moophayak, Kom Sukontason, Kim N. Irvine, Jeffery K. Tomberlin, Hiromu Kurahashi, Theeraphap Chareonviriyaphap, Pradya Somboon, and Kabkaew L. Sukontason. 2018. "Spatial Distribution of Forensically Significant Blow Flies in Subfamily Luciliinae (Diptera: Calliphoridae), Chiang Mai Province, Northern Thailand: Observations and Modeling Using GIS" Insects 9, no. 4: 181. https://doi.org/10.3390/insects9040181
APA StyleKlong-klaew, T., Ngoen-klan, R., Moophayak, K., Sukontason, K., Irvine, K. N., Tomberlin, J. K., Kurahashi, H., Chareonviriyaphap, T., Somboon, P., & Sukontason, K. L. (2018). Spatial Distribution of Forensically Significant Blow Flies in Subfamily Luciliinae (Diptera: Calliphoridae), Chiang Mai Province, Northern Thailand: Observations and Modeling Using GIS. Insects, 9(4), 181. https://doi.org/10.3390/insects9040181




