Testing the Insecticidal Activity of Nanostructured Alumina on Sitophilus oryzae (L.) (Coleoptera: Curculionidae) Under Laboratory Conditions Using Galvanized Steel Containers
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Culture
2.2. Insecticides Dust Used for Bioassays
2.3. Laboratory Efficacy Tests: Adult Survival, Grain Damage and Progeny Production Assessment
2.4. Experimental Design and Bioassay Conditions
2.5. Data and Statistical Analyses
3. Results
3.1. Parental Survival (Adults)
3.2. Grain Damage (Grain Weight Loss and Frass Production)
3.3. F1 Production (Transgenerational Effects of NSA on Sitophilus Oryzae)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ebeling, W. Sorptive dusts for pest control. Annu. Rev. Entomol. 1971, 16, 123–158. [Google Scholar] [CrossRef] [PubMed]
- Fields, P.; Korunic, Z. The effect of grain moisture content and temperature on the efficacy of diatomaceous earths from different geographical locations against stored-products beetles. J. Stored Prod. Res. 2000, 36, 1–13. [Google Scholar] [CrossRef]
- Subramanyam, B.; Roesli, R. Alternatives to pesticides in stored-product IMP. In Inert Dusts; Kluwer Academic Publishers: Boston, MA, USA, 2000; pp. 321–379. [Google Scholar]
- Korunić, Z. Diatomaceous earths: Natural insecticides. Pestic. Fitomed. 2013, 28, 77–95. [Google Scholar] [CrossRef]
- Lee, B.H.; Annis, P.C.; Choi, W.S. Fumigant toxicity of essential oils from the Myrtaceae family and 1, 8-cineole against 3 major stored-grain insects. J. Stored Prod. Res. 2004, 40, 553–564. [Google Scholar] [CrossRef]
- Rees, D. Insects of Stored Products; CSIRO Publishing: Canaberra, Australia, 2004; pp. 1–181. [Google Scholar]
- Liu, Z.L.; Ho, S.H. Bioactivity of the essential oil extracted from Evodia rutaecarpa Hook f. et Thomas against the grain storage insects, Sitophilus zeamais Motsch. and Tribolium castaneum (Herbst). J. Stored Prod. Res. 1999, 35, 317–328. [Google Scholar] [CrossRef]
- Arthur, F.H. Toxicity of Diatomaceous Earth to Red Flour Beetles and Confused Flour Beetles (Coleoptera: Tenebrionidae): Effects of Temperature and Relative Humidity. J. Econ. Entomol. 2000, 93, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Arthur, F.H. Survival of Sitophilus oryzae (L.) on wheat treated with diatomaceous earth: Impact of biological and environmental parameters on product efficacy. J. Stored Prod. Res. 2002, 38, 305–313. [Google Scholar] [CrossRef]
- Altieri, M.; Nicholls, C. Biodiversity and Pest Management in Agroecosystems, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 1–252. [Google Scholar]
- Athanassiou, C.G.; Kavallieratos, N.G.; Economou, L.P.; Dimizas, C.B.; Vayias, B.J.; Tomanović, S.; Milutinović, M. Persistence and efficacy of three diatomaceous earth populations against Sitophuilus oryzae (Coleoptera: Curculionidae) on wheat and barley. J. Econ. Entomol. 2005, 98, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Kordali, S.; Aslan, I.; Çalmaşur, O.; Cakir, A. Toxicity of essential oils isolated from three Artemisia species and some of their major components to granary weevil, Sitophilus granarius (L.) (Coleoptera: Curculionidae). Ind. Crops Prod. 2006, 23, 162–170. [Google Scholar] [CrossRef]
- Barik, T.K.; Sahu, B.; Swain, V. Nanosilica—From medicine to pest control. Parasitol. Res. 2008, 103, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Stadler, T.; Buteler, M.; Weaver, D.K. Novel use of nanostructured alumina as an insecticide. Pest Manag. Sci. 2010, 66, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Stefanazzi, N.; Stadler, T.; Ferrero, A. Composition and toxic, repellent and feeding deterrent activity of essential oils against the stored-grain pests Tribolium castaneum (Coleoptera: Tenebrionidae) and Sitophilus oryzae (Coleoptera: Curculionidae). Pest Manag. Sci. 2011, 67, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Souto, R.N.P.; Harada, A.Y.; Andrade, E.H.A.; Maia, J.G.S. Insecticidal activity of piper essential oils from the Amazon against the fire Ant Solenopsis saevissima (Smith)(Hymenoptera: Formicidae). Neotrop. Entomol. 2012, 41, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Bohinc, T.; Vayias, B.; Bartol, T.; Trdan, S. Assessment of insecticidal efficacy of diatomaceous earth and powders of common lavender and field horsetail against bean weevil adults. Neotrop. Entomol. 2013, 42, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, C.; Murugan, K. Adulticidal, repellent, and ovicidal properties of indigenous plant extracts against the malarial vector, Anopheles stephensi (Diptera: Culicidae). Parasitol. Res. 2013, 112, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Buteler, M.; López García, G.P.; Pochettino, A.A.; Stefanazzi, N.; Ferrero, A.A.; Stadler, T. Insecticidal activity of volcanic ash against Sitophilus oryzae L. (Coleoptera: Curculionidae) under laboratory conditions. Austral Ecol. 2014, 24, 17–22. [Google Scholar]
- Mishra, P.; Balaji, A.; Dhal, P.; Suresh Kumar, R.; Magdassi, S.; Margulis, K.; Tyagi, B.K.; Mukherjee, A.; Chandrasekaran, N. Stability of nano-sized permethrin in its colloidal state and its effect on the physiological and biochemical profile of Culex tritaeniorhynchus larvae. Bull. Entomol. Res. 2017, 107, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Pérez-de-Luque, A.; Rubiales, D. Nanotechnology for parasitic plant control. Pest Manag. Sci. 2009, 65, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Bhaumik, A.; Rani, P.U.; Mandal, S.; Epidi, T.T. Nano-particles—A recent approach to insect pest control. Afr. J. Biotechnol. 2010, 9, 3489–3493. [Google Scholar]
- Khot, L.R.; Sankaran, S.; Maja, J.M.; Ehsani, R.; Schuster, E.W. Applications of nanomaterials in agricultural production and crop protection: A review. Crop Prot. 2012, 35, 64–70. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A. Role of nanotechnology in agriculture with special reference to management of insect pests. Appl. Microbiol. Biotechnol. 2012, 94, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Kah, M.; Hofmann, T. Nanopesticide research: Current trends and future priorities. Environ. Int. 2014, 63, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Buteler, M.; Sofie, S.W.; Weaver, D.K.; Driscoll, D.; Muretta, J.; Stadler, T. Development of nanoalumina dust as insecticide against Sitophilus oryzae and Rhyzopertha dominica. Int. J. Pest Manag. 2015, 61, 80–89. [Google Scholar] [CrossRef]
- Cicek, S.; Nadaroglu, H. The use of nanotechnology in the agriculture. Adv. Nano Res. 2015, 3, 207–223. [Google Scholar] [CrossRef]
- Govindarajan, M.; Benelli, G. Facile biosynthesis of silver nanoparticles using Barleria cristata: Mosquitocidal potential and biotoxicity on three non-target aquatic organisms. Parasitol. Res. 2016, 115, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lombi, E.; Zhao, F.J.; Kopittke, P.M. Nanotechnology: A new opportunity in plant sciences. Trends Plant Sci. 2016, 21, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Korunić, Z. Review Diatomaceous earths, a group of natural insecticides. J. Stored Prod. Res. 1998, 34, 87–97. [Google Scholar] [CrossRef]
- Dowdy, A.K. Mortality of red flour beetle, Tribolium castaneum (Coleoptera: Tenebrionidae) exposed to high temperature and diatomaceous earth combinations. J. Stored Prod. Res. 1999, 35, 175–182. [Google Scholar] [CrossRef]
- Arthur, F.H.; Throne, J.E. Efficacy of diatomaceous earth to control internal infestations of rice weevil and maize weevil (Coleoptera: Curculionidae). J. Econ. Entomol. 2003, 96, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Frederick, J.L.; Subramanyam, B. Influence of temperature and application rate on efficacy of a diatomaceous earth formulation against Tribolium castaneum adults. J. Stored Prod. Res. 2016, 69, 86–90. [Google Scholar] [CrossRef]
- McGaughey, W.H. Diatomaceous earth for confused flour beetle and rice weevil control in rough, brown, and milled rice. J. Econ. Entomol. 1972, 65, 1427–1428. [Google Scholar] [CrossRef] [PubMed]
- Malek, M.; Parveen, B. Effect of insects infestation on the weight loss and viability of stored BE paddy. Bangladesh J. Zool. 1989, 17, 1–83. [Google Scholar]
- Golob, P. Current status and future perspectives for inert dusts for control of stored product insects. J. Stored Prod. Res. 1997, 33, 69–79. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Athanassiou, C.G.; Pashalidou, F.G.; Andris, N.S.; Tomanović, Ž. Influence of grain type on the insecticidal efficacy of two diatomaceous earth formulations against Rhyzopertha dominica (F) (Coleoptera: Bostrychidae). Pest Manag. Sci. 2005, 61, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Athanassiou, C.G.; Kavallieratos, N.G.; Meletsis, C.M. Insecticidal effect of three diatomaceous earth formulations, applied alone or in combination, against three stored-product beetle species on wheat and maize. J. Stored Prod. Res. 2007, 43, 330–334. [Google Scholar] [CrossRef]
- Vardeman, E.A.; Arthur, F.H.; Nechols, J.R.; Campbell, J.F. Efficacy of surface applications with diatomaceous earth to control Rhyzopertha dominica (F.)(Coleoptera: Bostrichidae) in stored wheat. J. Stored Prod. Res. 2007, 43, 335–341. [Google Scholar] [CrossRef]
- Debnath, N.; Das, S.; Seth, D.; Chandra, R.; Bhattacharya, S.C.; Goswami, A. Entomotoxic effect of silica nanoparticles against Sitophilus oryzae (L.). J. Pest Sci. 2011, 84, 99–105. [Google Scholar] [CrossRef]
- Stadler, T.; Buteler, M.; Weaver, D.K.; Sofie, S. Comparative toxicity of nanostructured alumina and a commercial inert dust for Sitophilus oryzae (L.) and Rhyzopertha dominica (F.) at varying ambient humidity levels. J. Stored Prod. Res. 2012, 48, 81–90. [Google Scholar] [CrossRef]
- Tefera, T.; Mugo, S.N.; Likhayo, P. Effects of insect population density and storage time on grain damage and weight loss in maize due to the maize weevil Sitophilus zeamais and the larger grain borer Prostephanus truncatus. Afr. J. Agric. Res. 2011, 6, 2249–2254. [Google Scholar]
- Yusuf, B.L.; He, Y. Design, development and techniques for controlling grains post-harvest losses with metal silo for small and medium scale farmers. Afr. J. Biotechnol. 2011, 10, 14552–14561. [Google Scholar]
- Yu, X.; Raeesi, A.; Ghaednia, H.; Heydariha, J.; Das, S.; Xie, S. Behavior of a large steel field silo structure subject to grain loading. J. Perform. Constr. Facil. 2017, 31, 4017038. [Google Scholar] [CrossRef]
- Maonga, B.B.; Assa, M.M.; Haraman, E.M.K. Adoption of small metallic grain silos in Malawi: A farm level cross-sectional study. Int. J. Dev. Sustain. 2013, 2, 1534–1548. [Google Scholar]
- Winston, P.W.; Bates, D.H. Saturated solutions for the control of humidity in biological research. Ecology 1960, 41, 232–237. [Google Scholar] [CrossRef]
- Toniolo, J.C.; Lima, M.D.; Takimi, A.S.; Bergmann, C.P. Synthesis of alumina powders by the glycine-nitrate combustion process. Mater. Res. Bull. 2005, 40, 561–571. [Google Scholar] [CrossRef]
- Karasev, V.V.; Onischuk, A.A.; Glotov, O.G.; Baklanov, A.M.; Maryasov, A.G.; Zarko, V.E.; Panfilova, V.N.; Levykin, A.I.; Sabelfeld, K.K. Formation of charged aggregates of Al2O3 nanoparticles by combustion of aluminum droplets in air. Combust. Flame 2004, 138, 40–54. [Google Scholar] [CrossRef]
- SAS Institute. Sas/Sat®9.3 User’s Guide. Available online: https://support.sas.com/documentation/cdl/en/statug/63962/HTML/default/viewer.htm#titlepage.htm (accessed on 30 April 2018).
- Lorini, I.; Beckel, H. Efficacy of “diatomaceous earth” to control the main stored grain pests. In Proceedings of the 9th International Working Conference on Stored-Product Protection, São Paulo, Brazil, 15–18 October 2006; pp. 863–867. [Google Scholar]
- Desmarchelier, J.M.; Dines, J.C. Dryacide treatment of stored wheat: Its efficacy against insects, and after processing. Anim. Prod. Sci. 1987, 27, 309–312. [Google Scholar] [CrossRef]
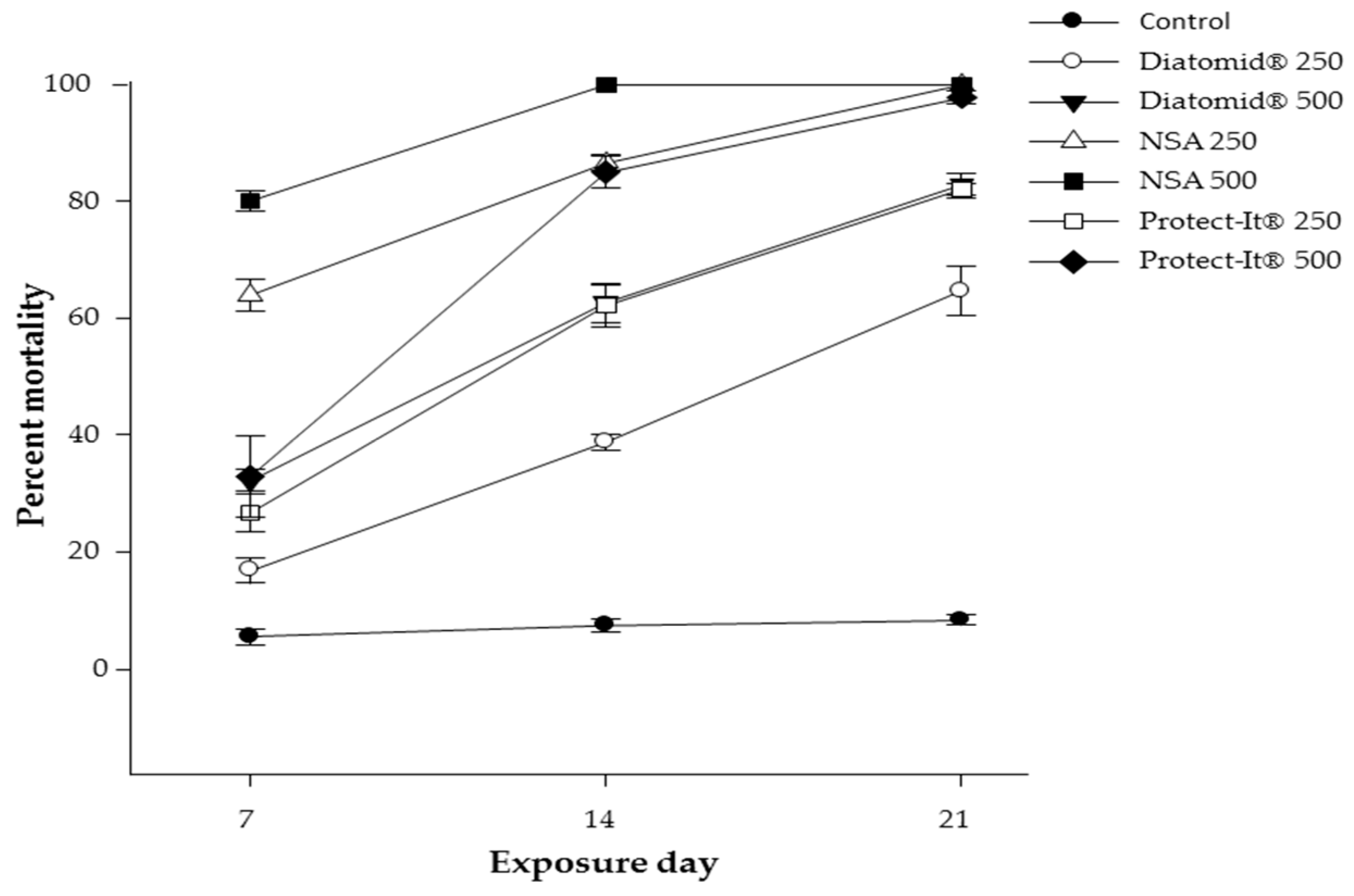
| Treatment (ppm) | Percent Mortality | Letters |
|---|---|---|
| Control (0) | 8.4 | A |
| DiatomiD® (250) | 64.6 | B |
| Protect-It® (250) | 82 | C |
| DiatomiD® (500) | 82.7 | C |
| Protect-It® (500) | 97.8 | D |
| NSA (250) | 100 | D |
| NSA (500) | 100 | D |
| Treatment (ppm) | % Wheat Weight Loss | Frass Grams (SE) | N° Adults F1 (SE) | Multiplication Rate of F1 |
|---|---|---|---|---|
| Control | 17.46 | 1.85 (0.15) | 2171 (49.1) | 0.30 |
| DiatomiD® 250 | 1.64 | 0.92 (0.02) | 1363 (15.6) | 0.24 |
| DiatomiD® 500 | 0.25 | 0.46 (0.12) | 816 (47.2) | 0.17 |
| Protect-It®250 | 0.19 | 0.32 (0.04) | 574 (41.3) | 0.12 |
| Protect-It®500 | 0.23 | 0.27 (0.05) | 434 (26.4) | 0.09 |
| NSA 250 | 0.15 | 0.22 (0.03) | 306 (14.6) | 0.05 |
| NSA 500 | 0.12 | 0.13 (0.002) | 214 (14.6) | 0.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-García, G.P.; Buteler, M.; Stadler, T. Testing the Insecticidal Activity of Nanostructured Alumina on Sitophilus oryzae (L.) (Coleoptera: Curculionidae) Under Laboratory Conditions Using Galvanized Steel Containers. Insects 2018, 9, 87. https://doi.org/10.3390/insects9030087
López-García GP, Buteler M, Stadler T. Testing the Insecticidal Activity of Nanostructured Alumina on Sitophilus oryzae (L.) (Coleoptera: Curculionidae) Under Laboratory Conditions Using Galvanized Steel Containers. Insects. 2018; 9(3):87. https://doi.org/10.3390/insects9030087
Chicago/Turabian StyleLópez-García, Guillermo Pablo, Micaela Buteler, and Teodoro Stadler. 2018. "Testing the Insecticidal Activity of Nanostructured Alumina on Sitophilus oryzae (L.) (Coleoptera: Curculionidae) Under Laboratory Conditions Using Galvanized Steel Containers" Insects 9, no. 3: 87. https://doi.org/10.3390/insects9030087
APA StyleLópez-García, G. P., Buteler, M., & Stadler, T. (2018). Testing the Insecticidal Activity of Nanostructured Alumina on Sitophilus oryzae (L.) (Coleoptera: Curculionidae) Under Laboratory Conditions Using Galvanized Steel Containers. Insects, 9(3), 87. https://doi.org/10.3390/insects9030087





