Does the Pollen Diet Influence the Production and Expression of Antimicrobial Peptides in Individual Honey Bees?
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Bee Rearing and Feeding
2.3. Preparation of Pollen Diets and Nutrition Factors
2.4. Sample Pre-Treatment
2.5. Quantification of Apidaecin 1 Isoforms
2.6. Protein Assay
2.7. RNA Isolation and cDNA Preparation
2.8. Analysis of Gene Expression
2.9. Quantification of Relative Gene Expression Level
2.10. Statistical Analysis
3. Results
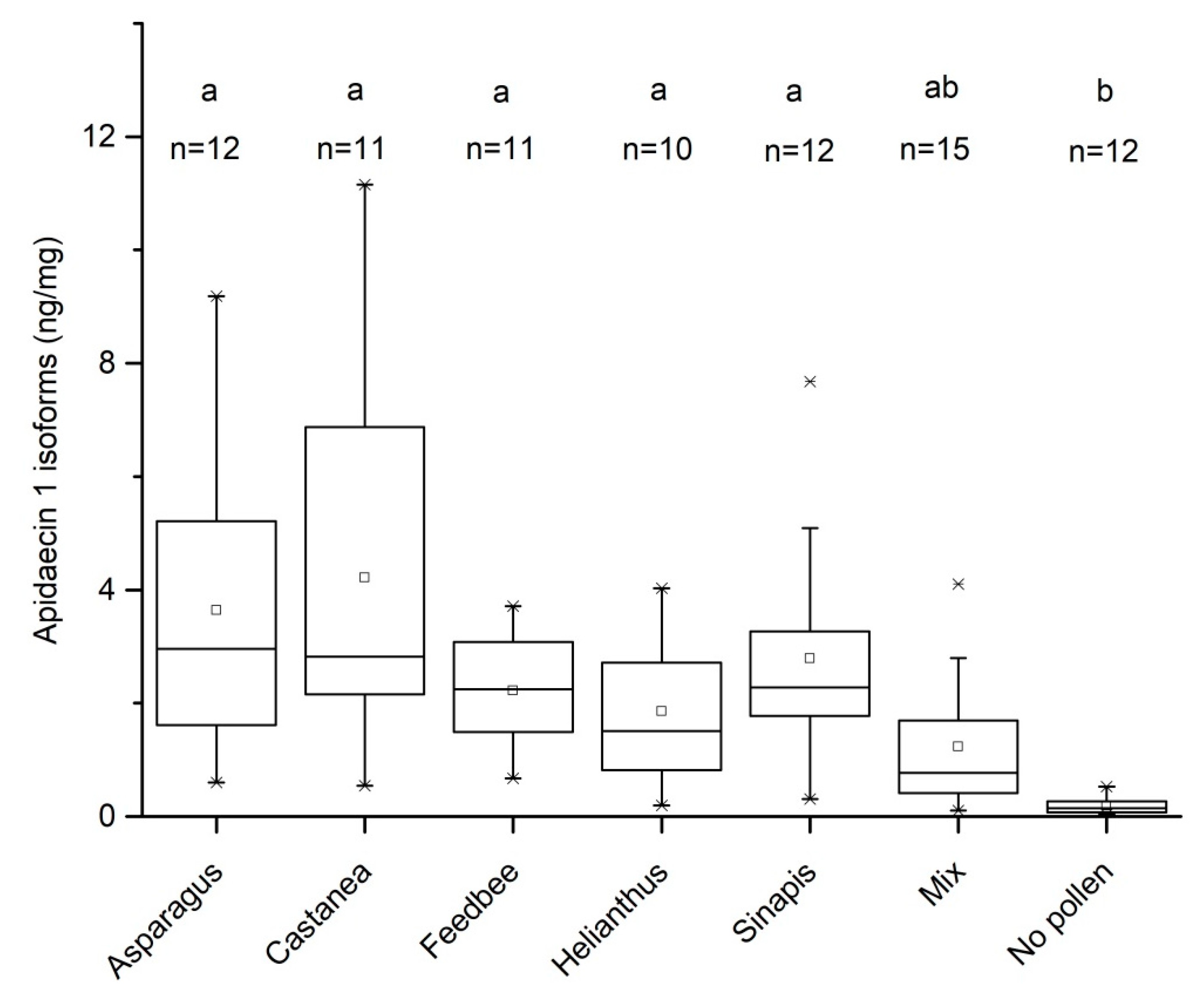
3.1. Effect of Pollen Diet on the Levels of Apidaecin 1 Isoforms in Bee Thorax
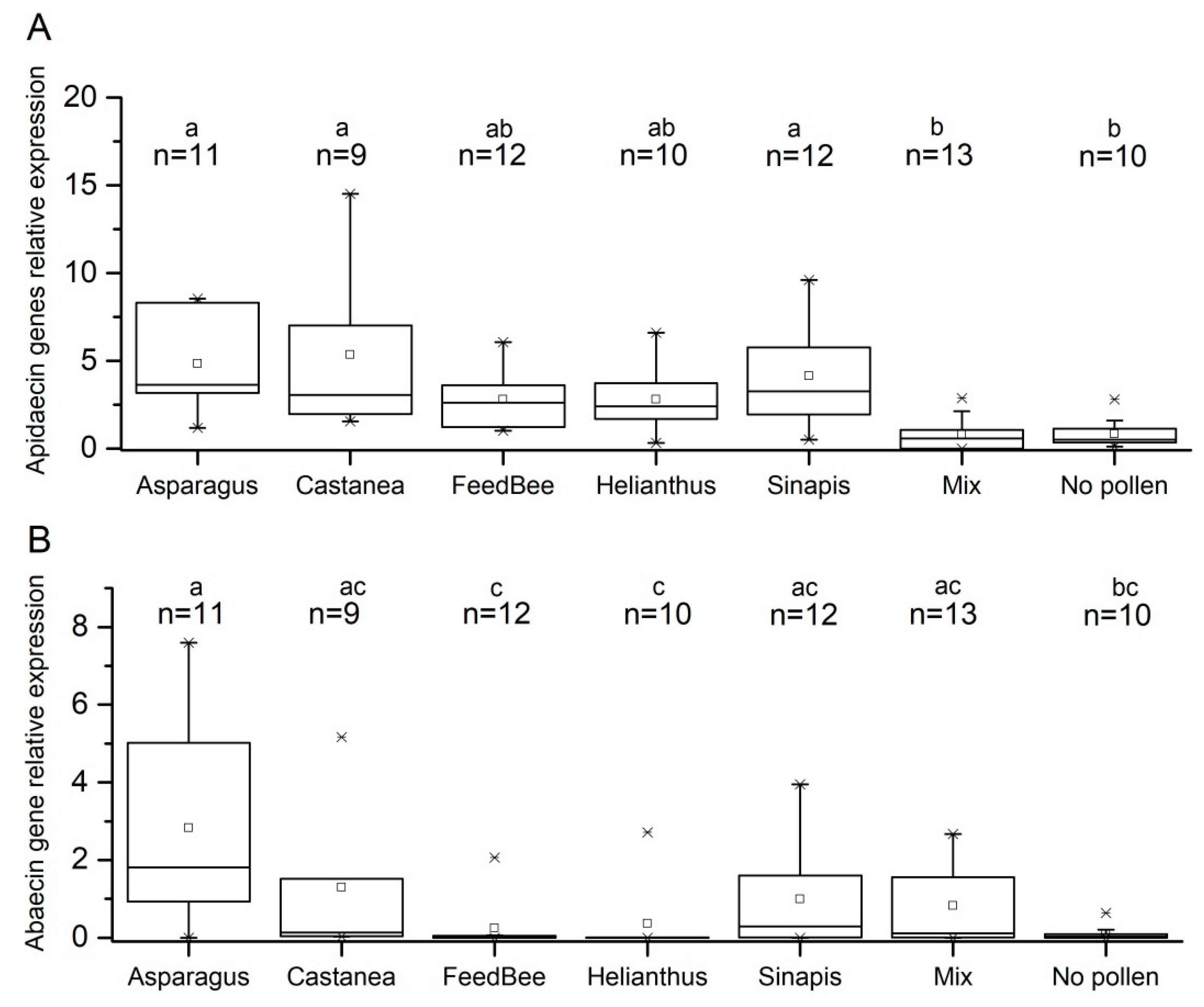
3.2. Effect of Pollen Diet on Expression of Apidaecin and Abaecin Genes
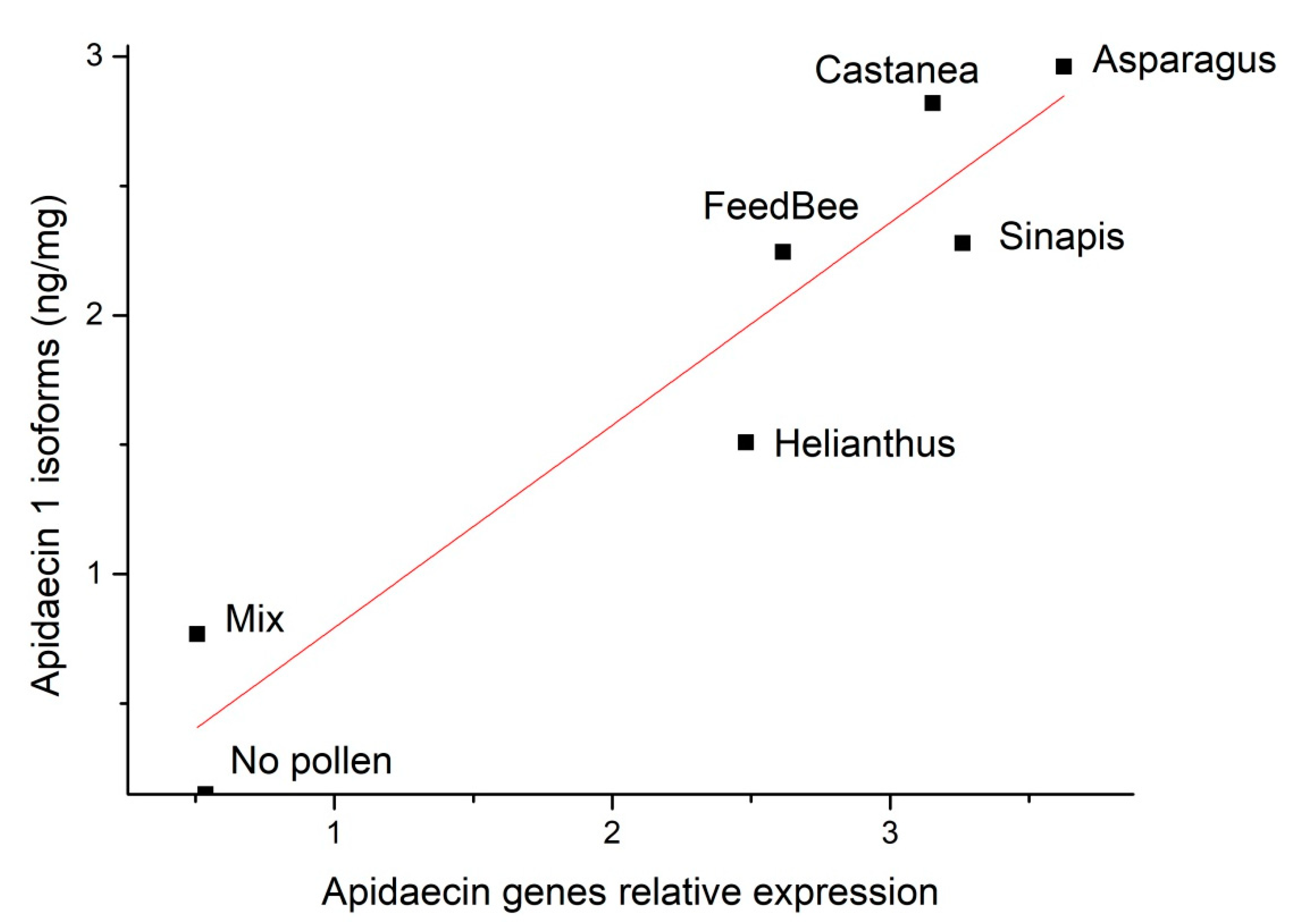
3.3. Correlation of Apidaecin 1 Isoforms Concentration in Thoraces and Gene Expression in Abdomens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMP | antimicrobial peptide |
| Cq | quantification cycle |
| CI | Confidence interval |
| FA | formic acid |
| GITC | guanidium isothiocyanate |
| GOI | gene of interest |
| HKG | housekeeping gene |
| MALDI-TOF MS | matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry |
| nLC-MS | nanoflow liquid chromatography coupled with mass spectrometry |
| qPCR | quantitive polymerase chain reaction |
| RP-HPLC | reversed-phase high performance liquid chromatography |
| TFA | trifluoroacetic acid |
| UHR-Q-TOF | ultra-high resolution quadrupole-time-of-flight |
| WCX | weak cation exchange |
References
- Brodschneider, R.; Crailsheim, K. Nutrition and health in honey bees. Apidologie 2010, 41, 278–294. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Salignon, M.; Le Conte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.-L.; Alaux, C. Influence of pollen nutrition on honey bee health: Do pollen quality and diversity matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef] [PubMed]
- Crailsheim, K.; Schneider, L.H.W.; Hrassnigg, N.; Buhlmann, G.; Brosch, U.; Gmeinbauer, R.; Schöffmann, B. Pollen consumption and utilization in worker honeybees (Apis mellifera carnica)—Dependence on individual age and function. J. Insect Physiol. 1992, 38, 409–419. [Google Scholar] [CrossRef]
- Wheeler, M.M.; Robinson, G.E. Diet-dependent gene expression in honey bees: Honey vs. Sucrose or high fructose corn syrup. Sci. Rep. 2014, 4, 5726. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Aronstein, K.; Chen, Y.P.; Hetru, C.; Imler, J.L.; Jiang, H.; Kanost, M.; Thompson, G.J.; Zou, Z.; Hultmark, D. Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol. Biol. 2006, 15, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Barribeau, S.M.; Sadd, B.M.; du Plessis, L.; Brown, M.J.F.; Buechel, S.D.; Cappelle, K.; Carolan, J.C.; Christiaens, O.; Colgan, T.J.; Erler, S.; et al. A depauperate immune repertoire precedes evolution of sociality in bees. Genome Biol. 2015, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Wilson-Rich, N.; Spivak, M.; Fefferman, N.H.; Starks, P.T. Genetic, individual, and group facilitation of disease resistance in insect societies. Annu. Rev. Entomol. 2009, 54, 405–423. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Spivak, M. Socialized medicine: Individual and communal disease barriers in honey bees. J. Invertebr. Pathol. 2010, 103, S62–S72. [Google Scholar] [CrossRef] [PubMed]
- De Grandi-Hoffman, G.; Chen, Y. Nutrition, immunity and viral infections in honey bees. Curr. Opin. Insect Sci. 2015, 10, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Gätschenberger, H.; Azzami, K.; Tautz, J.; Beier, H. Antibacterial immune competence of honey bees (Apis mellifera) is adapted to different life stages and environmental risks. PLoS ONE 2013, 8, e66415. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, N.; Corona, M.; Neumann, P.; Dainat, B. Overwintering is associated with reduced expression of immune genes and higher susceptibility to virus infection in honey bees. PLoS ONE 2015, 10, e0129956. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Gorenflo, A.; Siede, R.; Meixner, M.; Büchler, R. The neonicotinoids thiacloprid, imidacloprid, and clothianidin affect the immunocompetence of honey bees (Apis mellifera L.). J. Insect Physiol. 2016, 86, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Casteels-Josson, K.; Capaci, T.; Casteels, P.; Tempst, P. Apidaecin multipeptide precursor structure—A putative mechanism for amplification of the insect antibacterial response. EMBO J. 1993, 12, 1569–1578. [Google Scholar] [PubMed]
- Casteels, P.; Ampe, C.; Riviere, L.; Van Damme, J.; Elicone, C.; Fleming, M.; Jacobs, F.; Tempst, P. Isolation and characterization of abaecin, a major antibacterial response peptide in the honeybee (Apis mellifera). Eur. J. Biochem. 1990, 187, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Casteels, P.; Ampe, C.; Jacobs, F.; Vaeck, M.; Tempst, P. Apidaecins: Antibacterial peptides from honeybees. EMBO J. 1989, 8, 2387–2391. [Google Scholar] [PubMed]
- Fujiwara, S.; Imai, J.; Fujiwara, M.; Yaeshima, T.; Kawashima, T.; Kobayashi, K. A potent antibacterial protein in royal jelly. Purification and determination of the primary structure of royalisin. J. Biol. Chem. 1990, 265, 11333–11337. [Google Scholar] [PubMed]
- Klaudiny, J.; Albert, T.; Bachanova, K.; Kopernick, J.; Simuth, J. Two structurally different defensin genes, one of them encoding a novel defensin isoform, are expressed in honeybee Apis mellifera. Insect Biochem. Mol. 2005, 35, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Vilmos, P.; Kurucz, É. Insect immunity: Evolutionary roots of the mammalian innate immune system. Immunol. Lett. 1998, 62, 59–66. [Google Scholar] [CrossRef]
- Danihlík, J.; Aronstein, K.; Petřivalský, M. Antimicrobial peptides: A key component of honey bee innate immunity. J. Apic. Res. 2016, 54, 123–136. [Google Scholar] [CrossRef]
- Casteels, P.; Ampe, C.; Jacobs, F.; Tempst, P. Functional and chemical characterization of hymenoptaecin, an antibacterial polypeptide that is infection-inducible in the honeybee (Apis mellifera). J. Biol. Chem. 1993, 268, 7044–7054. [Google Scholar] [PubMed]
- Chaimanee, V.; Chantawannakul, P.; Chen, Y.; Evans, J.D.; Pettis, J.S. Differential expression of immune genes of adult honey bee (Apis mellifera) after inoculated by Nosema ceranae. J. Insect Physiol. 2012, 58, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Siede, R.; Meixner, M.D.; Büchler, R. Comparison of transcriptional changes of immune genes to experimental challenge in the honey bee (Apis mellifera). J. Apic. Res. 2012, 51, 320–328. [Google Scholar] [CrossRef]
- Jefferson, J.M.; Dolstad, H.A.; Sivalingam, M.D.; Snow, J.W. Barrier immune effectors are maintained during transition from nurse to forager in the honey bee. PLoS ONE 2013, 8, e54097. [Google Scholar] [CrossRef] [PubMed]
- Bilikova, K.; Hanes, J.; Nordhoff, E.; Saenger, W.; Klaudiny, J.; Simuth, J. Apisimin, a new serine-valine-rich peptide from honeybee (Apis mellifera L.) royal jelly: Purification and molecular characterization. FEBS Lett. 2002, 528, 125–129. [Google Scholar] [CrossRef]
- Baracchi, D.; Francese, S.; Turillazzi, S. Beyond the antipredatory defence: Honey bee venom function as a component of social immunity. Toxicon 2011, 58, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Danihlík, J.; Šebela, M.; Petřivalský, M.; Lenobel, R. A sensitive quantification of the peptide apidaecin 1 isoforms in single bee tissues using a weak cation exchange pre-separation and nanocapillary liquid chromatography coupled with mass spectrometry. J. Chromatogr. A 2014, 1374, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R.; Alaux, C.; Costa, C.; Csaki, T.; Doublet, V.; Eisenhardt, D.; Fries, I.; Kuhn, R.; McMahon, D.P.; Medrzycki, P.; et al. Standard methods for maintaining adult Apis mellifera in cages under in vitro laboratory conditions. J. Apic. Res. 2013, 52, 1–36. [Google Scholar] [CrossRef]
- Saffari, A.; Kevan, P.G.; Atkinson, J. Consumption of three dry pollen substitutes in commercial apiaries. J. Apic. Sci. 2010, 54, 13–20. [Google Scholar]
- Omar, E.; Abd-Ella, A.A.; Khodairy, M.M.; Moosbeckhofer, R.; Crailsheim, K.; Brodschneider, R. Influence of different pollen diets on the development of hypopharyngeal glands and size of acid gland sacs in caged honey bees (Apis mellifera). Apidologie 2017, 48, 425–436. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Evans, J.D.; Schwarz, R.S.; Chen, Y.P.; Budge, G.; Cornman, R.S.; De la Rua, P.; de Miranda, J.R.; Foret, S.; Foster, L.; Gauthier, L.; et al. Standard methods for molecular research in Apis mellifera. J. Apic. Res. 2013, 52, 1–54. [Google Scholar] [CrossRef]
- Lourenço, A.P.; Mackert, A.; dos Santos Cristino, A.; Simões, Z.L.P. Validation of reference genes for gene expression studies in the honey bee, Apis mellifera, by quantitative real-time RT-PCR. Apidologie 2008, 39, 372–385. [Google Scholar] [CrossRef]
- Pfaffl, M.; Tichopad, A.; Prgomet, C.; Neuvians, T. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: Bestkeeper—Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The miqe guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time rt-pcr. Nucl. Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3. [Google Scholar] [CrossRef]
- Paoli, P.P.; Donley, D.; Stabler, D.; Saseendranath, A.; Nicolson, S.W.; Simpson, S.J.; Wright, G.A. Nutritional balance of essential amino acids and carbohydrates of the adult worker honeybee depends on age. Amino Acids 2014, 46, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- De Groot, A.P. Protein and amino acid requirements of the honeybee (Apis mellifera L.). Physiol. Comp. Oecol. 1953, 3, 197–285. [Google Scholar]
- Nicolson, S.; Human, H. Chemical composition of the ‘low quality’ pollen of sunflower (Helianthus annuus, Asteraceae). Apidologie 2013, 44, 144–152. [Google Scholar] [CrossRef]
- Corby-Harris, V.; Jones, B.; Walton, A.; Schwan, M.; Anderson, K. Transcriptional markers of sub-optimal nutrition in developing Apis mellifera nurse workers. BMC Genom. 2014, 15, 134. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.M.; Mao, W.; Pollock, H.S.; Niu, G.; Schuler, M.A.; Berenbaum, M.R. Ecologically appropriate xenobiotics induce cytochrome p450s in Apis mellifera. PLoS ONE 2012, 7, e31051. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Schuler, M.A.; Berenbaum, M.R. Honey constituents up-regulate detoxification and immunity genes in the western honey bee Apis mellifera. Proc. Natl. Acad. Sci. USA 2013, 110, 8842–8846. [Google Scholar] [CrossRef] [PubMed]
- Doublet, V.; Poeschl, Y.; Gogol-Döring, A.; Alaux, C.; Annoscia, D.; Aurori, C.; Barribeau, S.M.; Bedoya-Reina, O.C.; Brown, M.J.F.; Bull, J.C.; et al. Unity in defence: Honeybee workers exhibit conserved molecular responses to diverse pathogens. BMC Genom. 2017, 18, 207. [Google Scholar] [CrossRef] [PubMed]
- Flenniken, M.L.; Andino, R. Non-specific dsRNA-mediated antiviral response in the honey bee. PLoS ONE 2013, 8, e77263. [Google Scholar] [CrossRef] [PubMed]
- Selbach, M.; Schwanhausser, B.; Thierfelder, N.; Fang, Z.; Khanin, R.; Rajewsky, N. Widespread changes in protein synthesis induced by micrornas. Nature 2008, 455, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Asgari, S. MicroRNA functions in insects. Insect Biochem. Mol. Biol. 2013, 43, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Alaux, C.; Dantec, C.; Parrinello, H.; Le Conte, Y. Nutrigenomics in honey bees: Digital gene expression analysis of pollen’s nutritive effects on healthy and Varroa-parasitized bees. BMC Genom. 2011, 12, 496. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.O.; Thoenes, S.C.; Levin, M.D. Survival of honey bees, Apis mellifera (Hymenoptera: Apidae), fed various pollen sources. Ann. Entomol. Soc. Am. 1987, 80, 176–183. [Google Scholar] [CrossRef]
- Campana, B.J.; Moeller, F.E. Honey bees: Preference for and nutritive value of pollen from five plant sources. J. Econ. Entomol. 1977, 70, 39–41. [Google Scholar] [CrossRef]
- Höcherl, N.; Siede, R.; Illies, I.; Gätschenberger, H.; Tautz, J. Evaluation of the nutritive value of maize for honey bees. J. Insect Physiol. 2012, 58, 278–285. [Google Scholar] [CrossRef] [PubMed]
- De Jong, D.; da Silva, E.J.; Kevan, P.G.; Atkinson, J.L. Pollen substitutes increase honey bee haemolymph protein levels as much as or more than does pollen. J. Apic. Res. 2009, 48, 34–37. [Google Scholar] [CrossRef]
- Boncristiani, H.; Underwood, R.; Schwarz, R.; Evans, J.D.; Pettis, J.; vanEngelsdorp, D. Direct effect of acaricides on pathogen loads and gene expression levels in honey bees Apis mellifera. J. Insect Physiol. 2012, 58, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.M. Honey bee toxicology. Ann. Rev. Entomol. 2015, 60, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Schmehl, D.R.; Teal, P.E.A.; Frazier, J.L.; Grozinger, C.M. Genomic analysis of the interaction between pesticide exposure and nutrition in honey bees (Apis mellifera). J. Insect Physiol. 2014, 71, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Ambika Manirajan, B.; Ratering, S.; Rusch, V.; Schwiertz, A.; Geissler-Plaum, R.; Cardinale, M.; Schnell, S. Bacterial microbiota associated with flower pollen is influenced by pollination type, and shows a high degree of diversity and species-specificity. Environ. Microbiol. 2016, 18, 5161–5174. [Google Scholar] [CrossRef] [PubMed]
- McFrederick, Q.S.; Thomas, J.M.; Neff, J.L.; Vuong, H.Q.; Russell, K.A.; Hale, A.R.; Mueller, U.G. Flowers and wild megachilid bees share microbes. Microb. Ecol. 2017, 73, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Kwong, W.K.; Moran, N.A. Gut microbial communities of social bees. Nat. Rev. Microbiol. 2016, 14, 374–384. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danihlík, J.; Škrabišová, M.; Lenobel, R.; Šebela, M.; Omar, E.; Petřivalský, M.; Crailsheim, K.; Brodschneider, R. Does the Pollen Diet Influence the Production and Expression of Antimicrobial Peptides in Individual Honey Bees? Insects 2018, 9, 79. https://doi.org/10.3390/insects9030079
Danihlík J, Škrabišová M, Lenobel R, Šebela M, Omar E, Petřivalský M, Crailsheim K, Brodschneider R. Does the Pollen Diet Influence the Production and Expression of Antimicrobial Peptides in Individual Honey Bees? Insects. 2018; 9(3):79. https://doi.org/10.3390/insects9030079
Chicago/Turabian StyleDanihlík, Jiří, Mária Škrabišová, René Lenobel, Marek Šebela, Eslam Omar, Marek Petřivalský, Karl Crailsheim, and Robert Brodschneider. 2018. "Does the Pollen Diet Influence the Production and Expression of Antimicrobial Peptides in Individual Honey Bees?" Insects 9, no. 3: 79. https://doi.org/10.3390/insects9030079
APA StyleDanihlík, J., Škrabišová, M., Lenobel, R., Šebela, M., Omar, E., Petřivalský, M., Crailsheim, K., & Brodschneider, R. (2018). Does the Pollen Diet Influence the Production and Expression of Antimicrobial Peptides in Individual Honey Bees? Insects, 9(3), 79. https://doi.org/10.3390/insects9030079