Effects of Endosymbiont Disruption on the Nutritional Dynamics of the Pea Aphid Acyrthosiphon pisum
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants and Insects
2.2. Antibiotic Screening for Endosymbiont Disruption in the Pea Aphid
2.3. Effects of Buchnera Disruption on the Growth of Pea Aphid
2.4. Quantification of the Dynamic Changes of Pea Aphid Nutrition
2.4.1. Protein Detection
2.4.2. Carbohydrate Detection
2.4.3. Lipid Detection
2.5. Statistical Analysis
3. Results
3.1. Endosymbiont Disruption in Pea Aphids
3.2. Effects of Buchnera Disruption on the Growth of Pea Aphid
3.3. Quantification of the Dynamic Changes of Proteins
3.4. Quantification for the Dynamic Changes of Carbohydrates
3.5. Quantification of the Dynamic Changes to Lipids
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frago, E.; Dicke, M.; Godfray, H.C.J. Insect symbionts as hidden players in insect-plant interactions. Trends Ecol. Evol. 2012, 27, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Weinert, L.A.; Araujo-Jnr, E.V.; Ahmed, M.Z.; Welch, J.J. The incidence of bacterial endosymbionts in terrestrial arthropods. Proc. R. Soc. B 2015, 282, 20150249. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. How multi-partner endosymbioses function. Nat. Rev. Microbiol. 2016, 14, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, E.A.; Douglas, A.E. Symbiotic bacteria enable insect to use a nutritionally inadequate diet. Proc. R. Soc. B 2009, 276, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Brumin, M.; Kontsedalov, S.; Ghanim, M. Rickettsia influences thermotolerance in the whitefly Bemisia tabcai B biotype. Insect Sci. 2011, 18, 57–66. [Google Scholar] [CrossRef]
- Haine, E.R. Symbiont-mediated protection. Proc. R. Soc. B 2008, 275, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Kontsedalov, S.; Zchori-Fein, E.; Chiel, E.; Gottlieb, Y.; Inbar, M.; Ghanim, M. The presence of Rickettsia is associated with increased susceptibility of Bemisia tabaci (Homoptera: Aleyrodidae) to insecticides. Pest Manag. Sci. 2008, 64, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Oliver, K.M.; Russell, J.A.; Moran, N.A.; Hunter, M.S. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl. Acad. Sci. USA 2003, 100, 1803–1807. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.F.; Wong, C.A.N.; Chaston, J.M.; Colvin, J.; McKenzie, C.L.; Douglas, A.E. The bacterial communities in plant phloem-sap-feeding insects. Mol. Ecol. 2014, 23, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. Nutritional interaction in insect-microbial symbiosis: Aphid and their symbiotic bacteria Buchnera. Annu. Rev. Entomol. 1998, 43, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.W.; Poliakov, A.; Haribal, M.; Jander, G.; van Wijk, K.J.; Douglas, A.E. Matching the supply of bacterial nutrients to the nutritional demand of the animal host. Proc. R. Soc. B 2014, 281, 20141163. [Google Scholar] [CrossRef] [PubMed]
- Santos-Garcia, D.; Vargas-Chavez, C.; Moya, A.; Latorre, A.; Silva, F.J. Genome Evolution in the primary endosymbiont of whiteflies sheds light on their divergence. Genome Biol. Evol. 2015, 7, 873–888. [Google Scholar] [CrossRef] [PubMed]
- Santos-Garcia, D.; Farnier, P.A.; Beitia, F.; Zchori-Fein, E.; Vavre, F.; Mouton, L.; Mova, A.; Latorre, A.; Silva, F.J. Complete genome sequence of “Candidatus Portiera aleyrodidarum” BT-QVLC, an obligate symbiont that supplies amino acids and carotenoids to Bemisia tabaci. J. Bacteriol. 2012, 194, 6654–6655. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.F.; Xia, F.F.; Johnson, K.W.; Brown, C.D.; Bartom, E.; Tuteja, J.H.; Grossman, R.L.; Brumin, M.; White, K.P.; Ghanim, M. Comparison of the genome sequences of “Candidatus Portiera aleyrodidarum” Primary endosymbionts of the whitefly Bemisia tabaci B and Q Biotypes. Appl. Environ. Microb. 2013, 79, 1757–1759. [Google Scholar] [CrossRef] [PubMed]
- Nakabachi, A.; Ueoka, R.; Oshima, K.; Teta, R.; Mangoni, A.; Gurgui, M.; Oldhan, N.J.; Echten-Deckert, G.V.; Okamura, K.; Yamamoto, K.; et al. Defensive bacteriome symbiont with a drastically reduced genome. Curr. Biol. 2013, 23, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.L.; Hall, A.A.G.; Riegler, M. Symbionts in waiting: The dynamics of incipient endosymbiont complementation and replacement in minimal bacterial communities of psyllids. Microbiome 2017, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Pais, R.; Lohs, C.; Wu, Y.N.; Wang, J.W.; Aksoy, S. The obligate mutualist Wigglesworthia glossinidia influences reproduction, digestion, and immunity processes of its host, the tsetse fly. Appl. Environ. Microb. 2008, 74, 5965–5974. [Google Scholar] [CrossRef] [PubMed]
- Losey, J.E.; Eubanks, M.D. Implications of pea aphid host-plant specialization for the potential colonization of vegetables following post-harvest emigration from forage crops. Environ. Entomol. 2000, 29, 1283–1288. [Google Scholar] [CrossRef]
- Ryalls, J.M.W.; Riegler, M.; Moore, B.D.; Johnson, S.N. Biology and trophic interactions of lucerne aphids. Agric. For. Entomol. 2013, 15, 335–350. [Google Scholar] [CrossRef]
- Karley, A.J.; Douglas, A.E.; Parker, W.E. Amino acid composition and nutritional quality of potato leaf phloem sap for aphids. J. Exp. Biol. 2002, 205, 3009–3018. [Google Scholar] [PubMed]
- Douglas, A.E. Phloem-sap feeding by animals: Problems and solutions. J. Exp. Bot. 2006, 57, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, T.L.; Douglas, A.E. The impact of aposymbiosis on amino acid metabolism of pea aphids (Acyrthosiphon pisum). Entomol. Exp. Appl. 1996, 80, 279–282. [Google Scholar] [CrossRef]
- Douglas, A.E. Reproductive failure and the free amino acid pools in pea aphids (Acyrthosiphon pisum) lacking symbiotic bacteria. J. Insect Physiol. 1996, 42, 247–255. [Google Scholar] [CrossRef]
- Wilkinson, T.L.; Douglas, A.E. Why aphids (Acyrthosiphon pisum) lacking symbiotic bacteria have elevated levels of the amino acid glutamine. J. Insect Phys. 1995, 41, 921–927. [Google Scholar] [CrossRef]
- Wilkinson, T.L.; Ishikawa, H. On the functional significance of symbiotic microorganisms in the Homoptera: A comparative study of Acyrthosiphon pisum and Nilaparvata lugens. Physiol. Entomol. 2008, 26, 86–93. [Google Scholar] [CrossRef]
- Miura, T.; Braendle, C.; Shingleton, A.; Sisk, G.; Kambhampati, S.; Stern, D.L. A comparison of parthenogenetic and sexual embryogenesis of the pea aphid Acyrthosiphon pisum (Hemiptera: Aphidoidea). J. Exp. Zool. Part B 2003, 295, 59–81. [Google Scholar] [CrossRef] [PubMed]
- Caillaud, C.M.; Rahbe, Y. Aposymbiosis in a cereal aphid: Reproductive failure and influence on plant utilization. Ecol. Entomol. 1999, 24, 111–114. [Google Scholar] [CrossRef]
- Douglas, A.E. Reproductive diapause and the bacterial symbiosis in the sycamore aphid Drepanosiphum platanoidis. Ecol. Entomol. 2000, 25, 256–261. [Google Scholar] [CrossRef]
- Machado-Assefh, C.R.; Lopez-Isasmendi, G.; Tjallingii, W.F.; Jander, G.; Alvarez, A.E. Disrupting Buchnera aphidicola, the endosymbiotic bacteria of Myzus persicae, delays host plant acceptance. Arthropod Plant Interact. 2015, 9, 529–541. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebroug, N.J.; Farr, A.L.; Randdall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 267–275. [Google Scholar]
- Van Handel, E. Microseparation of glycogen, sugars, and lipids. Anal. Biochem. 1965, 11, 266–271. [Google Scholar] [CrossRef]
- Van Handel, E.; Day, J. Assay of lipids, glycogen and sugars in individual mosquitoes: Correlations with wing length in field-collected Aedes vexans. J. Am. Mosq. Control 1988, 4, 549–550. [Google Scholar]
- Van Handel, E. Rapid determination of total lipids in mosquitoes. J. Am. Mosq. Control 1985, 1, 302–304. [Google Scholar]
- Williams, C.M.; Thomas, R.H.; MacMillan, H.A.; Marshall, K.E.; Sinclair, B.J. Triacylglyceride measurement in small quantities of homogenised insect tissue: Comparisons and caveats. J. Insect Physiol. 2011, 57, 1602–1613. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E.; Minto, L.B.; Wilkinson, T.L. Quantifying nutrient production by the microbial symbionts in an aphid. J. Exp. Biol. 2001, 204, 349–358. [Google Scholar] [PubMed]
- Viñuelas, J.; Febvay, G.; Duport, G.; Colella, S.; Fayard, J.M.; Charles, H.; Rahbe, Y.; Calevro, F. Multimodal dynamic response of the Buchnera aphidicola pLeu plasmid to variations in leucine demand of its host, the pea aphid Acyrthosiphon pisum. Mol. Microbiol. 2011, 81, 1271–1285. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, T.L.; Koga, R.; Fukatsu, T. Role of host nutrition in symbiont regulation: Impact of dietary nitrogen on proliferation of obligate and facultative bacterial endosymbionts of the pea aphid Acyrthosiphon pisum. Appl. Environ. Microb. 2007, 73, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.M.; Li, X.R.; Zhang, Y.H.; Coates, B.; Zhou, X.G.; Cheng, D.F. Bacterial symbionts, Buchnera, and starvation on wing dimorphism in English grain aphid, Sitobion avenae (F.) (Homoptera: Aphididae). Front Physiol. 2015, 6, 155. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Wilkinson, T.L.; Douglas, A.E. The aphid-bacterial symbiosis: A comparison between pea aphids and black bean aphids. Entomol. Exp. Appl. 1996, 80, 275–278. [Google Scholar] [CrossRef]
- Hansen, A.K.; Moran, N.A. Aphid genome expression reveals host-symbiont cooperation in the production of amino acids. Proc. Natl. Acad. Sci. USA 2011, 108, 2849–2854. [Google Scholar] [CrossRef] [PubMed]
- Sandstrom, J.; Pettersson, J. Amino acid composition of phloem sap and the relation to intraspecific variation in pea aphid Acyrthosiphon pisum performance. J. Insect Physiol. 1994, 40, 947–955. [Google Scholar] [CrossRef]
- Raikhel, A.S.; Dhadialla, T.S. Accumulation of yolk proteins in insect oocytes. Annu. Rev. Entomol. 1992, 37, 217–251. [Google Scholar] [CrossRef] [PubMed]
- Ahsaei, S.M.; Tabadkani, S.M.; Hosseininaveh, V.; Allahyari, H.; Bigham, M. Differential accumulation of energy by the colour morphs of the pea aphid Acyrthosiphon pisum (Hemiptera: Aphididae) mirrors their ecological adaptations. Eur. J. Entomol. 2013, 110, 241–245. [Google Scholar] [CrossRef]
- Hansford, R.G.; Johnson, R.N. The nature and control of the tricarboxylate cycle in beetle flight muscle. Biochem. J. 1975, 148, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Febvay, G.; Rahbe, Y.; Rynkiewicz, M.; Guillaud, J.; Bonnot, G. Fate of dietary sucrose and neosynthesis of amino acids in the pea aphid, Acyrthosiphon pisum, reared on different diets. J. Exp. Biol. 1999, 202, 2639–2652. [Google Scholar] [PubMed]
- Lv, N. Study on the Intracellular Bacterial Symbiont of Two Color Morphs of Acyrthosiphon pisum Harris. Master’s Thesis, Gansu Agricultural University, Lanzhou, China, 2015. [Google Scholar]
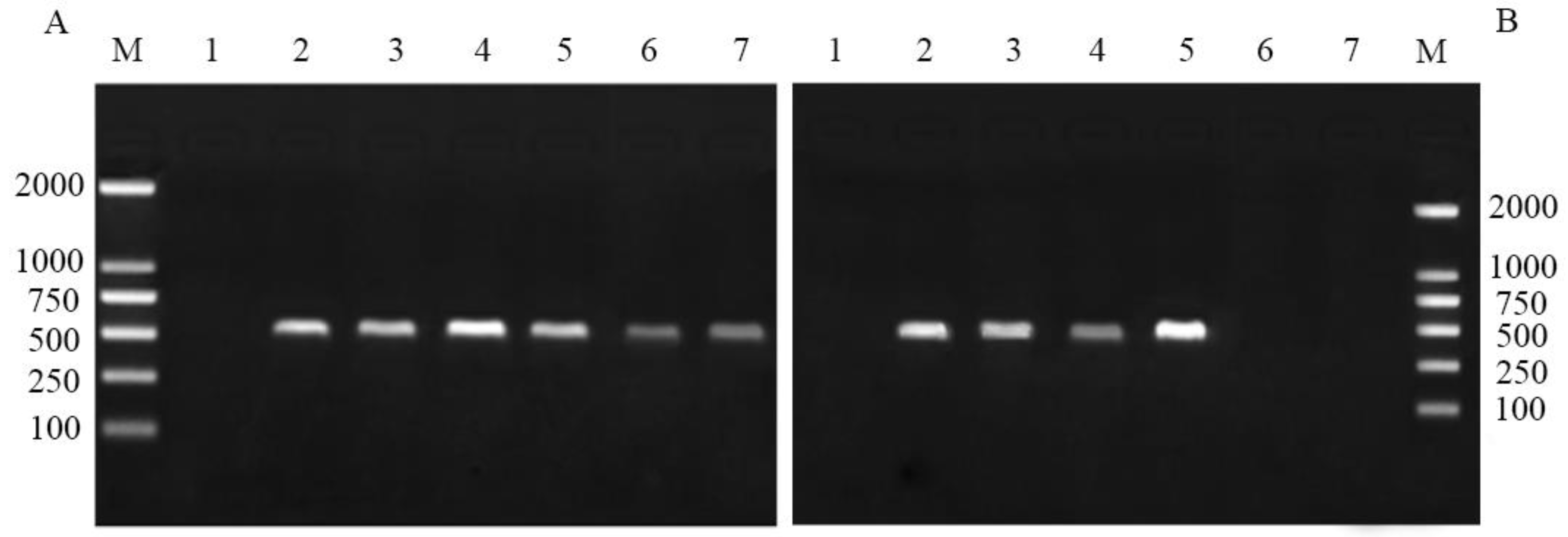
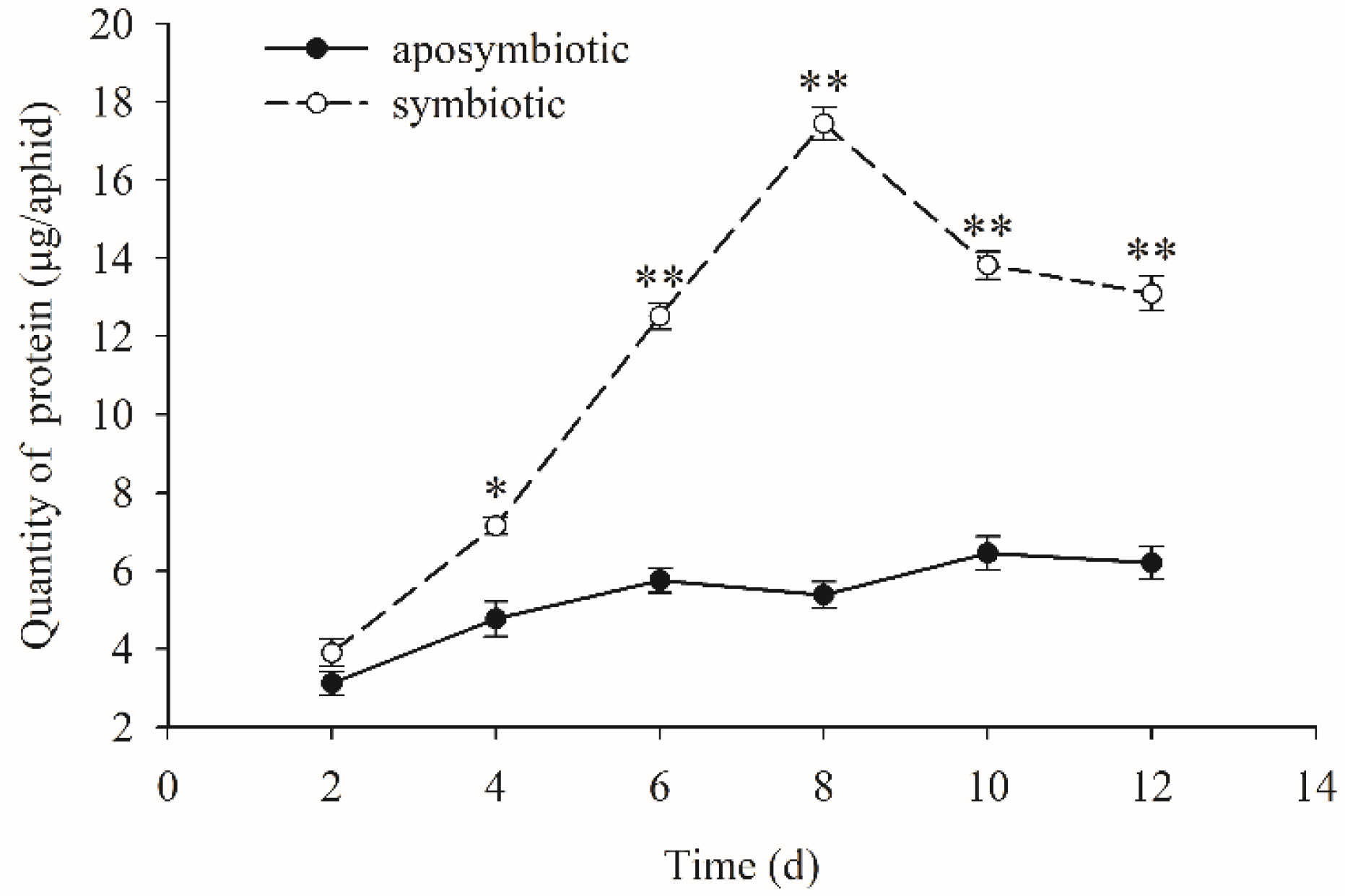
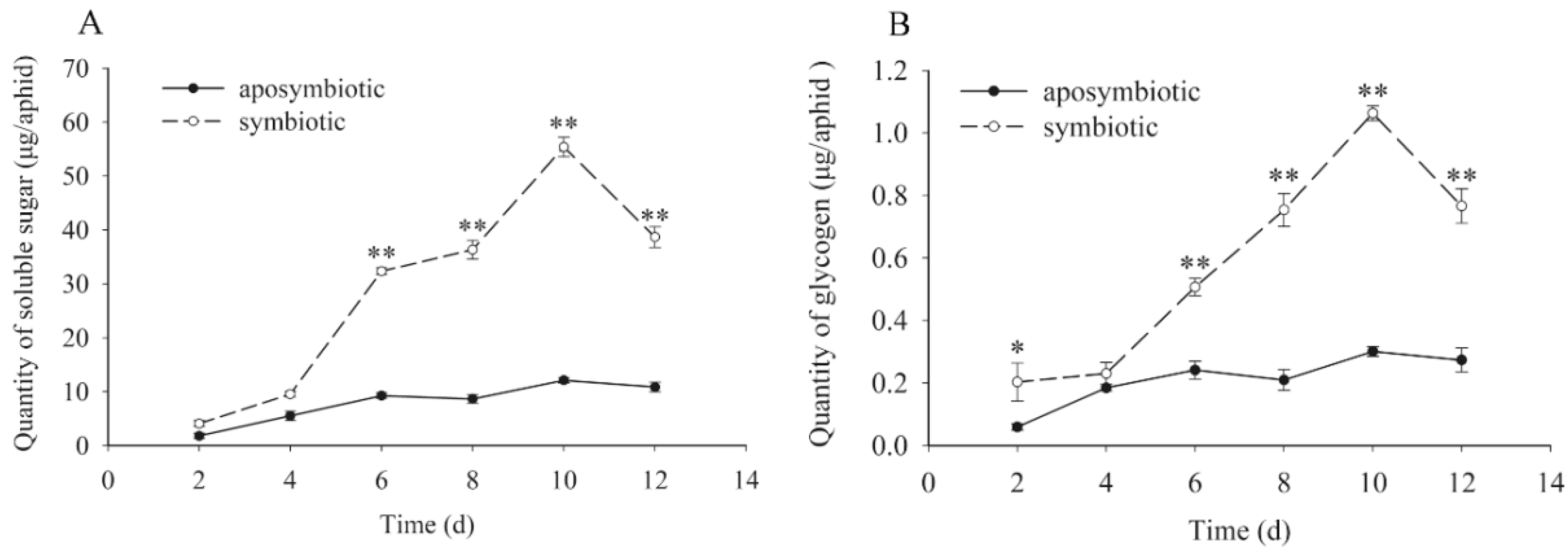
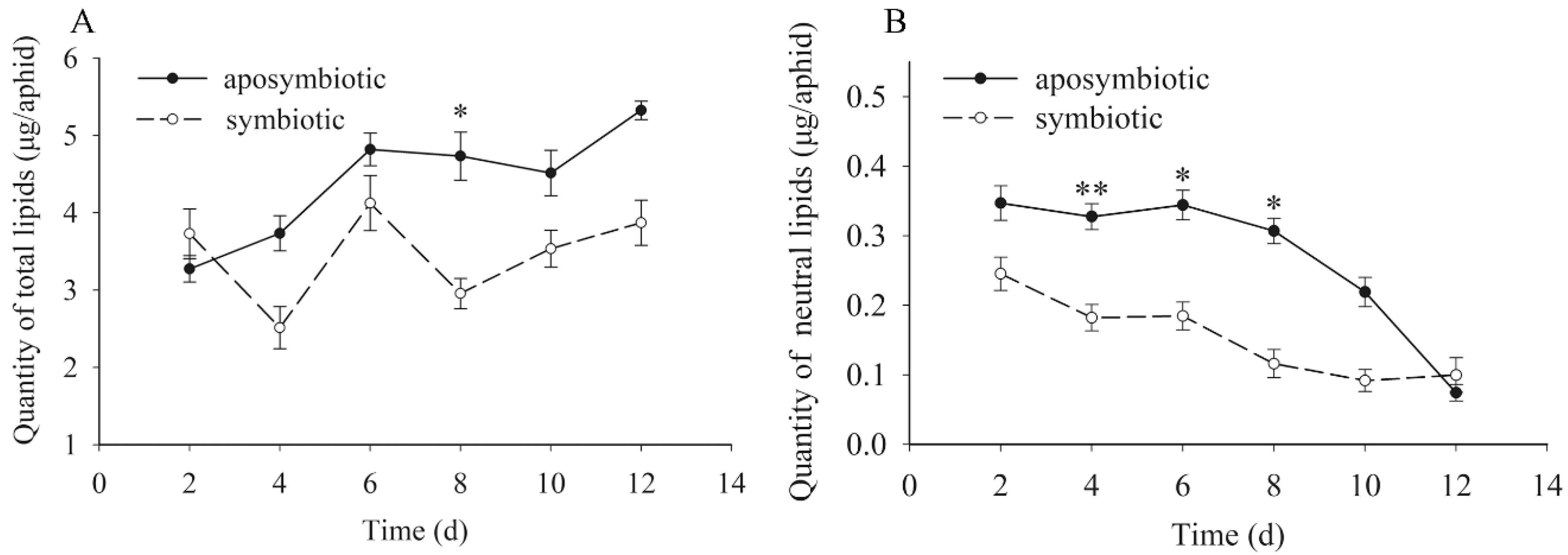
| Symbiotic Pea Aphids | Aposymbiotic Pea Aphids | ||
|---|---|---|---|
| Length (μm) | 3333.26 ± 70.19 | 1788.64 ± 44.07 * | t = 28.314, df = 58, p < 0.05 |
| Width (μm) | 1411.80 ± 37.30 | 705.23 ± 32.59 * | t = 16.447, df = 58, p < 0.05 |
| Weight (mg) | 3.525 ± 0.125 | 1.238 ± 0.132 * | t = 27.691, df = 58, p < 0.05 |
| Number of offspring | 60.57 ± 1.98 | 0 * | t = 121.911, df = 58, p < 0.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, N.; Wang, L.; Sang, W.; Liu, C.-Z.; Qiu, B.-L. Effects of Endosymbiont Disruption on the Nutritional Dynamics of the Pea Aphid Acyrthosiphon pisum. Insects 2018, 9, 161. https://doi.org/10.3390/insects9040161
Lv N, Wang L, Sang W, Liu C-Z, Qiu B-L. Effects of Endosymbiont Disruption on the Nutritional Dynamics of the Pea Aphid Acyrthosiphon pisum. Insects. 2018; 9(4):161. https://doi.org/10.3390/insects9040161
Chicago/Turabian StyleLv, Ning, Lei Wang, Wen Sang, Chang-Zhong Liu, and Bao-Li Qiu. 2018. "Effects of Endosymbiont Disruption on the Nutritional Dynamics of the Pea Aphid Acyrthosiphon pisum" Insects 9, no. 4: 161. https://doi.org/10.3390/insects9040161
APA StyleLv, N., Wang, L., Sang, W., Liu, C.-Z., & Qiu, B.-L. (2018). Effects of Endosymbiont Disruption on the Nutritional Dynamics of the Pea Aphid Acyrthosiphon pisum. Insects, 9(4), 161. https://doi.org/10.3390/insects9040161





