Identity and Seasonal Abundance of Beneficial Arthropods Associated with Big Sagebrush (Artemisia tridentata) in Central Washington State, USA
Abstract
1. Introduction
2. Materials and Methods
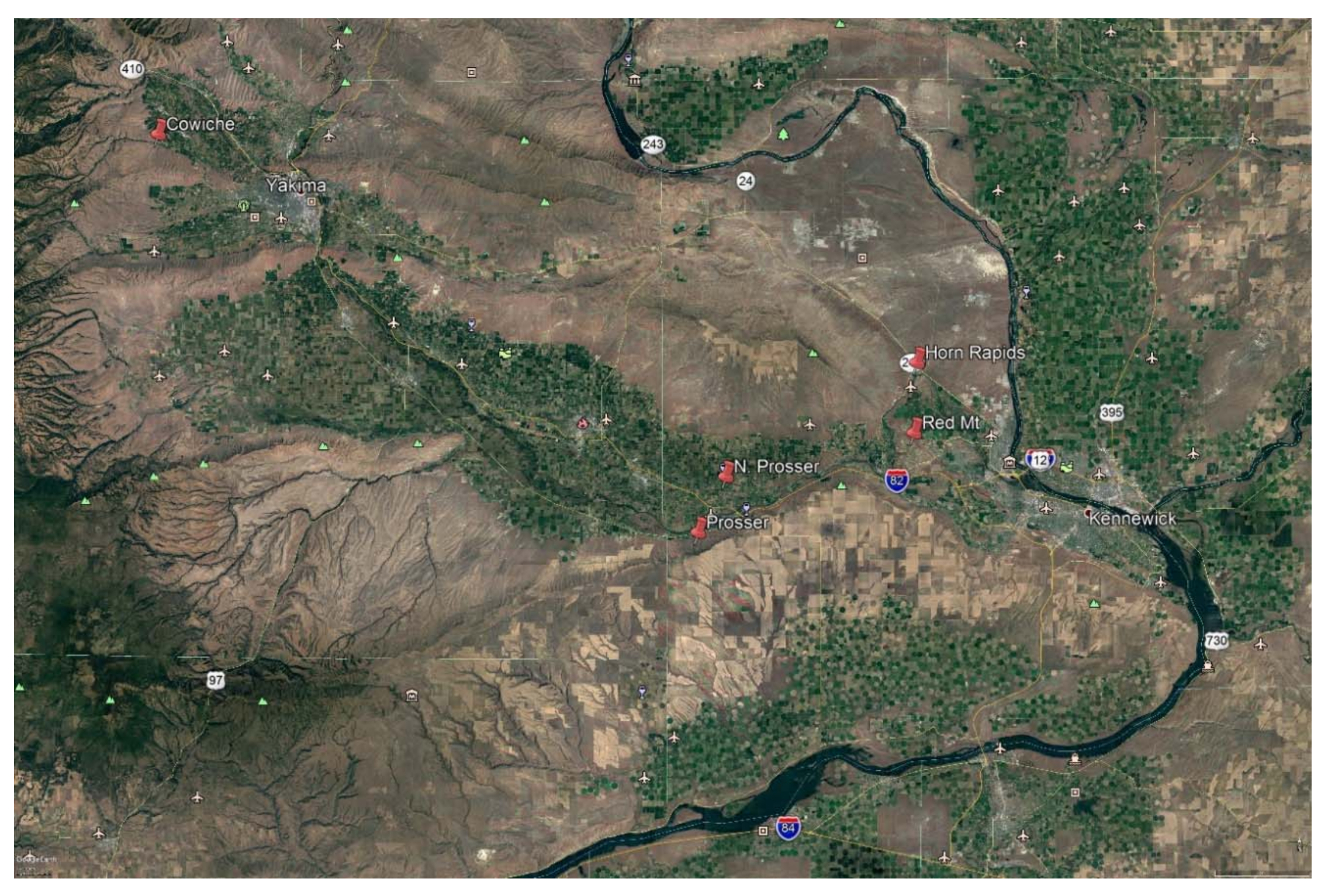
2.1. Sites
2.2. Traps and Trapping
2.3. Arthropod Identification
2.4. Data Analysis
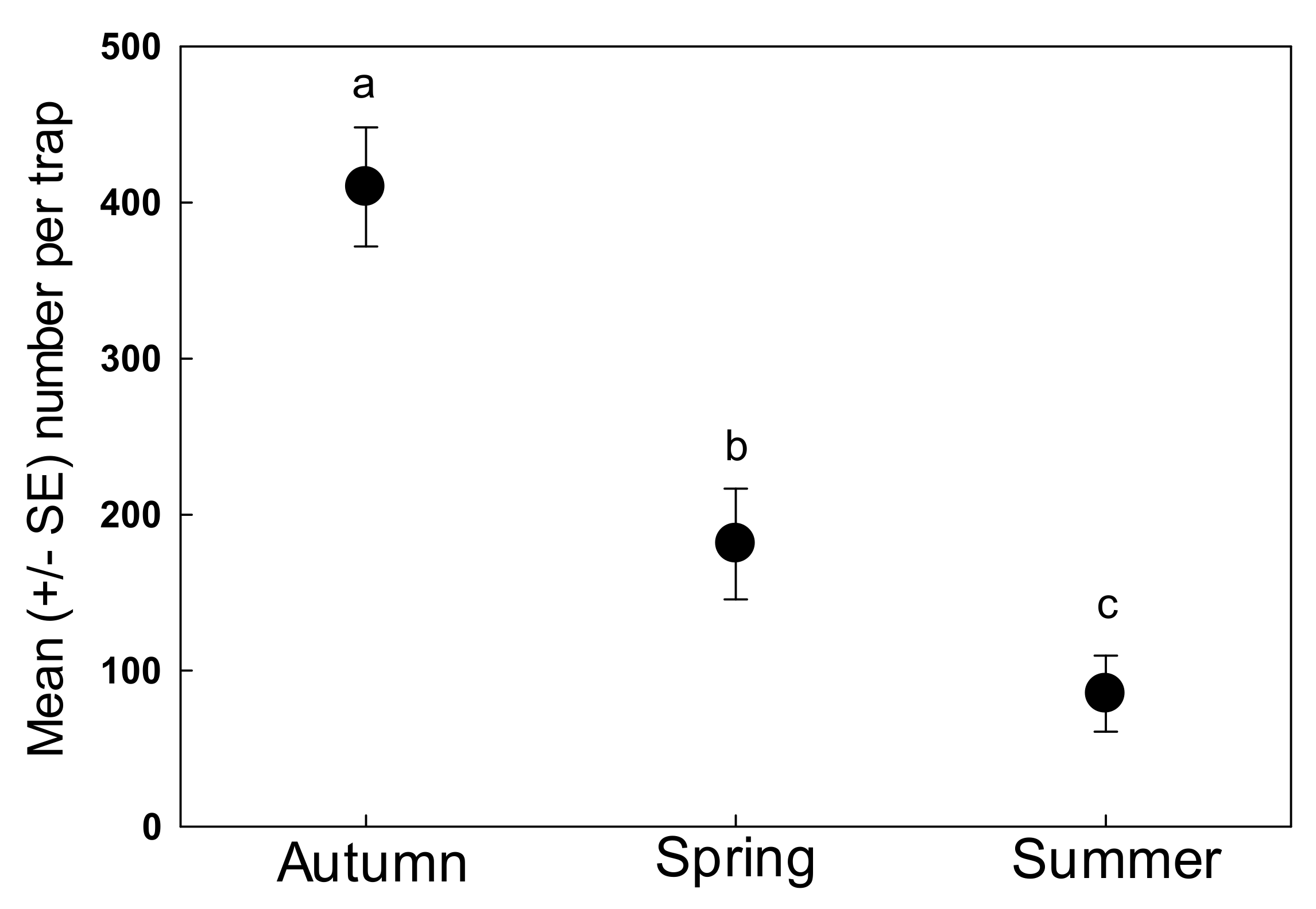
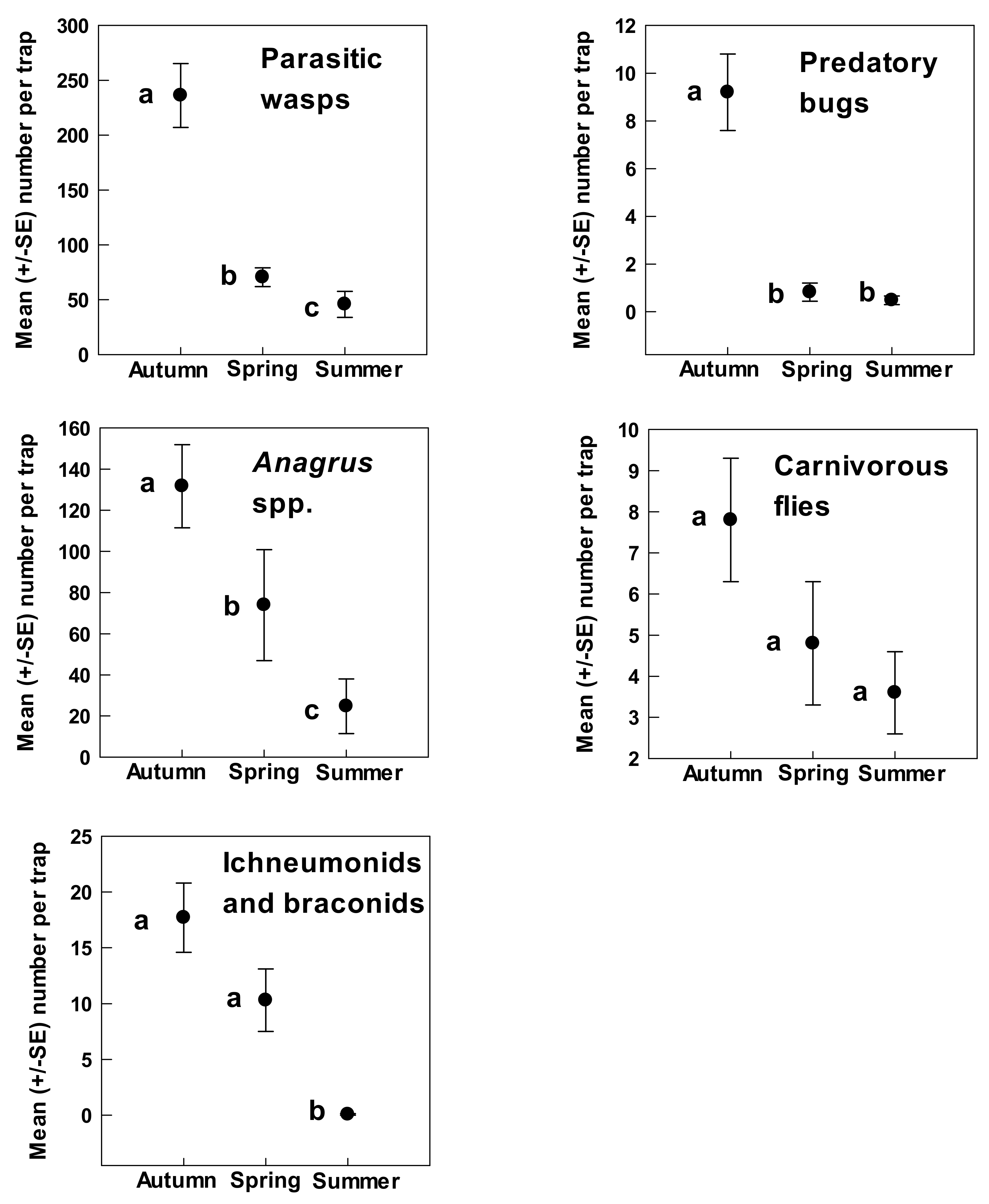
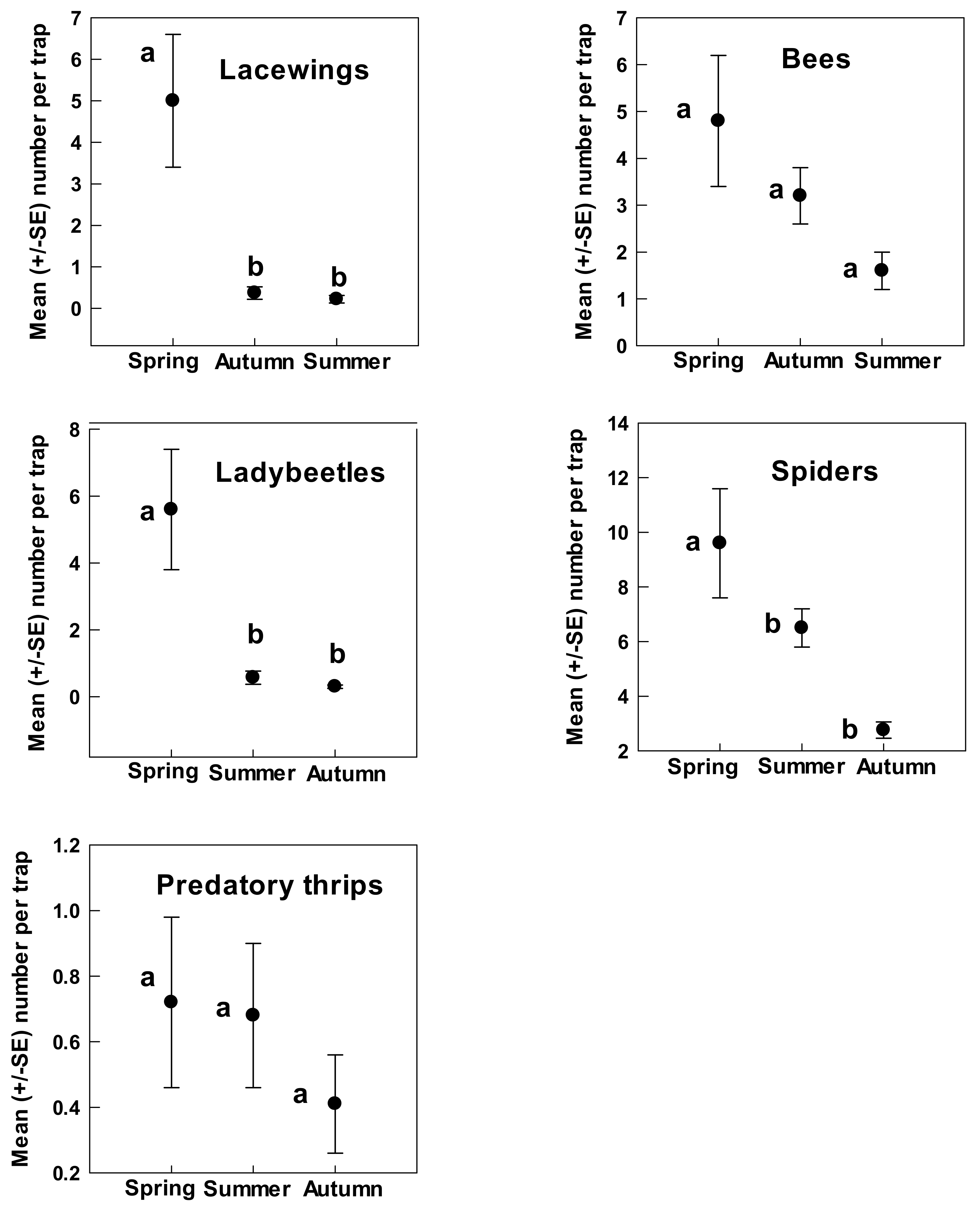
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- West, N.E. Western intermountain sagebrush steppe. In Ecosystems of the World: Temperate Deserts and Semi-Deserts; West, N.E., Ed.; Elsevier: Amsterdam, The Netherlands, 1983; pp. 351–374. [Google Scholar]
- West, N.E. Intermountain deserts, shrub steppes and woodlands. In North American Terrestrial Vegetation; Barbour, M.G., Billings, W.D., Eds.; Cambridge University Press: Cambridge, UK, 1989; pp. 209–230. [Google Scholar]
- Davies, K.W.; Boyd, C.S.; Beck, J.L.; Bates, J.D.; Svejcar, T.J.; Gregg, M.A. Saving the sagebrush sea: An ecosystem conservation plan for big sagebrush plant communities. Biol. Conserv. 2011, 144, 2573–2584. [Google Scholar] [CrossRef]
- Sanford, M.P.; Huntly, N.J. Seasonal patterns of arthropod diversity and abundance on big sagebrush, Artemisia tridentata. West. N. Am. Nat. 2010, 70, 67–76. [Google Scholar] [CrossRef]
- Takahashi, M.; Huntly, N. Herbivorous insects reduce growth and reproduction of big sagebrush (Artemisia tridentata). Arthropod-Plant Interact. 2010, 4, 257–266. [Google Scholar] [CrossRef]
- Takahashi, M. Dynamics of the Interactions between Big Sagebrush (Artemisia tridentata) and Its Associated Arthropods in Southeastern Idaho: Food Webs and Effects of Herbivory in a Changing Climate. Ph.D. Thesis, ProQuest Dissertations Publishing, Idaho State University, Pocatello, ID, USA, 2012. [Google Scholar]
- Christiansen, T.A.; Lockwood, J.A.; Powell, A. Arthropod community dynamics in undisturbed and intensively managed mountain brush habitats. Great Basin Nat. 1989, 49, 134–139. [Google Scholar]
- Spawton, K.A.; Wetzel, W.C. Gall insect community on big sagebrush varies with plant size but not plant age. Environ. Entomol. 2015, 44, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Wiens, J.A.; Cates, R.G.; Rotenberry, J.T.; Cobb, N.; Van Horne, B.; Redak, R.A. Arthropod dynamics on sagebrush (Artemisia tridentata): Effects of plant chemistry and avian predation. Ecol. Monogr. 1991, 6, 299–321. [Google Scholar] [CrossRef]
- Abraham, B.J. Spatial and temporal patterns in a sagebrush steppe spider community (Arachnida, Araneae). J. Arachnol. 1983, 11, 31–50. [Google Scholar]
- Bolshakova, V.L.J.; Evans, E.W. Phenology of the sagebrush defoliating moth Aroga websteri (Lepidoptera: Gelechiidae), with application to population irruptions. Ann. Entomol. Soc. Am. 2016, 109, 424–431. [Google Scholar] [CrossRef]
- Miliczky, E.; Horton, D.R. Natural enemy fauna (Insecta, Araneae) found on native sagebrush steppe plants in eastern Washington with reference to species also found in adjacent apple and pear orchards. Pan-Pac. Entomol. 2007, 83, 50–65. [Google Scholar] [CrossRef]
- Dobler, F.C.; Eby, J.; Perry, C.; Richardson, S.; Vander Haegen, M. Status of Washington’s Shrub Steppe Ecosystem; Extent, Ownership and Wildlife/Vegetation Relationships; Phase One Completion Report; Washington Department of Fish and Wildlife: Olympia, WA, USA, 1996; p. 39. [Google Scholar]
- Gurr, G.M.; Wratten, S.D.; Barbosa, P. Success in conservation biological control of arthropods. In Biological Control: Measures of Success; Gurr, G.M., Wratten, S.D., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 105–132. [Google Scholar]
- Fiedler, A.K.; Landis, D.A.; Wratten, S.D. Maximizing ecosystem services from conservation biological control: The role of habitat management. Biol. Conserv. 2008, 45, 254–271. [Google Scholar] [CrossRef]
- Gurr, G.M.; Scarratt, S.L.; Wratten, S.D.; Berndt, L.; Irvin, N. Ecological engineering, habitat manipulation and pest management. In Ecological Engineering for Pest Management: Advances in Habitat Manipulation for Arthropods; Gurr, G.M., Wratten, S.D., Altieri, M.A., Eds.; CSIRO Publishing: Melbourne, Australia, 2004; pp. 1–12. [Google Scholar]
- Cunningham, R.B.; Lindenmayer, D.B.; Crane, M.; Michael, D.; Macgregor, C. Reptile and arboreal marsupial response to replanted vegetation in agricultural landscapes. Ecol. Appl. 2007, 17, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Fahrig, L.; Baudry, J.; Brotons, L.; Burel, F.G.; Crist, T.O.; Fuller, R.J.; Sirami, C.; Siriwardena, G.M.; Martin, J.L. Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol. Lett. 2011, 14, 101–112. [Google Scholar] [CrossRef] [PubMed]
- James, D.G.; Lauby, G.; Seymour, L.; Buckley, K. Beauty with Benefits: Butterfly conservation in Washington State, USA, wine grape vineyards. J. Insect Conserv. 2015, 19, 341–348. [Google Scholar] [CrossRef]
- James, D.G.; Seymour, L.; Lauby, G.; Buckley, K. Beneficial insects attracted to native flowering buckwheats (Eriogonum Michx) in central Washington. Environ. Entomol. 2014, 43, 942–948. [Google Scholar] [CrossRef] [PubMed]
- James, D.G.; Lauby, G.; Seymour, L.; Buckley, K. Beneficial insects associated with stinging nettle (Urtica dioica Linnaeus) in central Washington State. Pan-Pac. Entomol. 2015, 91, 1–9. [Google Scholar] [CrossRef]
- James, D.G.; Seymour, L.; Lauby, G.; Buckley, K. Beneficial insect attraction to milkweeds (Asclepias speciosa, Asclepias fascicularis) in Washington State, USA. Insects 2016, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Prischmann, D.A.; James, D.G.; Storm, C.P.; Wright, L.C.; Snyder, W.E. Identity, abundance and phenology of Anagrus spp. (Hymenoptera: Mymaridae) and leafhoppers (Homoptera: Cicadellidae) associated with grape, blackberry and wild rose in Washington State. Ann. Entomol. Soc. Am. 2007, 100, 41–52. [Google Scholar] [CrossRef]
- Frank, S.D.; Shrewsbury, P.M.; Esiekpe, O. Spatial and temporal variation in natural enemy assemblages on Maryland native plant species. Environ. Entomol. 2008, 37, 476–486. [Google Scholar] [CrossRef]
- Xavier, S.S.; Olson, D.M.; Coffin, A.W.; Strickland, T.C.; Schmidt, J.M. Perennial grass and native wildflowers: A synergistic approach to habitat management. Insects 2017, 8, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Prischmann, D.A.; James, D.G.; Gringras, S.N.; Snyder, W.E. Diversity and abundance of insects and spiders on managed and unmanaged grapevines in southcentral Washington State. Pan-Pac. Entomol. 2005, 81, 131–144. [Google Scholar]
- Woods, J.L.; Dreves, A.J.; James, D.G.; Lee, J.C.; Walsh, D.B.; Gent, D.H. Development of biological control of Tetranychus urticae Koch (Acari: Tetranychidae) and Phorodon humuli (Schrank) in Oregon hop yards. J. Econ. Entomol. 2014, 107, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Barral, M.P.; Rey Benayas, J.M.; Meli, P.; Maceira, N.O. Quantifying the impacts of ecological restoration on biodiversity and ecosystem services in agroecosystems: A global meta-analysis. Agric. Ecosyst. Environ. 2015, 202, 223–231. [Google Scholar] [CrossRef]
- Liu, B.; Yang, L.; Zeng, Y.; Yang, F.; Yang, Y.; Lu, Y. Secondary crops and non-crop habitats within landscapes enhance the abundance and diversity of generalist predators. Agric. Ecosyst. Environ. 2018, 258, 30–39. [Google Scholar] [CrossRef]
- Quinn, M.A. Influence of habitat fragmentation and crop system on Columbia Basin shrubsteppe communities. Ecol. Appl. 2004, 14, 1634–1655. [Google Scholar] [CrossRef]
| Beneficial Insect Categories | Species, Genera, or Family Included |
|---|---|
| Neuroptera (Lacewings) | Chrysoperla plorabunda (Fitch) Chrysopa nigricornis Burmeister Chrysopa coloradensis Banks Chrysopa oculata Say |
| Coccinellidae (Ladybeetles) | Harmonia axyridis (Pallas) Coccinella septempunctata L. Coccinella transversogutatta Mulsant Hippodamia convergens (Guerin-Meneville) Stethorus picipes Casey Stethorus punctillum (Weise) |
| Heteroptera (Predatory true bugs) | Deraeocoris brevis (Uhler) Geocoris pallens Stal Orius spp. |
| Aeolothripidae (Predatory thrips) | Franklinothrips spp. Aeolothrips spp. |
| Diptera (Predatory and parasitic flies) | Empididae Syrphidae Dolichopodidae Tachinidae |
| Hymenoptera: Ichneumonidae and Braconidae (Ichneumonid and braconid wasps) | Ichneumonidae Braconidae |
| Mymaridae (Fairy wasps) | Anagrus spp. |
| Other Hymenoptera | Pteromalidae, Eulophidae, Trichogrammatidae, Scelionidae |
| Araneae (Spiders) | Linyphiidae, Oxyopidae, Clubionidae, Thomisidae, Salticidae |
| Apoidea (Bees) | Apis mellifera L., Andrenidae, Halictidae, Megachilidae, Apidae, Colletidae |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
James, D.G.; Seymour, L.; Lauby, G.; Buckley, K. Identity and Seasonal Abundance of Beneficial Arthropods Associated with Big Sagebrush (Artemisia tridentata) in Central Washington State, USA. Insects 2018, 9, 76. https://doi.org/10.3390/insects9030076
James DG, Seymour L, Lauby G, Buckley K. Identity and Seasonal Abundance of Beneficial Arthropods Associated with Big Sagebrush (Artemisia tridentata) in Central Washington State, USA. Insects. 2018; 9(3):76. https://doi.org/10.3390/insects9030076
Chicago/Turabian StyleJames, David G., Lorraine Seymour, Gerry Lauby, and Katie Buckley. 2018. "Identity and Seasonal Abundance of Beneficial Arthropods Associated with Big Sagebrush (Artemisia tridentata) in Central Washington State, USA" Insects 9, no. 3: 76. https://doi.org/10.3390/insects9030076
APA StyleJames, D. G., Seymour, L., Lauby, G., & Buckley, K. (2018). Identity and Seasonal Abundance of Beneficial Arthropods Associated with Big Sagebrush (Artemisia tridentata) in Central Washington State, USA. Insects, 9(3), 76. https://doi.org/10.3390/insects9030076






