Monitoring Nutrient Status of Brown Marmorated Stink Bug Adults and Nymphs on Summer Holly
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection
2.2. Nutrient Bioassay
2.3. Statistical Analyses
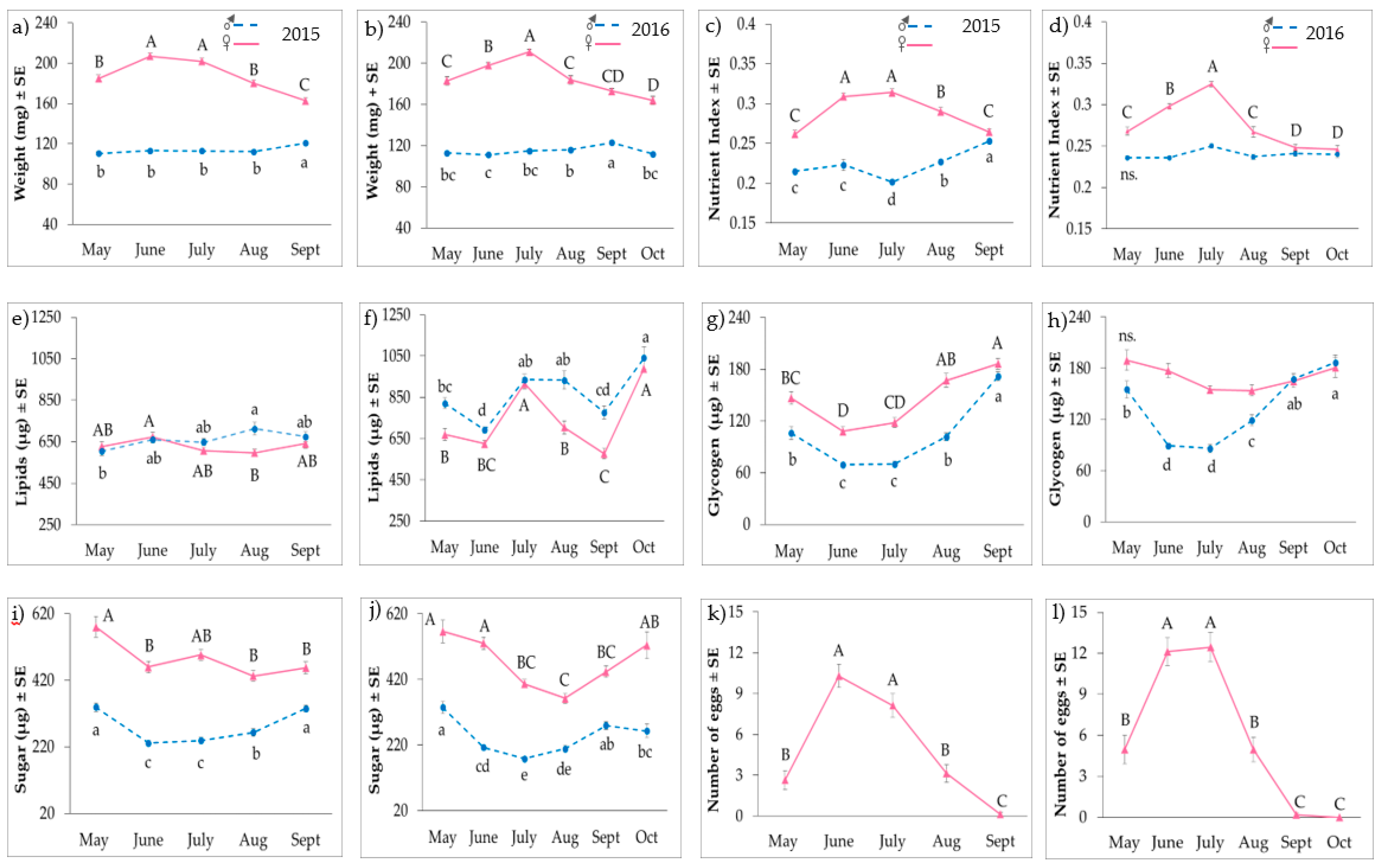
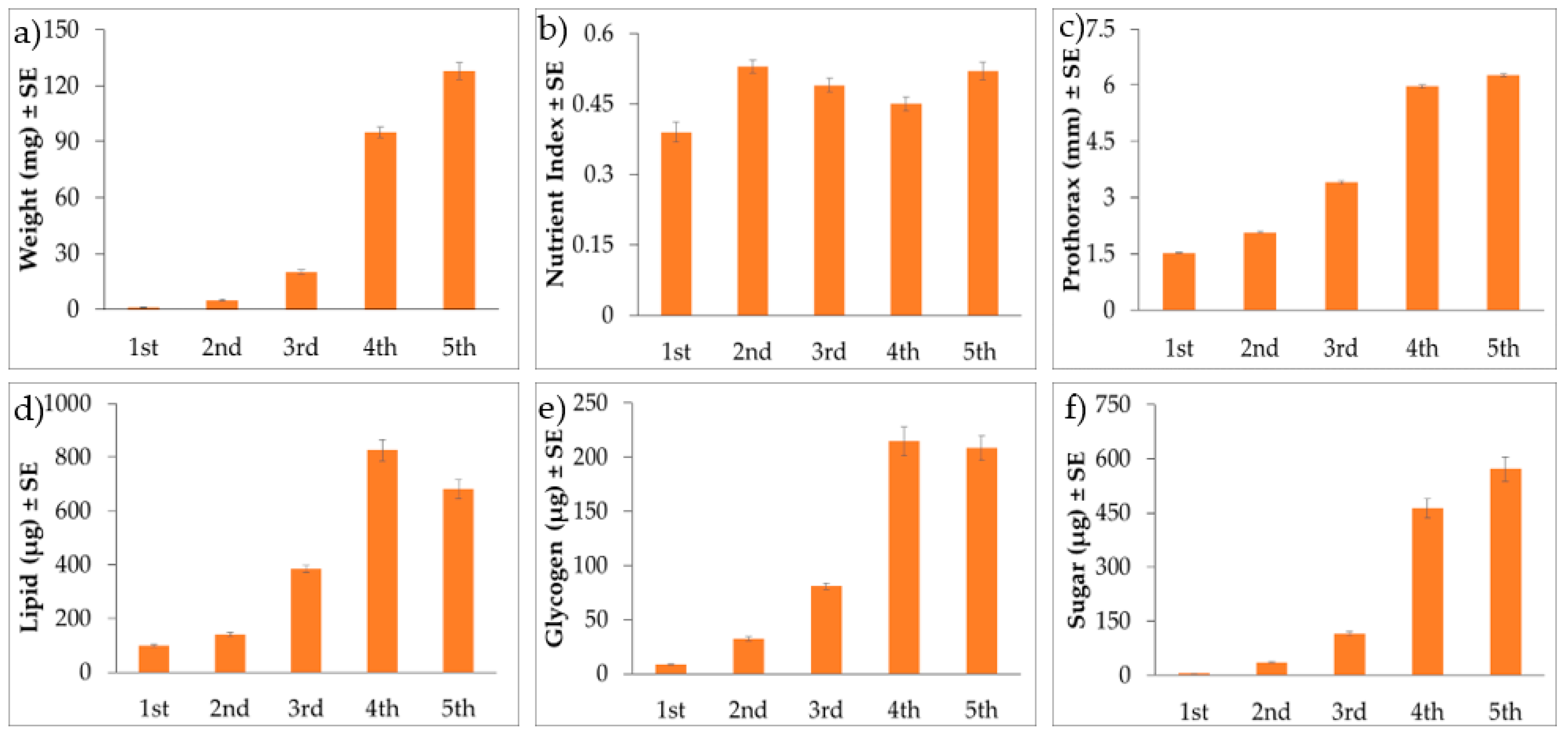
3. Results
3.1. Adult Nutrient Profiles
3.2. Reproductive Status
3.3. Nymphal BMSB
4. Discussion
4.1. Adult BMSB
4.2. Reproductive Status
4.3. Nymphal BMSB
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haye, T.; Gariepy, T.; Hoelmer, K.; Rossi, J.-P.; Streito, J.-C.; Tassus, X.; Desneux, N. Range expansion of the invasive brown marmorated stinkbug. Halyomorpha halys: An increasing threat to field, fruit and vegetable crops worldwide. J. Pest Sci. 2015, 88, 665–673. [Google Scholar] [CrossRef]
- Leskey, T.C.; Hamilton, G.C.; Nielsen, A.L.; Polk, D.F.; Rodriguez-Saona, C.; Bergh, J.C.; Herbert, D.A.; Kuhar, T.P.; Pfeiffer, D.; Dively, G.P.; et al. Pest status of the brown marmorated stink bug, Halyomorpha halys in the USA. Outlooks Pest Manag. 2012, 23, 218–226. [Google Scholar] [CrossRef]
- Wermelinger, B.; Wyniger, D.; Forster, B. First records of an invasive bug in Europe: Halyomorpha halys Stål (Heteroptera: Pentatomidae), a new pest on woody ornamentals and fruit trees? Mitt. Schweiz. Entomol. Ges. 2008, 81, 1–8. [Google Scholar]
- StopBMSB.Org. Where is BMSB? USDA-NIFA SCRI Coordinated Agricultural Project Northeastern IPM Center. 2017. Available online: http://www.stopbmsb.org/where-is-bmsb/ (accessed on 22 May 2018).
- Bergmann, E.; Bernhard, K.; Bernon, G.; Bickerton, M.; Gill, S.; Gonzales, C.; Hamilton, G.C.; Hedstrom, C.; Kamminga, K.; Koplinka-Loehr, C.; et al. Host Plants of the Brown Marmorated Stink Bug in the U.S. USDA-NIFA SCRI Coordinated Agricultural Project Northeastern IPM Center. 2014. Available online: http://www.stopbmsb.org/where-is-bmsb/host-plants/ (accessed on 25 January 2017).
- Wiman, N.; Dalton, D.; Brewer, L.; Shearer, P.; Walton, V. How to Monitor for Brown Marmorated Stink Bug in Specialty Crops. Oregon State University Extension Service, em9138. March 2016. Available online: https://catalog.extension.oregonstate.edu/sites/catalog/files/project/pdf/em9138.pdf (accessed on 5 September 2018).
- Inkley, D.B. Characteristics of home invasion by the brown marmorated stink bug (Hemiptera: Pentatomidae). J. Entomol. Sci. 2012, 47, 125–130. [Google Scholar] [CrossRef]
- Acebes-Doria, A.L.; Leskey, T.C.; Bergh, J.C. Host plant effects on Halyomorpha halys (Hemiptera: Pentatomidae) nymphal development and survivorship. Environ. Entomol. 2016, 45, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Funayama, K. A new rearing method using carrots as food for the brown-marmorated stink bug, Halyomorpha halys (Stål) (Heteroptera: Pentatomidae). Appl. Entomol. Zool. 2006, 41, 415–418. [Google Scholar] [CrossRef]
- Funayama, K. Importance of apple fruits as food for the brown-marmorated stink bug, Halyomorpha halys (Stål) (Heteroptera: Pentatomidae). Appl. Entomol. Zool. 2004, 39, 617–623. [Google Scholar] [CrossRef]
- Mainali, B.P.; Kim, H.J.; Yoon, Y.N.; Oh, I.S.; Bae, S.D. Evaluation of apple and orange fruits as food sources for the development of Halyomorpha halys (Hemiptera: Pentatomidae). Korean J. Appl. Entomol. 2014, 53, 473–477. [Google Scholar] [CrossRef]
- Skillman, V.P.; Wiman, N.G.; Lee, J.C. Nutrient declines in overwintering Halyomorpha halys populations. Entomol. Exp. Appl. 2018, in press. [Google Scholar]
- Hahn, D.A.; Denlinger, D.L. Meeting the energetic demands of insect diapause: Nutrient storage and utilization. J. Insect Physiol. 2007, 53, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Beenakkers, A.M.T.; Van der Horst, D.J.; Van Marrewijk, W.J.A. Insect flight muscle metabolism. Insect Biochem. 1984, 14, 243–260. [Google Scholar] [CrossRef]
- Funayama, K. Nutritional states of post-overwintering adults of the brown-marmorated stink bug, Halyomorpha halys (Stål) (Heteroptera: Pentatomidae). Jpn. J. Appl. Entomol. Zool. 2012, 56, 12–15. [Google Scholar] [CrossRef]
- Skillman, V.P. Nutrient Profile and Nursery Feeding Damage of Halyomorpha halys (Hempitera: Pentatomidae). Master’s Thesis, Oregon State University, Corvallis, OR, USA, 2017. [Google Scholar]
- Acebes-Doria, A.L. Host Plant Effects on the Biology, Behavior and Ecology of Brown Marmorated Stink Bug, Halyomorpha halys (Stål) (Hemiptera: Pentatomidae). Ph.D. Dissertation, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2016. [Google Scholar]
- Martinson, H.M.; Venugopal, P.D.; Bergmann, E.J.; Shrewsbury, P.M.; Raupp, M.J. Fruit availability influences the seasonal abundance of invasive stink bugs in ornamental tree nurseries. J. Pest Sci. 2015, 88, 461–468. [Google Scholar] [CrossRef]
- Nielsen, A.L.; Fleischer, S.; Hamilton, G.C.; Hancock, T.; Krawczyk, G.; Lee, J.C.; Ogburn, E.; Pote, J.M.; Raudenbush, A.; Rucker, A.; et al. Phenology of Halyomorpha halys described using female reproductive development. Ecol. Evol. 2017, 7, 6680–6690. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.L.; Shearer, P.W.; Hamilton, G.C. Toxicity of insecticides to Halymorpha halys (Hemiptera: Pentatomidae) using glass-vial bioassays. J. Econ. Entomol. 2008, 101, 1439–1442. [Google Scholar] [CrossRef] [PubMed]
- Biddinger, D.; Tooker, J.; Surcica, A.; Krawczyk, G. Survey of native biocontrol agents of the brown marmorated stink bug in Pennsylvania fruit orchards and adjacent habitat. Pa. Fruit News 2012, 42, 47–54. [Google Scholar]
- Lowenstein, D.L.; Andrews, H.; Rudolph, E.; Sullivan, E.; Marshall, C.M.; Wiman, N.G. Astata unicolor (Hymenoptera: Crabronidae) population in Oregon with observations of predatory behavior on Pentatomidae. Ann. Entomol. Soc. Am. 2018, 111, 122–126. [Google Scholar] [CrossRef]
- Skillman, V.P.; Lee, J.C. Nutrient content of brown marmorated stink bug eggs and comparisons between experimental uses. J. Insect Sci. 2017, 17, 120. [Google Scholar] [CrossRef]
- Van Handel, E. Rapid determination of glycogen and sugars in mosquitos. J. Am. Mosq. Control. Assoc. 1985, 1, 299–301. [Google Scholar] [PubMed]
- Van Handel, E. Rapid determination of total lipids in mosquitoes. J. Am. Mosq. Control. Assoc. 1985, 1, 302–304. [Google Scholar] [PubMed]
- Seagraves, M.P.; Kajita, Y.; Weber, D.C.; Obrycki, J.J.; Lundgren, J.G. Sugar feeding by coccinellids under field conditions: The effects of sugar sprays in soybean. BioControl 2011, 56, 305–314. [Google Scholar] [CrossRef]
- Tochen, S.; Walton, V.M.; Lee, J.C. Impact of floral feeding on adult Drosophila suzukii survival and nutrient status. J. Pest Sci. 2016, 89, 793–802. [Google Scholar] [CrossRef]
- Olson, D.M.; Fabamiro, H.; Lundgren, J.G.; Heimpel, G.E. Effects of sugar feeding on carbohydrate and lipid metabolism in a parasitoid wasp. Physiol. Entomol. 2000, 25, 17–26. [Google Scholar] [CrossRef]
- Fadamiro, H.Y.; Chen, L.I.; Onagbola, E.O.; Graham, L.F. Lifespan and patterns of accumulation and mobilization of nutrients in the sugar-fed phorid fly, Pseudacteon tricuspis. Physiol. Entomol. 2005, 30, 212–224. [Google Scholar] [CrossRef]
- Yuval, B.; Kaspi, R.; Shloush, S.; Warburg, M.S. Nutritional reserves regulate male participation in Mediterranean fruit fly leks. Ecol. Entomol. 1998, 23, 211–215. [Google Scholar] [CrossRef]
- SAS 9.3; SAS Institute Inc.: Cary, NC, USA, 2012.
- Wiman, N.G.; Walton, V.M.; Shearer, P.W.; Rondon, S.I.; Lee, J.C. Factors affecting flight capacity of brown marmorated stink bug, Halymorpha halys (Hemiptera: Pentotomidae). J. Pest Sci. 2015, 88, 37–47. [Google Scholar] [CrossRef]
- Lee, D.H.; Nielsen, A.L.; Leskey, T.C. Dispersal capacity and behavior of nymphal stages of Halyomorpha halys (Hemiptera: Pentatomidae) evaluated under laboratory and field conditions. J. Insect Behav. 2014, 27, 639–651. [Google Scholar] [CrossRef]
- Lee, D.H.; Leskey, T.C. Flight behavior of foraging and overwintering brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae). Bull. Entomol. Res. 2015, 105, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.L.; Hamilton, C.G.; Matadha, D. Developmental Rate Estimation and Life Table Analysis for Halyomorpha halys (Hemiptera: Pentatomidae). Environ. Entomol. 2008, 37, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, T.C.; Chatterjee, M. Seasonal changes in feeding, fat body and ovarian conditions of Nezara viridula L. (Heteroptera: Pentatomidae). Insect Sci. Appl. 1985, 6, 633–635. [Google Scholar] [CrossRef]
- Steele, J.E. Glycogen phosphorylase in insects. Insect Biochem. 1982, 12, 131–147. [Google Scholar] [CrossRef]
- Nielsen, A.L.; Hamilton, G.C. Life history of the invasive species Halyomorpha halys (Hemiptera: Pentatomidae) in Northeastern United States. Ann. Entomol. Soc. Am. 2009, 102, 608–616. [Google Scholar] [CrossRef]
| Nymphal | Aliquot of Supernatant or Glycogen Mixture | ||
|---|---|---|---|
| Instar | Lipid | Glycogen | Sugar |
| 5th | 50 µL | 50/100 µL | 50 µL |
| 4th | 50 µL | 100 µL | 50 µL |
| 3rd | 50/100 µL | 100/150 µL | 50/100 µL |
| 2nd | 150/250 µL | 200/400 µL | 150/250 µL |
| 1st | 300 µL | 500 µL | 300 µL |
| Amount Aliquoted | Lipid and Sugar Evaporation Time | Additional Anthrone Added to Glycogen Aliquot | Multiplier Based on Amount Aliquoted |
|---|---|---|---|
| 50 µL | 1 min | 950 µL | 20 |
| 100 µL | 2 min | 900 µL | 10 |
| 150 µL | 3 min | 850 µL | 6.667 |
| 200 µL | 4 min | 800 µL | 5 |
| 250 µL | 5 min | 750 µL | 4 |
| 300 µL | 6 min | 700 µL | 3.333 |
| 400 µL | 7 min | 600 µL | 2.5 |
| 500 µL | 8 min | 500 µL | 2 |
| Year | Measurement | Effects | Female | Male | ||||
|---|---|---|---|---|---|---|---|---|
| df | F | p | df | F | p | |||
| 2015 | Weight | Month | 4633 | 41.60 | <0.0001 | 4661 | 9.32 | <0.0001 |
| Nutrient Index | Month | 4633 | 30.83 | <0.0001 | 4661 | 71.82 | <0.0001 | |
| Lipid | Month | 4612 | 2.06 | 0.0844 | 4660 | 2.49 | 0.0419 | |
| Prothorax | 1612 | 0.59 | 0.4432 | 1660 | 2.53 | 0.1124 | ||
| Glycogen | Month | 4632 | 25.89 | <0.0001 | 4660 | 94.38 | <0.0001 | |
| Prothorax | 1632 | 0.04 | 0.8452 | 1660 | 6.62 | 0.0103 | ||
| Sugar | Month | 4632 | 4.50 | 0.0014 | 4660 | 38.59 | <0.0001 | |
| Prothorax | 1632 | 7.98 | 0.0049 | 1660 | 47.22 | <0.0001 | ||
| Egg | Month | 4632 | 280.85 | <0.0001 | . | . | . | |
| 2016 | Weight | Month | 5541 | 28.37 | <0.0001 | 5562 | 9.89 | <0.0001 |
| Nutrient Index | Month | 5541 | 53.59 | <0.0001 | 5562 | 0.88 | 0.4939 | |
| Lipid | Month | 5540 | 30.56 | <0.0001 | 5561 | 13.25 | <0.0001 | |
| Prothorax | 1540 | 4.26 | 0.0396 | 1561 | 1.38 | 0.2411 | ||
| Glycogen | Month | 5540 | 1.89 | 0.0943 | 5561 | 52.30 | <0.0001 | |
| Prothorax | 1540 | 3.19 | 0.0748 | 1561 | 10.50 | 0.0013 | ||
| Sugar | Month | 5540 | 1.89 | 0.0943 | 5561 | 24.28 | <0.0001 | |
| Prothorax | 1540 | 17.89 | <0.0001 | 1561 | 11.07 | 0.0009 | ||
| Egg | Month | 5540 | 39.41 | <0.0001 | . | . | . | |
| Year-Sex | Variable (x) | Variable (y) | df | F | p | r2 | Slope | y-Intercept |
|---|---|---|---|---|---|---|---|---|
| 2015-females | Egg load | Weight | 1620 | 278.30 | <0.0001 | 0.310 | 0.00242 | 0.175 |
| Egg load | Nutrient Index | 1620 | 201.10 | <0.0001 | 0.245 | 0.00314 | 0.274 | |
| Egg load | Lipid | 1620 | 7.73 | 0.0056 | 0.012 | −2.984 | 645.2 | |
| Egg load | Glycogen | 1620 | 4.43 | 0.0358 | 0.007 | −0.7696 | 148.6 | |
| Egg load | Sugar | 1620 | 1.32 | 0.251 | . | . | . | |
| 2015-males | Glycogen | Lipid | 1620 | 15.10 | <0.0001 | 0.024 | −0.452 | 696.2 |
| Sugar | Lipid | 1620 | 30.90 | <0.0001 | 0.048 | −0.220 | 735.5 | |
| Sugar | Glycogen | 1620 | 211.00 | <0.0001 | 0.254 | 0.173 | 62.43 | |
| Glycogen | Lipid | 1668 | 4.07 | 0.0442 | 0.006 | −0.305 | 696.4 | |
| Sugar | Lipid | 1668 | 20.70 | <0.0001 | 0.030 | −0.365 | 766.4 | |
| Sugar | Glycogen | 1668 | 392.00 | <0.0001 | 0.370 | 0.327 | 15.27 | |
| 2016-females | Egg load | Weight | 1549 | 219.50 | <0.0001 | 0.286 | 0.0018 | 0.176 |
| Egg load | Nutrient Index | 1549 | 277.10 | <0.0001 | 0.336 | 0.0030 | 0.259 | |
| Egg load | Lipid | 1549 | 2.48 | 0.116 | . | . | . | |
| Egg load | Glycogen | 1549 | 1.47 | 0.226 | . | . | . | |
| Egg load | Sugar | 1549 | 5.55 | 0.0188 | 0.01 | 2.20 | 452.65 | |
| 2016-males | Glycogen | Lipid | 1549 | 0.78 | 0.38 | . | . | . |
| Sugar | Lipid | 1549 | 21.20 | <0.0001 | 0.037 | −0.264 | 848.0 | |
| Sugar | Glycogen | 1549 | 161.00 | <0.0001 | 0.227 | 0.178 | 85.93 | |
| Glycogen | Lipid | 1571 | 0.01 | 0.959 | . | . | . | |
| Sugar | Lipid | 1571 | 27.20 | <0.0001 | 0.046 | −0.573 | 985.3 | |
| Sugar | Glycogen | 1571 | 522.00 | <0.0001 | 0.478 | 0.407 | 28.61 |
| Nymphal Instar | June | July | August | September | ||||
|---|---|---|---|---|---|---|---|---|
| 1st | 61.9% | 52 | 1.4% | 4 | . | . | . | . |
| 2nd | 38.1% | 32 | 43.4% | 124 | 6.9% | 12 | . | . |
| 3rd | . | . | 50.7% | 145 | 40.2% | 70 | . | . |
| 4th | . | . | . | . | 31.6% | 55 | 66.7% | 30 |
| 5th | . | . | 4.5% | 13 | 21.3% | 37 | 33.3% | 15 |
| Total | . | 84 | . | 286 | . | 174 | . | 45 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skillman, V.P.; Wiman, N.G.; Lee, J.C. Monitoring Nutrient Status of Brown Marmorated Stink Bug Adults and Nymphs on Summer Holly. Insects 2018, 9, 120. https://doi.org/10.3390/insects9030120
Skillman VP, Wiman NG, Lee JC. Monitoring Nutrient Status of Brown Marmorated Stink Bug Adults and Nymphs on Summer Holly. Insects. 2018; 9(3):120. https://doi.org/10.3390/insects9030120
Chicago/Turabian StyleSkillman, Victoria P., Nik G. Wiman, and Jana C. Lee. 2018. "Monitoring Nutrient Status of Brown Marmorated Stink Bug Adults and Nymphs on Summer Holly" Insects 9, no. 3: 120. https://doi.org/10.3390/insects9030120
APA StyleSkillman, V. P., Wiman, N. G., & Lee, J. C. (2018). Monitoring Nutrient Status of Brown Marmorated Stink Bug Adults and Nymphs on Summer Holly. Insects, 9(3), 120. https://doi.org/10.3390/insects9030120





