Evaluating an Alleged Mimic of the Monarch Butterfly: Neophasia (Lepidoptera: Pieridae) Butterflies are Palatable to Avian Predators
Abstract
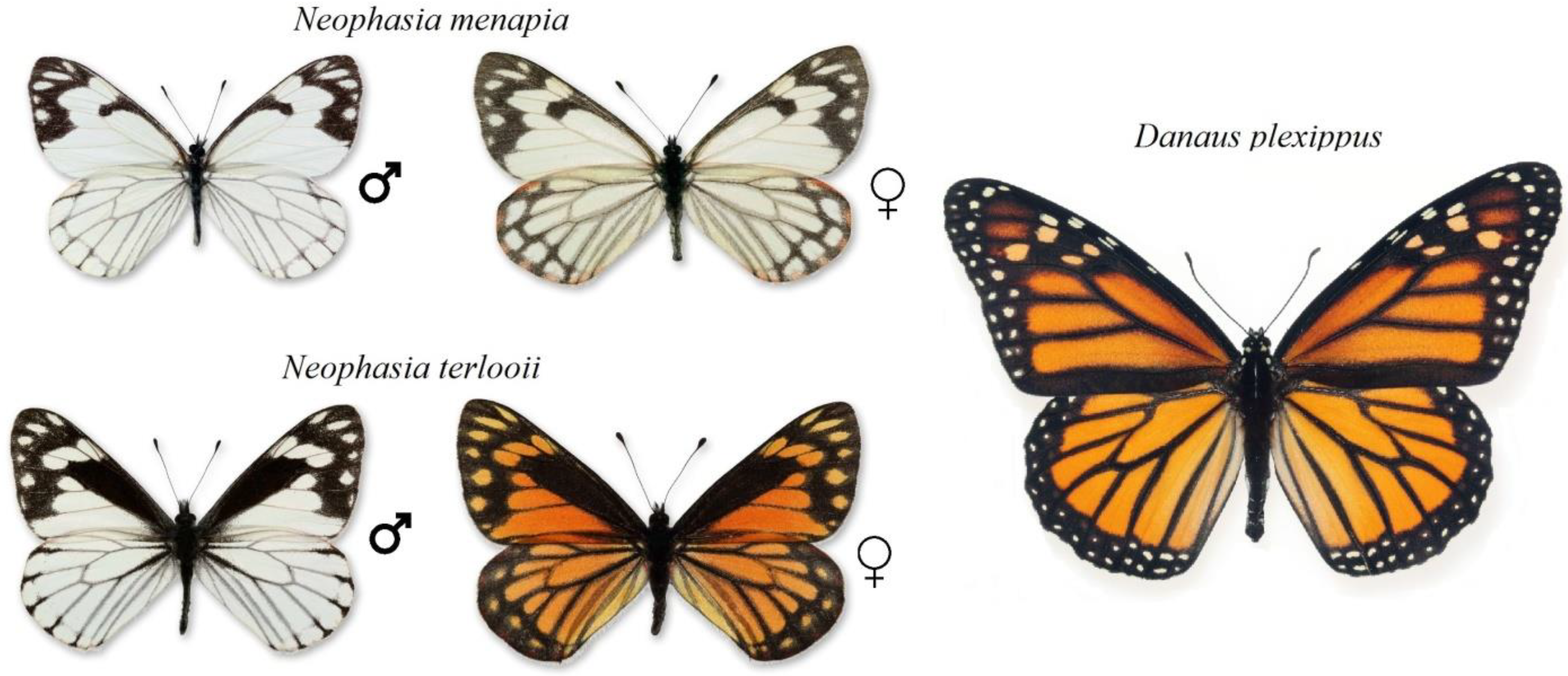
1. Introduction
2. Materials and Methods
2.1. Butterfly Collection
2.2. Aviary Setup
2.3. Presentation of Experimental Insects
2.4. Behavioral Observations
2.5. Statistical Analyses
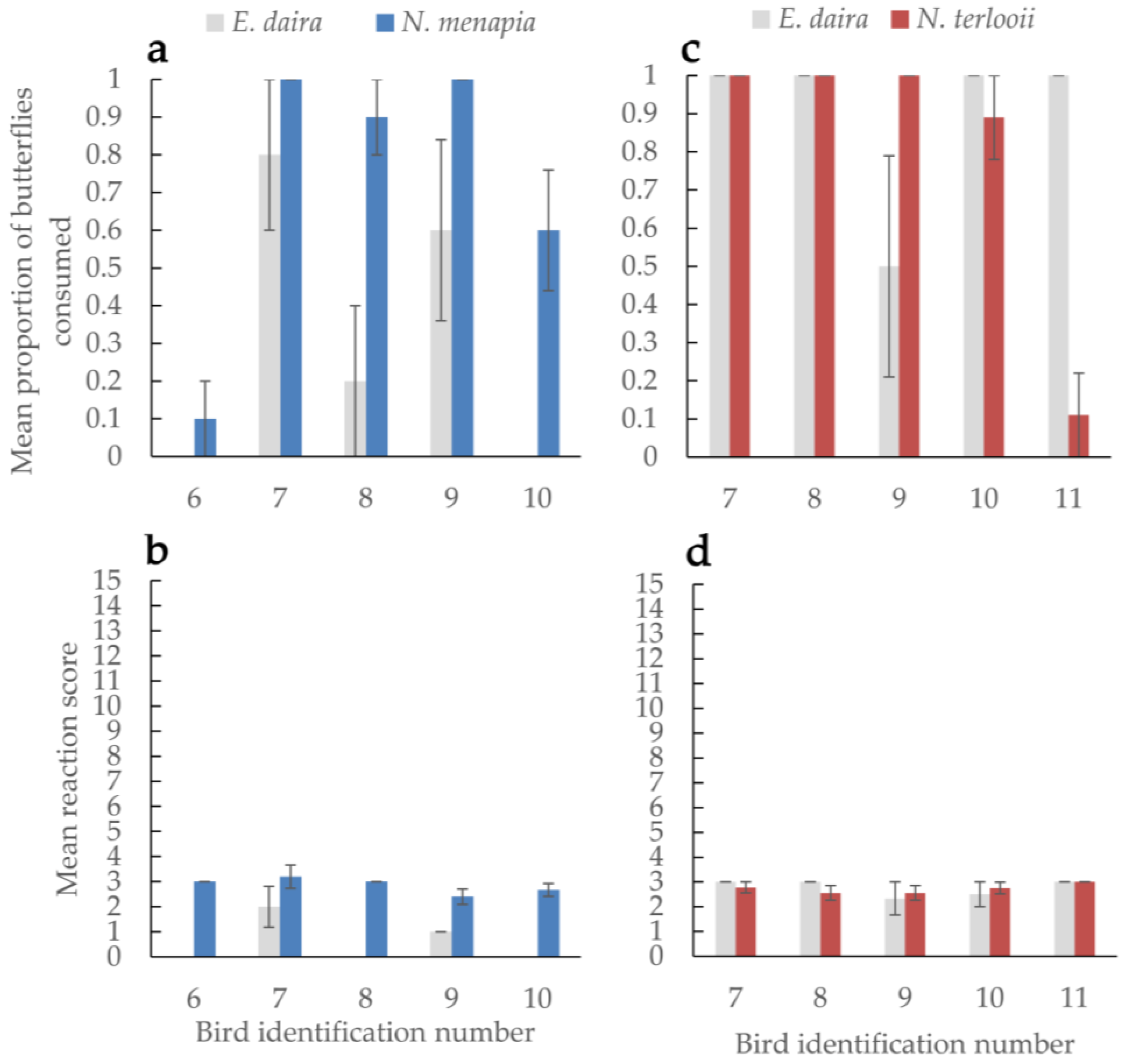
3. Results
4. Discussion
4.1. Batesian vs. Müllerian Mimicry
4.2. Predator‒Prey Dynamics
4.3. Sex-Limited Mimicry
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benson, W.W. Natural selection for Müllerian mimicry in Heliconius erato in Costa Rica. Science 1972, 176, 936–939. [Google Scholar] [CrossRef] [PubMed]
- Brower, A.V.Z. Rapid morphological radiation and convergence among races of the butterfly Heliconius erato inferred from patterns of mitochondrial DNA evolution. Proc. Natl. Acad. Sci. USA 1994, 91, 6491–6495. [Google Scholar] [CrossRef] [PubMed]
- Brower, A.V.Z. Parallel race formation and the evolution of mimicry in Heliconius butterflies: A phylogenetic hypothesis from mitochondrial DNA sequences. Evolution 1996, 50, 195–221. [Google Scholar] [CrossRef] [PubMed]
- Langham, G.M. Specialized avian predators repeatedly attack novel color morphs of Heliconius butterflies. Evolution 2004, 58, 2783–2787. [Google Scholar] [CrossRef] [PubMed]
- Müller, F. Ituna and Thyridia; a remarkable case of mimicry in butterflies. (R. Meldola translation). Proclam. Entomol. Soc. Lond. 1879, 1879, 20–29. [Google Scholar]
- Naisbit, R.E.; Jiggins, C.D.; Mallet, J. Disruptive sexual selection against hybrids contributes to speciation between Heliconius cydno and Heliconius melpomene. Proc. R. Soc. B-Biol. Sci. 2001, 268, 1849–1854. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, P.M.; Turner, J.R.G.; Brown, K.S.; Benson, W.W.; Singer, M.C. Genetics and the evolution of Müllerian mimicry in Heliconius butterflies. Philos. Trans. R. Soc. B-Biol. Sci. 1985, 308, 433–613. [Google Scholar] [CrossRef]
- Brower, J.V. Experimental studies of mimicry in some North American butterflies. 2. Battus philenor and Papilio troilus, P. polyxenes and P. glaucus. Evolution 1958, 12, 123–136. [Google Scholar] [CrossRef]
- Hazel, W.N. Sex-limited variability and mimicry in the swallowtail butterfly Papilio polyxenes Fabr. Heredity 1990, 65, 109–114. [Google Scholar] [CrossRef]
- Herrel, J.; Hazel, W. Female-limited variability in mimicry in the swallowtail butterfly Papilio polyxenes Fabr. Heredity 1995, 75, 106–110. [Google Scholar] [CrossRef]
- Krebs, R.A.; West, D.A. Female mate preference and the evolution of female-limited Batesian mimicry. Evolution 1988, 42, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Brower, J.V. Experimental studies of mimicry in some North American butterflies. 1. The monarch, Danaus plexippus, and viceroy, Limenitis archippus archippus. Evolution 1958, 12, 32–47. [Google Scholar] [CrossRef]
- Brower, J.V. Experimental studies of mimicry in some North American butterflies. 3. Danaus gilippus berenice and Limenitis archippus floridensis. Evolution 1958, 12, 273–285. [Google Scholar] [CrossRef]
- Ritland, D.B. Revising a classic butterfly mimicry scenario: Demonstration of Müllerian mimicry between Florida viceroys (Limenitis archippus floridensis) and queens (Danaus gilippus berenice). Evolution 1991, 45, 918–934. [Google Scholar] [CrossRef] [PubMed]
- Ritland, D.B.; Brower, L.P. The viceroy butterfly is not a Batesian mimic. Nature 1991, 350, 497–498. [Google Scholar] [CrossRef]
- Huheey, J.E. Mathematical models of mimicry. Am. Nat. 1988, 131 (Suppl. 1), S22–S41. [Google Scholar] [CrossRef]
- Malcolm, S.B. Mimicry: Status of a classical evolutionary paradigm. Trends Ecol. Evol. 1990, 5, 57–62. [Google Scholar] [CrossRef]
- Speed, M.P. Müllerian mimicry and the psychology of predation. Anim. Behav. 1993, 45, 571–580. [Google Scholar] [CrossRef]
- Turner, J.R.G. Mimicry: The palatability spectrum and its consequences. In The Biology of Butterflies; Vane-Wright, R.I., Ackery, P.R., Eds.; Academic Press: Cambridge, MA, USA, 1984; pp. 141–161. ISBN 9780691084992. [Google Scholar]
- Joron, M.; Mallet, J. Diversity in mimicry: Paradox or paradigm? Trends Ecol. Evol. 1998, 13, 461–466. [Google Scholar] [CrossRef]
- Poulton, E.B. Mimicry in North American butterflies: A reply. Proc. Acad. Natl. Sci. Phila. 1914, 66, 161–195. [Google Scholar]
- Butterflies and Moths of North America. Available online: http://www.butterfliesandmoths.org/ (accessed on 9 October 2016).
- Scott, J.A. The Butterflies of North America: A Natural History and Field Guide; Stanford University Press: Stanford, CA, USA, 1986; p. 583. ISBN 9780804720137. [Google Scholar]
- Bailowitz, R.A.; Brock, J.P. Butterflies of Southeastern Arizona; Sonoran Arthropod Studies, Inc.: Tucson, AZ, USA, 1991; p. 342. ISBN 9780962662904. [Google Scholar]
- Long, E.C.; Hahn, T.P.; Shapiro, A.M. Variation in wing pattern and palatability in a female-limited polymorphic mimicry system. Ecol. Evol. 2014, 4, 4543–4552. [Google Scholar] [CrossRef] [PubMed]
- Jones, F.M. Insect coloration and the relative acceptability of insects to birds. Trans. R. Entomol. Soc. Lond. 1932, 80, 345–371. [Google Scholar] [CrossRef]
- Jones, F.M. Further experiments on coloration and relative acceptability of insects to birds. Trans. R. Entomol. Soc. Lond. 1934, 82, 443–453. [Google Scholar] [CrossRef]
- Prudic, K.L.; Shapiro, A.M.; Clayton, N.S. Evaluating a putative mimetic relationship between two butterflies, Adelpha bredowii and Limenitis lorquini. Ecol. Entomol. 2002, 27, 68–75. [Google Scholar] [CrossRef]
- Brower, J.V.; Corvino, J.M. Plant poisons in a terrestrial food chain. Proc. Natl. Acad. Sci. USA 1967, 57, 893–898. [Google Scholar] [CrossRef] [PubMed]
- R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 10 August 2018).
- xlsx: Read, Write, Format Excel 2007 and Excel 97/2000/XP/2003 Files. R Package Version 0.5.7. Available online: http://CRAN.R-project.org/package=xlsx (accessed on 10 September 2016).
- aod: Analysis of Overdispersed Data. R Package Version 1.3. Available online: http://cran.r-project.org/package=aod (accessed on 10 September 2018).
- Skinner, H. Antigeny in Nearctic butterflies. Entomol. News 1913, 24, 23–27. [Google Scholar]
- Ödeen, A.; Hastad, O. The phylogenetic distribution of ultraviolet sensitivity in birds. BMC Evol. Biol. 2013, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Ritland, D.B. Variation in palatability of Queen butterflies (Danaus gilippus) and implications regarding mimicry. Ecology 1994, 75, 732–746. [Google Scholar] [CrossRef]
- Bent, A.C. Life histories of North American jays, crows, and titmice. US Natl. Mus. Bull. 1946, 191, 77–88. [Google Scholar]
- Wang, K.; Zhao, H.B. Birds generally carry a small repertoire of bitter taste receptor genes. Genome Biol. Evol. 2015, 7, 2705–2715. [Google Scholar] [CrossRef] [PubMed]
- Case, T.J.; Holt, R.D.; McPeek, M.A.; Keitt, T.H. The community context of species’ borders: Ecological and evolutionary perspectives. Oikos 2005, 108, 28–46. [Google Scholar] [CrossRef]
- Braby, M.F.; Pierce, N.E.; Vila, R. Phylogeny and historical biogeography of the subtribe Aporiina (Lepidoptera: Pieridae): Implications for the origin of Australian butterflies. Biol. J. Linn. Soc. 2007, 90, 413–440. [Google Scholar] [CrossRef]
- Ciesla, W. Forest insect damage from high-altitude color-IR photos. Am. Soc. Photogramm. 1974, 40, 683–689. [Google Scholar]
- Dewey, J.E.; Ciesla, W.M.; Meyer, H.E. Insect defoliation as a predisposing agent to a bark beetle outbreak in eastern Montana. Environ. Entomol. 1974, 3, 722. [Google Scholar] [CrossRef]
- Smith, D.A.S.; Owen, D.F.; Gordon, I.J.; Owiny, A.M. Polymorphism and evolution in the butterfly Danaus chrysippus L. (Lepidoptera: Danainae. Heredity 1993, 71, 242–251. [Google Scholar] [CrossRef]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.; et al. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Chang. Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Ohsaki, N. Preferential predation of female butterflies and the evolution of Batesian mimicry. Nature 1995, 378, 173–175. [Google Scholar] [CrossRef]
- Wickler, W. Mimicry in Plants and Animals; McGraw Hill: New York, NY, USA; World University Library: New York, NY, USA, 1968. [Google Scholar]
- Long, E.C.; Edwards, K.F.; Shapiro, A.M. A test of fundamental questions in mimicry theory using long-term datasets. Biol. J. Linn. Soc. 2015, 116, 487–494. [Google Scholar] [CrossRef]
- Bobisud, L.E. Optimal time of appearance of mimics. Am. Nat. 1978, 112, 962–965. [Google Scholar] [CrossRef]
- Huheey, J.E. The question of synchrony or ‘temporal sympatry’ in mimicry. Evolution 1980, 34, 614–616. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Kline, C.; Morris, S.M. Status of Danaus plexippus population in Arizona. J. Lepid. Soc. 2015, 69, 91–107. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halbritter, D.A.; Gordon, J.M.; Keacher, K.L.; Avery, M.L.; Daniels, J.C. Evaluating an Alleged Mimic of the Monarch Butterfly: Neophasia (Lepidoptera: Pieridae) Butterflies are Palatable to Avian Predators. Insects 2018, 9, 150. https://doi.org/10.3390/insects9040150
Halbritter DA, Gordon JM, Keacher KL, Avery ML, Daniels JC. Evaluating an Alleged Mimic of the Monarch Butterfly: Neophasia (Lepidoptera: Pieridae) Butterflies are Palatable to Avian Predators. Insects. 2018; 9(4):150. https://doi.org/10.3390/insects9040150
Chicago/Turabian StyleHalbritter, Dale A., Johnalyn M. Gordon, Kandy L. Keacher, Michael L. Avery, and Jaret C. Daniels. 2018. "Evaluating an Alleged Mimic of the Monarch Butterfly: Neophasia (Lepidoptera: Pieridae) Butterflies are Palatable to Avian Predators" Insects 9, no. 4: 150. https://doi.org/10.3390/insects9040150
APA StyleHalbritter, D. A., Gordon, J. M., Keacher, K. L., Avery, M. L., & Daniels, J. C. (2018). Evaluating an Alleged Mimic of the Monarch Butterfly: Neophasia (Lepidoptera: Pieridae) Butterflies are Palatable to Avian Predators. Insects, 9(4), 150. https://doi.org/10.3390/insects9040150




