First Case of Dual Size Asymmetry in an Identical Arthropod Organ: Different Asymmetries of the Combative (Sexual) and Cutting (Non-Sexual) Parts of Mandibles in the Horned Stored-Product Beetle Gnatocerus cornutus (Fabricius, 1798)
Abstract
1. Introduction
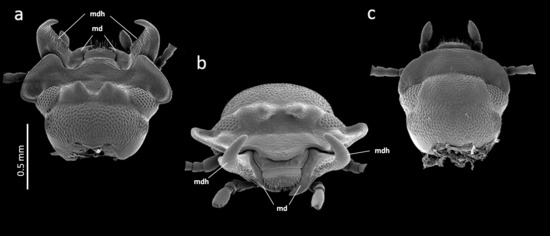
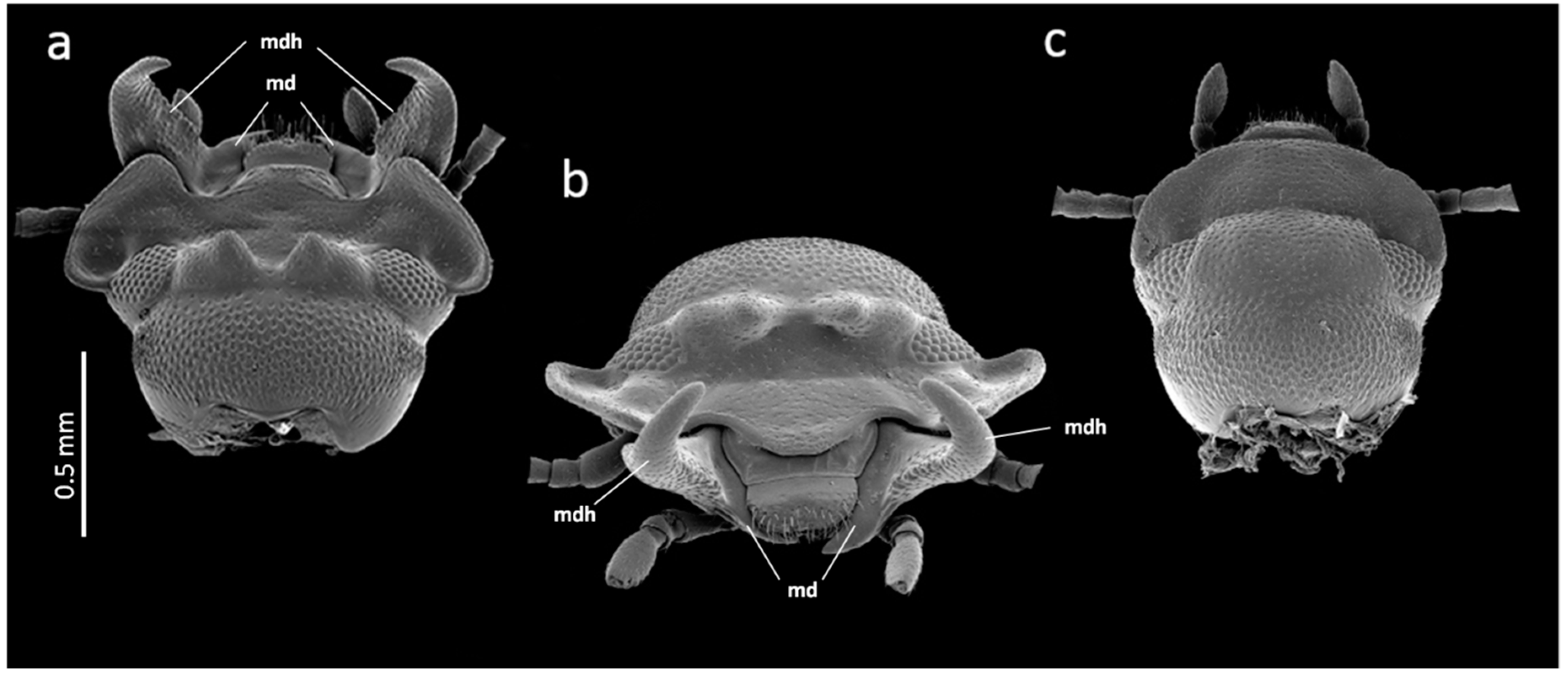
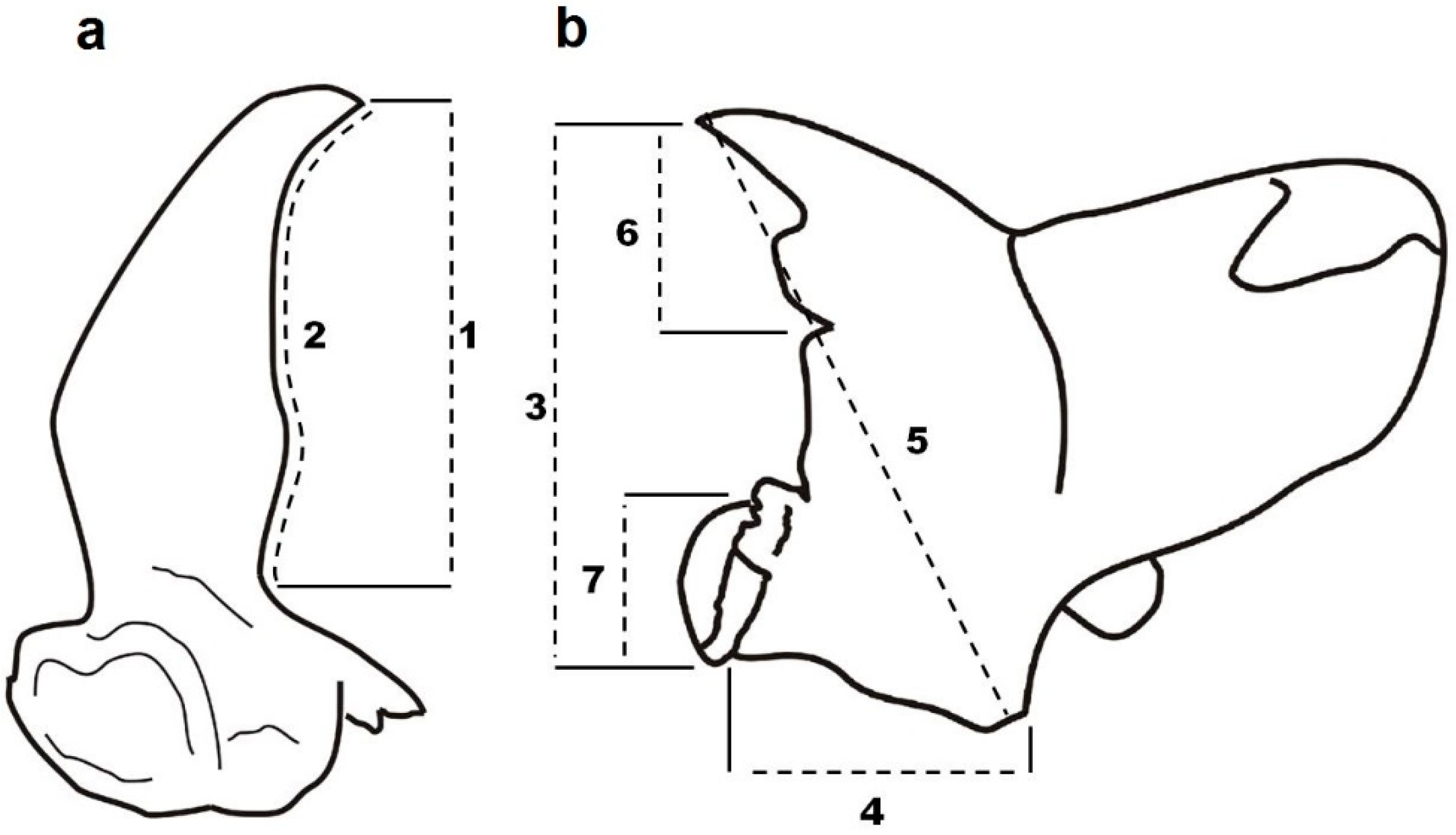
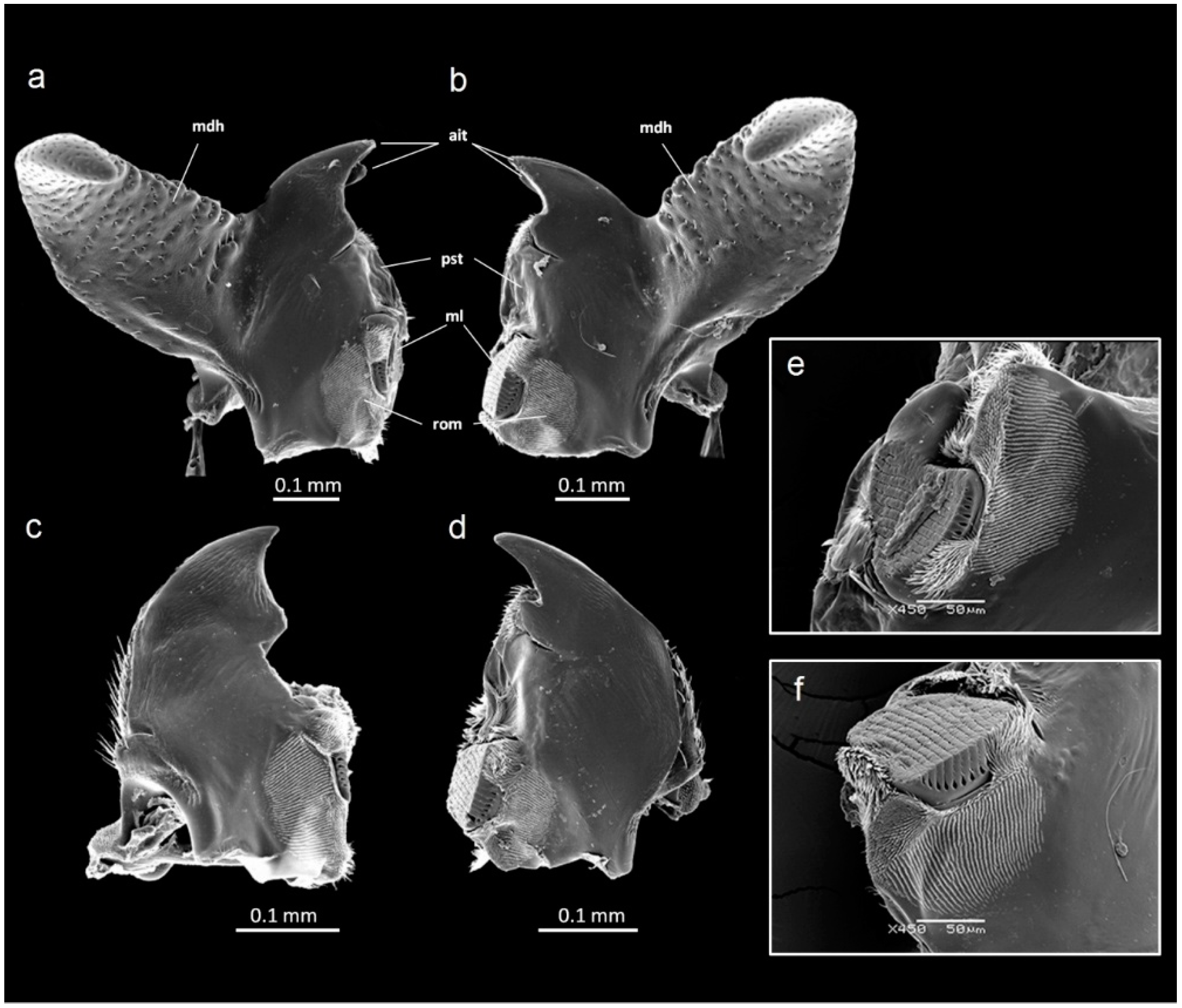
2. Materials and Methods
3. Results
3.1. Mandible Asymmetry
3.2. Horn Asymmetry
3.3. Sexual Differences
3.4. Relationship of Mandible and Horn Asymmetry
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kimura, G.; Takei, S.; Miyanoshita, A.; Tanikawa, T. Rediscovery of broad-horned flour beetle Gnatocerus (Gnatocerus) cornutus (Coleoptera: Tenebrionidae) from Fukuoka Prefecture, Japan. Med. Entomol. Zool. 2016, 67, 97–99. [Google Scholar] [CrossRef]
- Stejskal, V.; Hubert, J.; Aulicky, R.; Kucerova, Z. Overview of present and past and pest-associated risks in stored food and feed products: European perspective. J. Stored Prod. Res. 2015, 64, 122–132. [Google Scholar] [CrossRef]
- Hagstrum, D.; Subramanyam, B. Stored-Product Insect Resource; AACC Press: St. Paul, MN, USA, 2009; 509p. [Google Scholar]
- Okada, K.; Miyanoshita, A.; Miyatake, T. Intra-sexual dimorphism in male mandibles and male aggressive behavior in the broad-horned flour beetle Gnatocerus cornutus (Coleoptera: Tenebrionidae). J. Insect Behav. 2006, 19, 457–467. [Google Scholar] [CrossRef]
- Okada, K.; Miyatake, T. Effect of losing on male fights of broad-horned flour beetle, Gnatocerus cornutus. Behav. Ecol. Sociobiol. 2010, 64, 361–369. [Google Scholar] [CrossRef]
- Demuth, J.P.; Naidu, A.; Mydlarz, L.D. Sex, war and disease: The role of parasite infection on weapon development and mating success in a horned beetle (Gnathocerus cornutus). PLoS ONE 2012, 7, e28690. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.P. Analyzing fluctuating asymmetry with geometric morphometrics: Concepts, methods, and applications. Symmetry 2015, 7, 843–934. [Google Scholar] [CrossRef]
- Van Valen, L. A study of fluctuating asymmetry. Evolution 1962, 16, 125–142. [Google Scholar] [CrossRef]
- Palmer, A.R.; Strobeck, C. Fluctuating asymmetry, measurement, analysis, patterns. Annu. Rev. Ecol. Syst. 1986, 7, 391–421. [Google Scholar] [CrossRef]
- Watson, P.J.; Thornhill, R. Fluctuating asymmetry and sexual selection. Trends Ecol. Evol. 1994, 9, 21–25. [Google Scholar] [CrossRef]
- Inoda, T.; Hirata, Y.; Kamimura, S. Asymmetric mandibles of water-scavenger larvae improve feeding effectiveness on righthanded snails. Am. Nat. 2003, 162, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Bergamin, S.; Smits, A. How do the molluscivorous beetles Carabus granulatus and Phosphuga atrata (Insecta, Coleoptera) deal with sinistral and dextral prey? Vita Malacol. 2015, 13, 49–51. [Google Scholar]
- Kurtz, O.L.; Harris, K.L. Micro-Analytical Entomology for Food Sanitation Control; Association of Official Agricultural Chemists: Washington, DC, USA, 1962; 576p. [Google Scholar]
- Kvenberg, J.E. Scanning Electron Microscopic Study of Adult Stored Product Beetle Mandibles. J. Assoc. Off. Anal. Chem. 1977, 60, 1185–1209. [Google Scholar] [PubMed]
- Clissold, F.J. The biomechanics of chewing and plant fracture: Mechanisms and implications. Adv. Insect Phys. 2008, 34, 317–372. [Google Scholar] [CrossRef]
- Møller, A.P.; Pomiankowski, A. Fluctuating asymmetry and sexual selection. Genetica 1993, 89, 267–279. [Google Scholar] [CrossRef]
- Møller, A.P.; Thornhill, R. Bilateral symmetry and sexual selection: A meta-analysis. Am. Nat. 1998, 151, 174–192. [Google Scholar] [CrossRef] [PubMed]
- Mariappan, P.; Balasundaram, C.; Schmitz, B. Decapod crustacean chelipeds: An overview. J. Biosci. 2000, 25, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Rico-Guevara, A.; Hurme, K.J. Intrasexually selected weapons. Biol. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.P. Developmental stability, sexual selection and speciation. J. Evol. Biol. 1993, 6, 493–509. [Google Scholar] [CrossRef]
- Tomkins, J.L.; Simmons, L.W. Patterns of fluctuating asymmetry in earwig forceps: No evidence for honest signalling. Proc. R. Soc. Lond. B 1995, 259, 89–96. [Google Scholar] [CrossRef]
- Hunt, J.; Simmons, L.W. Patterns of fluctuating asymmetry in beetle horns: An experimental examination of the honest signalling hypothesis. Behav. Ecol. Sociobiol. 1997, 41, 109–114. [Google Scholar] [CrossRef]
- Hunt, J.; Simmons, L.W. Patterns of fluctuating asymmetry in beetle horns: No evidence for reliable signaling. Behav. Ecol. 1998, 9, 465–470. [Google Scholar] [CrossRef]
- Arrow, G.J. Horned Beetles; Dr. W. Junk: The Hague, The Netherlands, 1951; 181p. [Google Scholar]
- Emlen, D.J.; Marangelo, J.; Ball, B.; Cunningham, C.W. Diversity in the weapons of sexual selection: Horn evolution in the beetle genus Onthophagus (Coleoptera: Scarabaeidae). Evolution 2005, 59, 1060–1084. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, W.G. Beetle horn dimorphism: Making the best of a bad lot. Am. Nat. 1982, 119, 420–426. [Google Scholar] [CrossRef]
- Vendl, T.; Šípek, P.; Kouklík, O.; Kratochvíl, L. Hidden complexity in the ontogeny of sexual size dimorphism in male-larger beetles. Sci. Rep. 2018, 8, 5871. [Google Scholar] [CrossRef] [PubMed]
- Emlen, D.J.; Nijhout, H.F. The development and evolution of exaggerated morphologies in insects. Annu. Rev. Entomol. 2000, 45, 661–708. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Katsuki, M.; Sharma, M.D.; House, C.M.; Hosken, D.J. Sexual conflict over mating in Gnatocerus cornutus? Females prefer lovers not fighters. Proc. R. Soc. B 2014, 281, 20140281. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Miyatake, T. Genetic correlations between weapons, body shape and fighting behaviour in the horned beetle Gnatocerus cornutus. Anim. Behav. 2009, 77, 1057–1065. [Google Scholar] [CrossRef]
- Acorn, J.H.; Ball, G.E. The mandibles of some adult ground beetles: Structure, function, and the evolution of herbivory (Coleoptera: Carabidae). Can. J. Zool. 1991, 69, 638–650. [Google Scholar] [CrossRef]
- Samways, M.J.; Osborn, R.; Saunders, T.L. Mandible form relative to the main food type in ladybirds (Coleoptera: Coccinellidae). Biocontrol Sci. Technol. 1997, 7, 275–286. [Google Scholar] [CrossRef]
- Holter, P.; Scholtz, C.H. Re-establishment of biting mouthparts in desert-living dung beetles (Scarabaeidae: Scarabaeinae) feeding on plant litter—Old structures reacquired or new ones evolved? J. Morphol. 2011, 272, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Li, S.; Lu, Y.; Yang, H.; Tong, Y.; Yang, X. Mandible evolution in the Scarabaeinae (Coleoptera: Scarabaeidae) and adaptations to coprophagous habits. Front. Zool. 2015, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Doyen, J.T.; Tschinkel, W.R. Phenetic and cladistic relationships among tenebrionid beetles (Coleoptera). Syst. Entomol. 1982, 7, 127–183. [Google Scholar] [CrossRef]
- Leschen, R.A.B.; Steelman, C.D. Alphitobius diaperinus (Coleoptera: Tenebrionidae) larva and adult mouthparts. Entomol. News 1988, 99, 221–224. [Google Scholar]
- Král, D.; Hillert, O.; Drožová, D.; Šípek, P. Lethrus (Lethrus) schneideri sp. n. (Coleoptera: Geotrupidae) from Greece. ZooKeys 2013, 339, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.B.; Wheeler, Q.D. Asymmetrical male mandibular horns and mating behavior in Agathidium Panzer (Coleoptera: Leiodidae). J. Nat. Hist. 2005, 39, 779–792. [Google Scholar] [CrossRef]
- Eberhard, W.G. The function of horns in Podischnus agenor (Dynastinae) and other beetles. In Sexual Selection and Reproductive Competition in Insects; Blum, M.S., Blum, N.A., Eds.; Academic Press: New York, NY, USA, 1979; pp. 231–258. [Google Scholar]
- Okada, Y.; Fujisawa, H.; Kimura, Y.; Hasegawa, E. Morph-dependent form of asymmetry in mandibles of stag beetle Prosopocoilus inclinatus (Coleoptera: Lucanidae). Ecol. Entomol. 2008, 33, 684–689. [Google Scholar] [CrossRef]
- Okada, K.; Katsuki, M.; Okada, Y.; Miyatake, T. Immature performance linked with exaggeration of a sexually selected trait in an armed beetle. J. Evol. Biol. 2011, 24, 1737–1743. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Gotoh, H.; Miura, T.; Miyatake, T.; Okada, K. Juvenile hormone mediates developmental integration between exaggerated traits and supportive traits in the horned flour beetle Gnatocerus cornutus. Evol. Dev. 2012, 14, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Kiyose, K.; Katsuki, M.; Suzaki, Y.; Okada, K. Competitive males but not attractive males reduce female fitness in Gnatocerus cornutus. Anim. Behav. 2015, 109, 265–272. [Google Scholar] [CrossRef]
| Trait | Mean Asymmetry ± SE | t (df) | K-S Test | Larger Side | Asymmetry Pattern |
|---|---|---|---|---|---|
| Males | |||||
| Mandible length | ‒23.97 ± 1.70 | ‒14.12 (37) *** | 0.11 (n.s.) | L | DA |
| Mandible width | 22.05 ± 1.53 | 14.42 (37) *** | 0.13 (n.s.) | R | DA |
| Diagonal | ‒11.55 ± 1.57 | ‒7.37 (37) *** | 0.15 (n.s.) | L | DA |
| Incisor length | ‒18.13 ± 1.22 | ‒19.92 (37) *** | 0.11 (n.s.) | L | DA |
| Molar length | 4.18 ± 1.27 | 3.29 (37) ** | 0.11 (n.s.) | R | DA |
| Horn length 1 | 1.17 ± 2.09 | 0.56 (34) | 0.07 (n.s.) | - | FA |
| Horn length 2 | 4.46 ± 2.47 | 1.81 (34) | 0.11 (n.s.) | - | FA |
| Females | |||||
| Mandible length | ‒18.69 ± 2.03 | ‒9.19 (25) *** | 0.15 (n.s.) | L | DA |
| Mandible width | 18.08 ± 1.35 | 13.39 (25) *** | 0.15 (n.s.) | R | DA |
| Diagonal | ‒13.58 ± 2.29 | ‒11.41 (25) *** | 0.15 (n.s.) | L | DA |
| Incisor length | ‒19.35 ± 1.70 | ‒5.94 (25) *** | 0.14 (n.s.) | L | DA |
| Molar length | 4.58 ± 1.68 | 2.73 (25) * | 0.18 (n.s.) | R | DA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vendl, T.; Stejskal, V.; Aulicky, R. First Case of Dual Size Asymmetry in an Identical Arthropod Organ: Different Asymmetries of the Combative (Sexual) and Cutting (Non-Sexual) Parts of Mandibles in the Horned Stored-Product Beetle Gnatocerus cornutus (Fabricius, 1798). Insects 2018, 9, 151. https://doi.org/10.3390/insects9040151
Vendl T, Stejskal V, Aulicky R. First Case of Dual Size Asymmetry in an Identical Arthropod Organ: Different Asymmetries of the Combative (Sexual) and Cutting (Non-Sexual) Parts of Mandibles in the Horned Stored-Product Beetle Gnatocerus cornutus (Fabricius, 1798). Insects. 2018; 9(4):151. https://doi.org/10.3390/insects9040151
Chicago/Turabian StyleVendl, Tomas, Vaclav Stejskal, and Radek Aulicky. 2018. "First Case of Dual Size Asymmetry in an Identical Arthropod Organ: Different Asymmetries of the Combative (Sexual) and Cutting (Non-Sexual) Parts of Mandibles in the Horned Stored-Product Beetle Gnatocerus cornutus (Fabricius, 1798)" Insects 9, no. 4: 151. https://doi.org/10.3390/insects9040151
APA StyleVendl, T., Stejskal, V., & Aulicky, R. (2018). First Case of Dual Size Asymmetry in an Identical Arthropod Organ: Different Asymmetries of the Combative (Sexual) and Cutting (Non-Sexual) Parts of Mandibles in the Horned Stored-Product Beetle Gnatocerus cornutus (Fabricius, 1798). Insects, 9(4), 151. https://doi.org/10.3390/insects9040151