Nest Population Structure and Wood Litter Consumption by Microcerotermes indistinctus (Isoptera) in a Seasonally Dry Tropical Forest, Northeastern Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Nest Density
2.3. Colony Size
2.4. Quantification of Wood Consumption in the Laboratory
2.5. Litter Production
3. Results
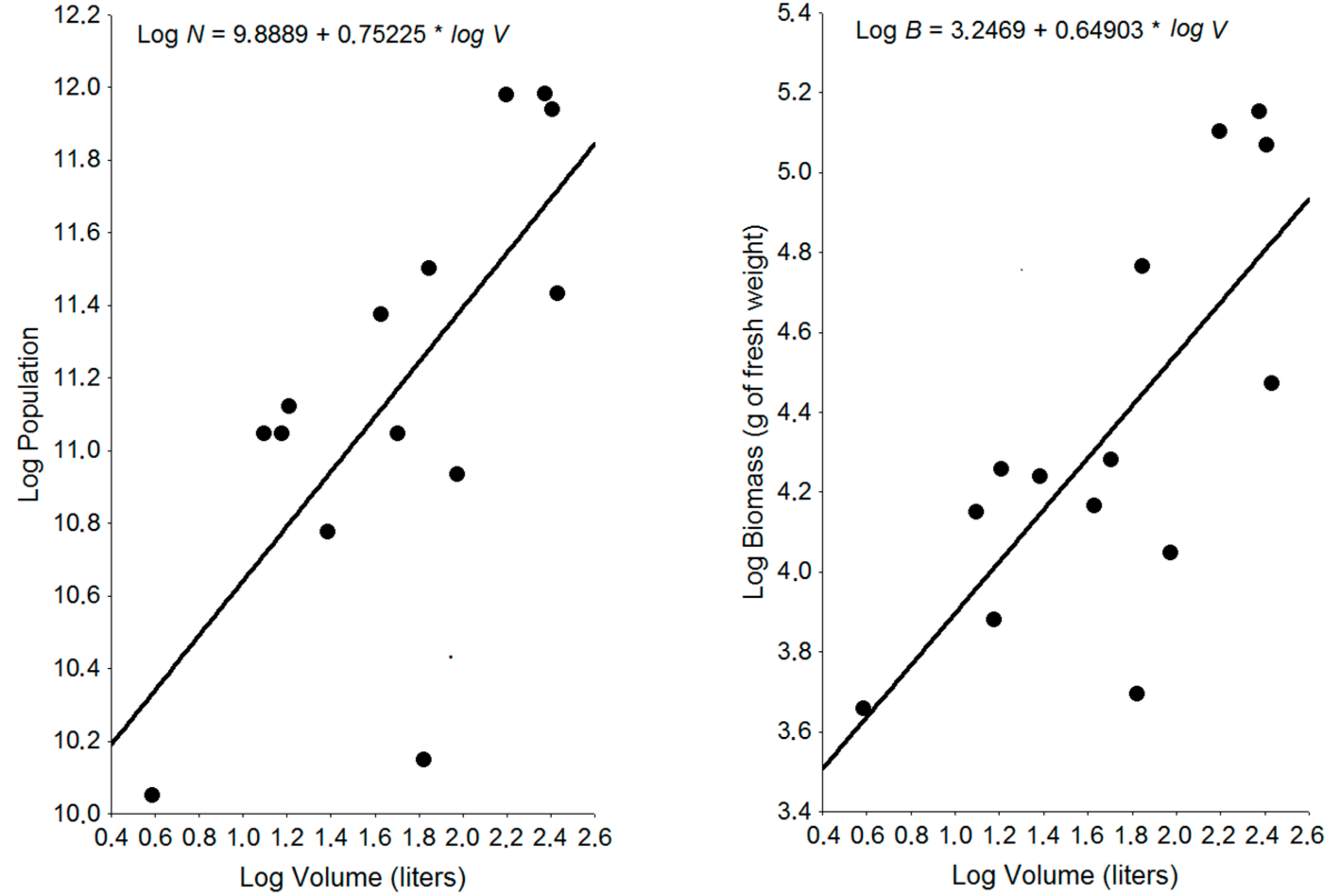
3.1. Nest Population
3.2. Quantification of Wood Consumption in the Laboratory
3.3. Wood Litter Production and the Impact of M. indistinctus
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krishna, K.; Grimaldi, D.A.; Krishna, V.; Engel, M.S. Treatise on the Isoptera of the world. Bull. Am. Mus. Nat. His. 2013, 377, 1–2704. [Google Scholar] [CrossRef]
- Lee, K.E.; Wood, T. Termites and Soils; Academic Press: New York, NY, USA, 1971; pp. 1–251. ISBN 0124408508. [Google Scholar]
- Wood, T.G.; Sands, W.A. The role of termites in ecosystems. In Production Ecology of Ants and Termites; Brian, M.V., Ed.; Cambridge University Press: Cambridge, UK, 1978; pp. 245–292. [Google Scholar]
- Wood, T.G. Food and feeding habits of termites. In Production Ecology of Ants and Termites; Brian, M.V., Ed.; Cambridge University Press: Cambridge, UK, 1978; pp. 55–80. [Google Scholar]
- Vasconcellos, A.; Moura, F.M.S. Wood litter consumption by three species of Nasutitermes termites in an area of the Atlantic Coastal Forest in Northeastern Brazil. J. Ins. Sci. 2010, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vasconcellos, A.; Bandeira, A.G.; Almeida, W.O.; Moura, F.M.S. Térmitas construtores de ninhos conspícuos em duas áreas de Mata Atlântica com diferentes níveis de perturbação antrópica. Neotrop. Entomol. 2008, 37, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Martius, C.; Ribeiro, J. Colony populations and biomass in nests of the Amazonian Forest termite Anoplotermes Banksi Emerson (Isoptera: Termitidae). Stud. Neotrop. Fauna Environ. 1996, 31, 82–86. [Google Scholar] [CrossRef]
- Vasconcellos, A. Biomass and abundance of termites in three remnant areas of Atlantic Forest in northeastern Brazil. Rev. Bras. Entomol. 2010, 54, 455–461. [Google Scholar] [CrossRef][Green Version]
- Nutting, W.L. Composition and size of some termite colonies in Arizona and Mexico. Ann. Entomol. Soc. Am. 1970, 63, 1105–1110. [Google Scholar] [CrossRef]
- Martius, C. Diversity and ecology of termites in Amazoniam forests. Pedobiology 1994, 38, 407–428. [Google Scholar]
- Martius, C. Decomposition of wood. In The Central-Amazonian Floodplain: Ecology of a Pulsing System; Junk, W.J., Ed.; Springer: Berlin, Germany, 1977; pp. 267–276. ISBN 978-3-662-03416-3. [Google Scholar]
- Torales, G.J.; Coronel, J.M. Qualitative and quantitative composition of colonies of Microcerotermes strunckii (Isoptera: Termitidae). Sociobiology 2004, 43, 523–534. [Google Scholar]
- Vasconcellos, A.; Araújo, V.F.P.; Moura, F.M.S.; Bandeira, A.G. Biomass and population structure of Constrictotermes cyphergaster (Silvestri) (Isoptera: Termitidae) in the dry forest of Caatinga, Northeastern Brazil. Neotrop. Entomol. 2007, 36, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Constantino, R. Termite Database. Available online: http://164.41.140.9/catal/ (accessed on 2 July 2018).
- Vasconcellos, A.; Bandeira, A.G.; Moura, F.M.S.; Araújo, V.F.P.; Gusmão, M.A.B.; Constantino, R. Termite assemblages in three habitats under different disturbance regimes in the semi-arid Caatinga of ne Brazil. J. Arid Environ. 2010, 74, 298–302. [Google Scholar] [CrossRef]
- Vasconcellos, A.; Moura, F.M.S. Térmitas de oito ecossistemas inseridos no domínio do semiárido brasileiro. In Artrópodes do Semiárido: Biodiversidade e Conservação, 1st ed.; Bravo, F., Calor, A., Eds.; Printmídia: Feira de Santana, Brazil, 2014; Volume 1, pp. 1–298. ISBN 978-85-62465-16-1. [Google Scholar]
- Lopes, M.C.A.; Araújo, V.F.P.; Vasconcellos, A. The effects of rainfall and vegetation on litterfall production in the semiarid region of northeastern Brazil. Braz. J. Biol. 2015, 75, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Veloso, A.L.; Sampaio, E.V.S.B.; Pareyn, F.G.C. Ecorregiões: Propostas Para o Bioma Caatinga, 1st ed.; Associação Plantas do Nordeste e The Nature Conservancy do Brasil: Recife, Brazil, 2002; pp. 1–76. [Google Scholar]
- Paes, J.B.; Morais, V.M.; Sobrinho, D.W.F.; Bakke, O.A. Resistência natural de nove madeiras do Semiárido Brasileiro a cupins subterrâneos, em ensaio de laboratório. Cerne 2001, 9, 36–47. [Google Scholar]
- Amorim, I.L.; Sampaio, E.V.S.B.; Araújo, E.L. Flora e estrutura da vegetação arbustivo-arbórea de uma área de Caatinga do Seridó, RN, Brasil. Acta Bot. Brasilica 2005, 19, 615–623. [Google Scholar] [CrossRef]
- Andrade, L.A.; Pereira, I.M.; Leite, U.T.; Barbosa, M.R.V. Análise da cobertura de duas fitofisionomias de caatinga, com diferentes históricos de uso, no município de São João do Cariri, Estado Da Paraíba. Cerne 2005, 11, 253–262. [Google Scholar]
- Lepage, M.; Darlington, J.P.E.C. Population dynamics of Termites. In Termites Evolution, Sociality, Simbioses, Ecology; Abe, T., Bignell, D.E., Higgashi, M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 333–361. ISBN 978-94-017-3223-9. [Google Scholar]
- Couto, A.A.V.O.; Arruda, A.R.; Silva, J.S.; Vega, E.S.F.; Moura, C.C.M.; Muniz, S.L.; Albuquerque, A.C. Parameters that influence the establishment and volume of Microcerotermes exiguus and Nasutitermes corniger nests in an Atlantic Forest fragment in Northeastern Brazil (Isoptera: Termitidae). Sociobiology 2012, 59, 903–910. [Google Scholar]
- Martius, C. The termites. In The Central Amazon Floodplain: Ecology of a Pulsing System; Junk, W.J., Ed.; Springer: Berlin, Germany, 1997; Volume 126, pp. 361–371. ISBN 978-3-662-03416-3. [Google Scholar]
- Noirot, C.; Darlington, J.P.E.C. Termite nests: Architecture, regulation and defense. In Termites: Evolution, Sociality, Symbioses, Ecology; Abe, T., Bignell, D.E., Higashi, M., Eds.; Kluwer Academic Press: Dordrecht, The Netherlands, 2000; pp. 121–139. ISBN 978-94-017-3223-9. [Google Scholar]
- Vasconcellos, A.; Bandeira, A.G. Populational and reproductive status of a polycalic colony of Nasutitermes Corniger (Isoptera, Termitidae) in the urban area of João Pessoa, NE Brazil. Sociobiology 2006, 47, 165–174. [Google Scholar]
- Myles, T.G. Review of secondary reproduction in termites (Insecta: Isoptera). Sociobiology 1999, 33, 1–91. [Google Scholar]
- Maynard, D.S.; Crowther, T.W.; King, J.R.; Warren, R.J.; Bradford, M.A. Temperate forest termites: Ecology, biogeography, and ecosystem impacts. Ecol. Entomol. 2015, 40, 199–210. [Google Scholar] [CrossRef]
- Vasconcellos, A.; Bandeira, A.G. Avaliação do consumo de madeira por espécies de Nasutitermes e Microcerotermes (Insecta, Isoptera, Termitidae). Rev. Nordestina Biol. 2000, 14, 17–24. [Google Scholar]
- Mélo, A.C.S.; Bandeira, A.G. Consumo de madeira por Heterotermes sulcatus (Isoptera: Rhinotermitidae) em ecossistema de Caatinga no Nordeste do Brasil. Oecol. Bras. 2007, 11, 350–355. [Google Scholar] [CrossRef][Green Version]
- Moura, F.M.S.; Vasconcellos, A.; Araújo, V.F.P.; Bandeira, A.G. Consumption of Vegetal Organic Matter by Constrictotermes cyphergaster (Isoptera, Termitidae, Nasutitermitinae) in an Area of Caatinga, Northeastern Brazil. Sociobiology 2008, 51, 181–189. [Google Scholar]
- Traniello, J.F.A.; Leuthold, R.H. Behavior and Ecology of Foraging in Termites. In Termites: Evolution, Sociality, Symbioses, Ecology; Abe, T., Bignell, D.E., Higashi, M., Eds.; Kluwer Academic Pub.: Dordrecht, The Netherlands, 2000; pp. 141–168. ISBN 978-94-017-3223-9. [Google Scholar]
- Ernesto, M.V.; Liberal, C.N.; Ferreira, A.S.; Alves, A.C.F.; Zeppelini, D.; Martins, C.F.; Colavite, A.P.; Duarte, A.J.C.; Vasconcellos, A. Hexapod decomposers of Serra de Santa Catarina, Paraíba, Brazil: An area with high potential for conservation of Caatinga biodiversity. Biota Neotrop. 2018, 18, 1–13. [Google Scholar] [CrossRef]
- Ohiagu, C.E.; Wood, T.G. Grass production and decomposition in Southern Guinea Savanna, Nigeria. Oecologia 1979, 40, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Collins, N.M. Termite populations and their role in litter removal in Malaysian rain forests. In Tropical Rain Forest: Ecology and Management; Sutton, S.L., Whitmore, T.C., Chadwick, A.C., Eds.; Blackwell Science: Oxford, UK, 1983; pp. 311–325. [Google Scholar]
- Silva, J.M.C.; Barbosa, L.C.F.; Leal, I.R.; Tabarelli, M. The Caatinga: Understanding the challenges. In Caatinga; Silva, J.M.C., Leal, I.R., Tabarelli, M., Eds.; Springer: Cham, Switzerland, 2018; Volume 1, pp. 3–19. ISBN 978-3-319-68338-6. [Google Scholar]
| Soldier | Worker | Rat. | Rep. | Pop. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nest | We (kg) | V (L) | Ind. | B (g) | Ind. | B (g) | S/W | L 1 | E 2 | W | S | Q | Total |
| A | 1.2 | 3.4 | 209 | 0.5 | 3643 | 6.6 | 1:17 | 2906 | 2857 | - | - | 1 | 6758 |
| B | 4.3 | 9.5 | 116 | 0.3 | 9008 | 16.2 | 1:78 | 116 | - | - | - | 1 | 9240 |
| C | 2.6 | 6.2 | 1962 | 4.3 | 19,953 | 35.9 | 1:10 | 3615 | 188 | - | - | 1 | 25,530 |
| D | 1.7 | 4.0 | 1364 | 3.0 | 36,907 | 66.4 | 1:27 | 9493 | 523 | 93 | - | - | 47,857 |
| E | 4.0 | 11.1 | 5992 | 13.2 | 81,010 | 145.8 | 1:13 | 55,407 | 10,895 | 77 | - | 1 | 153,381 |
| F | 0.7 | 1.8 | 1013 | 2.2 | 20,329 | 36.6 | 1:20 | 1,838 | 646 | - | - | - | 23,180 |
| G | 3.4 | 10.8 | 4077 | 9.0 | 91,004 | 163.8 | 1:22 | 65,012 | 36,646 | - | 14 | - | 160,107 |
| H | 3.9 | 11.4 | 2471 | 5.4 | 45,706 | 82.3 | 1:18 | 44,154 | 13,913 | - | - | - | 92,331 |
| I | 2.0 | 5.5 | 1593 | 3.5 | 38,281 | 68.9 | 1:24 | 22,898 | 20,210 | - | - | 1 | 62,772 |
| J | 1.9 | 7.2 | 947 | 2.1 | 30,647 | 55.2 | 1:32 | 24,488 | 8431 | - | - | - | 56,082 |
| L | 1.1 | 3.3 | 1021 | 2.3 | 25,618 | 46.1 | 1:25 | 36,113 | 18,918 | 95 | - | 1 | 62,847 |
| M | 1.1 | 3.0 | 1076 | 2.4 | 33,881 | 61.0 | 1:31 | 27,851 | 3050 | 35 | - | 1 | 62,843 |
| N | 1.7 | 5.1 | 1330 | 2.9 | 34,236 | 61.6 | 1:25 | 51,665 | 1330 | - | - | - | 87,231 |
| O | 3.4 | 9.0 | 5044 | 11.1 | 85,344 | 153.6 | 1:16 | 69,088 | 20,740 | - | - | 1 | 159,476 |
| P | 2.5 | 6.4 | 1982 | 4.4 | 62,728 | 112.9 | 1:31 | 34,125 | 24,213 | - | - | 1 | 98,835 |
| M | 2.4 | 6.5 | 2013 | 4.4 | 41,220 | 74.2 | 26 | 29,918 | 11,611 | 73,897 | |||
| SD | 1.2 | 3.2 | 1720 | 3.8 | 27,144 | 48.9 | 16 | 23,521 | 11,237 | 51,529 | |||
| Plant Species | Density (g/cm3) | Range of Wood Consumption | Mean Wood Consumption 1 |
|---|---|---|---|
| Cenostigma pyramidale | 0.96 | 2.7–76 | 5.2 ± 1.7 a |
| Mimosa tenuiflora | 0.92 | 1.1–3.8 | 2.4 ± 0.8 b |
| Croton sonderianus | 0.72 | 0.9–3.0 | 1.5 ± 0.6 b |
| Aspidosperma pyrifolium | 0.77 | 0.8–2.6 | 1.4 ± 0.5 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
R. B. Barca, R.; F. Lucena, E.; Vasconcellos, A. Nest Population Structure and Wood Litter Consumption by Microcerotermes indistinctus (Isoptera) in a Seasonally Dry Tropical Forest, Northeastern Brazil. Insects 2018, 9, 97. https://doi.org/10.3390/insects9030097
R. B. Barca R, F. Lucena E, Vasconcellos A. Nest Population Structure and Wood Litter Consumption by Microcerotermes indistinctus (Isoptera) in a Seasonally Dry Tropical Forest, Northeastern Brazil. Insects. 2018; 9(3):97. https://doi.org/10.3390/insects9030097
Chicago/Turabian StyleR. B. Barca, Reberth, Emanuelly F. Lucena, and Alexandre Vasconcellos. 2018. "Nest Population Structure and Wood Litter Consumption by Microcerotermes indistinctus (Isoptera) in a Seasonally Dry Tropical Forest, Northeastern Brazil" Insects 9, no. 3: 97. https://doi.org/10.3390/insects9030097
APA StyleR. B. Barca, R., F. Lucena, E., & Vasconcellos, A. (2018). Nest Population Structure and Wood Litter Consumption by Microcerotermes indistinctus (Isoptera) in a Seasonally Dry Tropical Forest, Northeastern Brazil. Insects, 9(3), 97. https://doi.org/10.3390/insects9030097





