“Sleepers” and “Creepers”: A Theoretical Study of Colony Polymorphisms in the Fungus Metarhizium Related to Insect Pathogenicity and Plant Rhizosphere Colonization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Isolates
2.2. Sleepers and Creepers Morphology Characterization in PDA Medium
2.3. Sleepers and Creepers Morphology Characterization in Insects
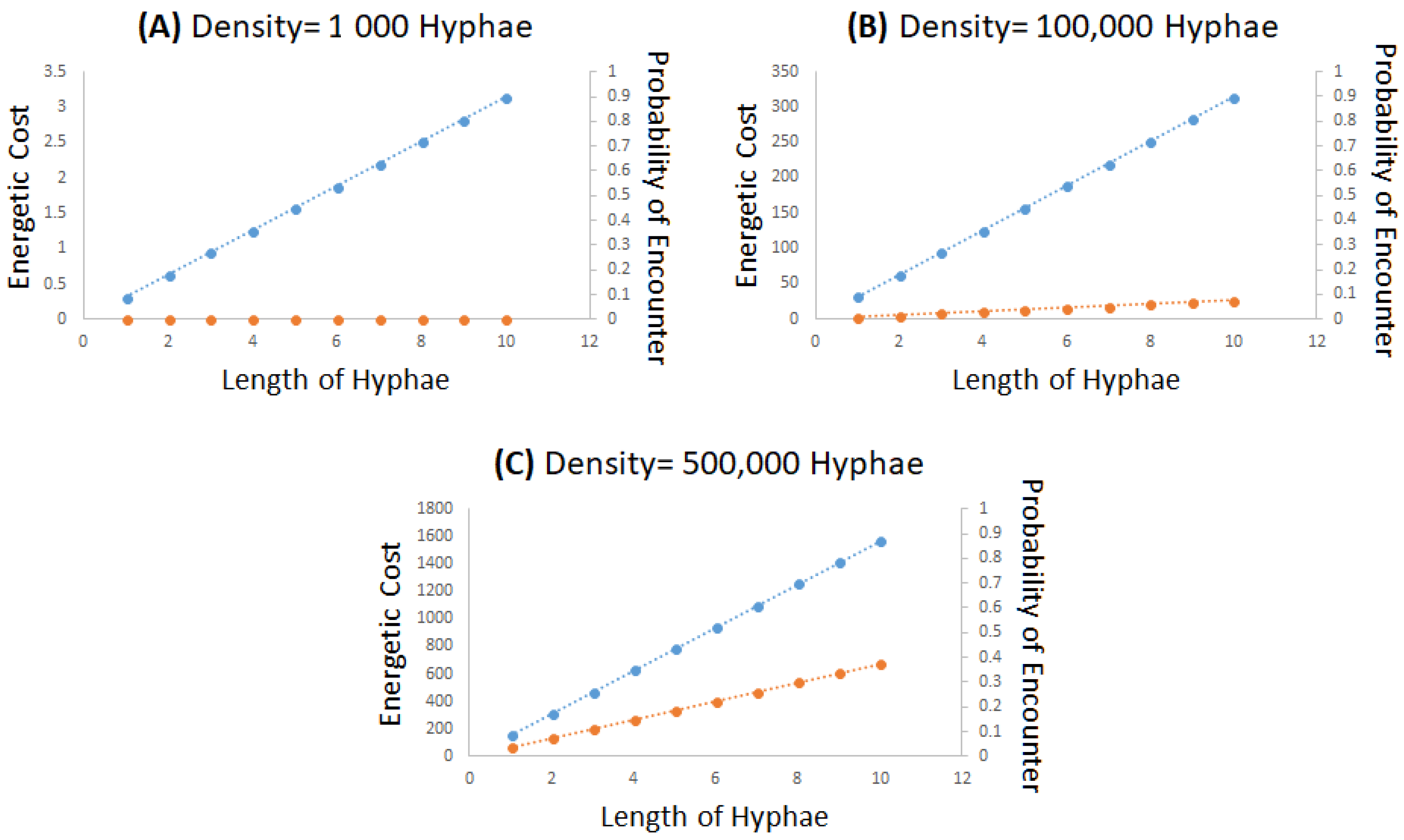
2.4. Estimation of Total Hyphal Volume
2.5. Effects of Passaging Creeper Phenotype
3. Results
3.1. Morphological Effect in Light and Darkness
3.2. Hypothetical Modeling
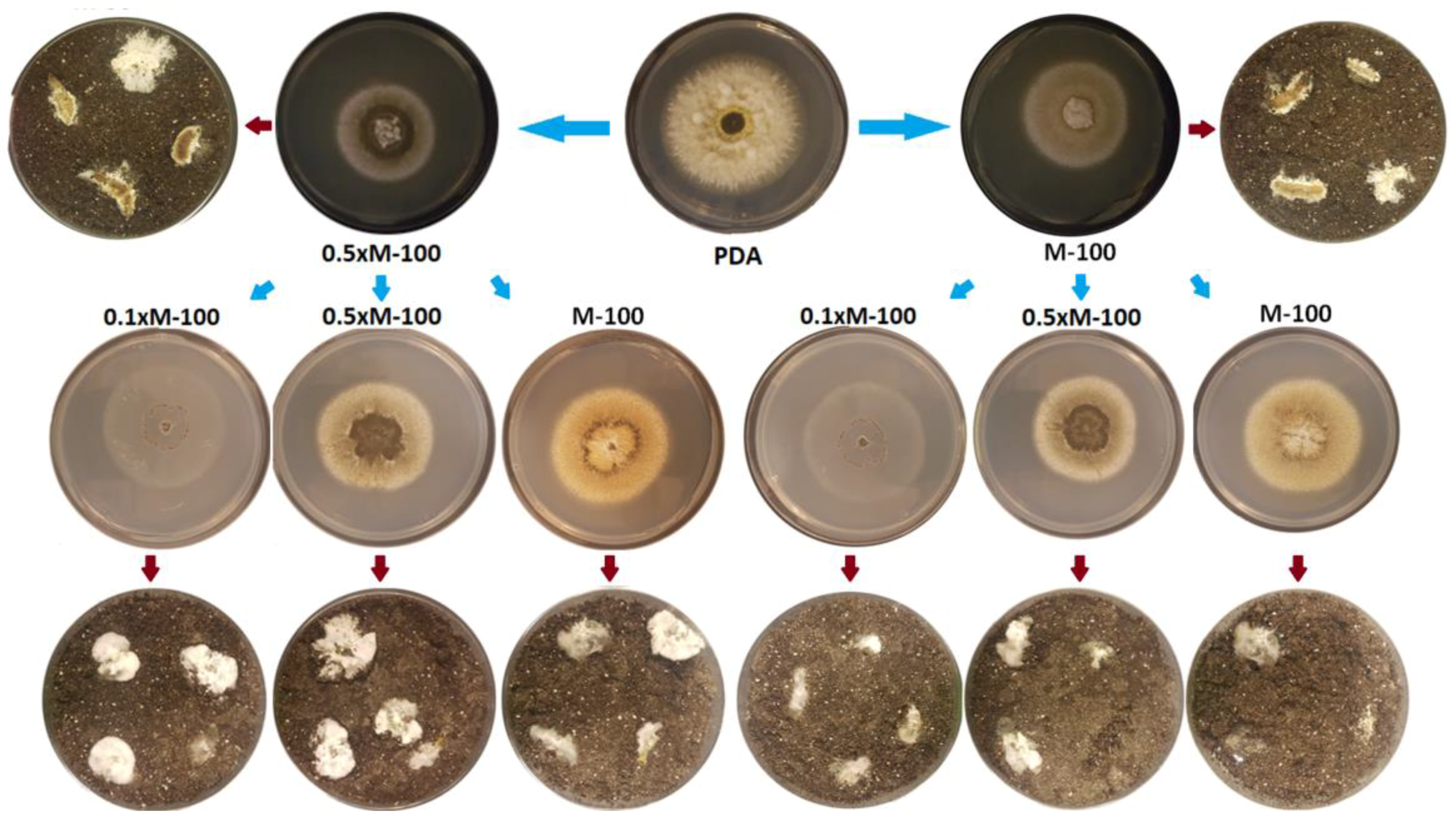
3.3. Minimal Media Effects on Creeper Phenotype
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liao, X.; O’Brien, T.; Fang, W.; St. Leger, R.J. The plant beneficial effects of Metarhizium species correlate with their association with roots. Appl. Microbiol. Biotechnol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Behie, S.W.; Jones, S.J.; Bidochka, M.J. Plant tissue localization of the endophytic insect pathogenic fungi Metarhizium and Beauveria. Fungal Ecol. 2015, 13, 112–119. [Google Scholar] [CrossRef]
- Kamp, A.M.; Bidochka, M.J. Conidia production by insect pathogenic fungi on commercially available agars. Lett. Appl. Microbiol. 2006, 35, 74–77. [Google Scholar] [CrossRef]
- Tangthirasunun, N.; Poeaim, S.; Soytong, K.; Sommartya, P.; Popoonsak, S. Variation in morphology and ribosomal DNA among isolates of Metarhizium anisopliae from Thailand. J. Agric. Technol. 2010, 6, 317–329. [Google Scholar]
- Simmons, A.M. Modes of response to environmental change and the elusive empirical evidence for bet hedging. Proc. Biol. Sci. 2011, 1712, 1601. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, J.F.; Rehner, S.A.; Humber, R.A. A Multilocus phylogeny of the Metarhizium anisopliae lineage. Mycologia 2009, 4, 512. [Google Scholar] [CrossRef]
- Levy, S.F.; Ziv, N.; Siegal, M.L. Bet hedging in yeast by heterogeneous, age-correlated expression of a stress protectant. PLoS Biol. 2012, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Meadows, R. Yeast survive by hedging their bets. PLoS Biol. 2012, 5, e1001327. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.K.; Smith, M.L.; Simons, A.M. Experimental evolution of bet hedging under manipulated environmental uncertainty in Neurospora crassa. Proc. Biol. Sci. 2014, 281, 20140706. [Google Scholar] [CrossRef] [PubMed]
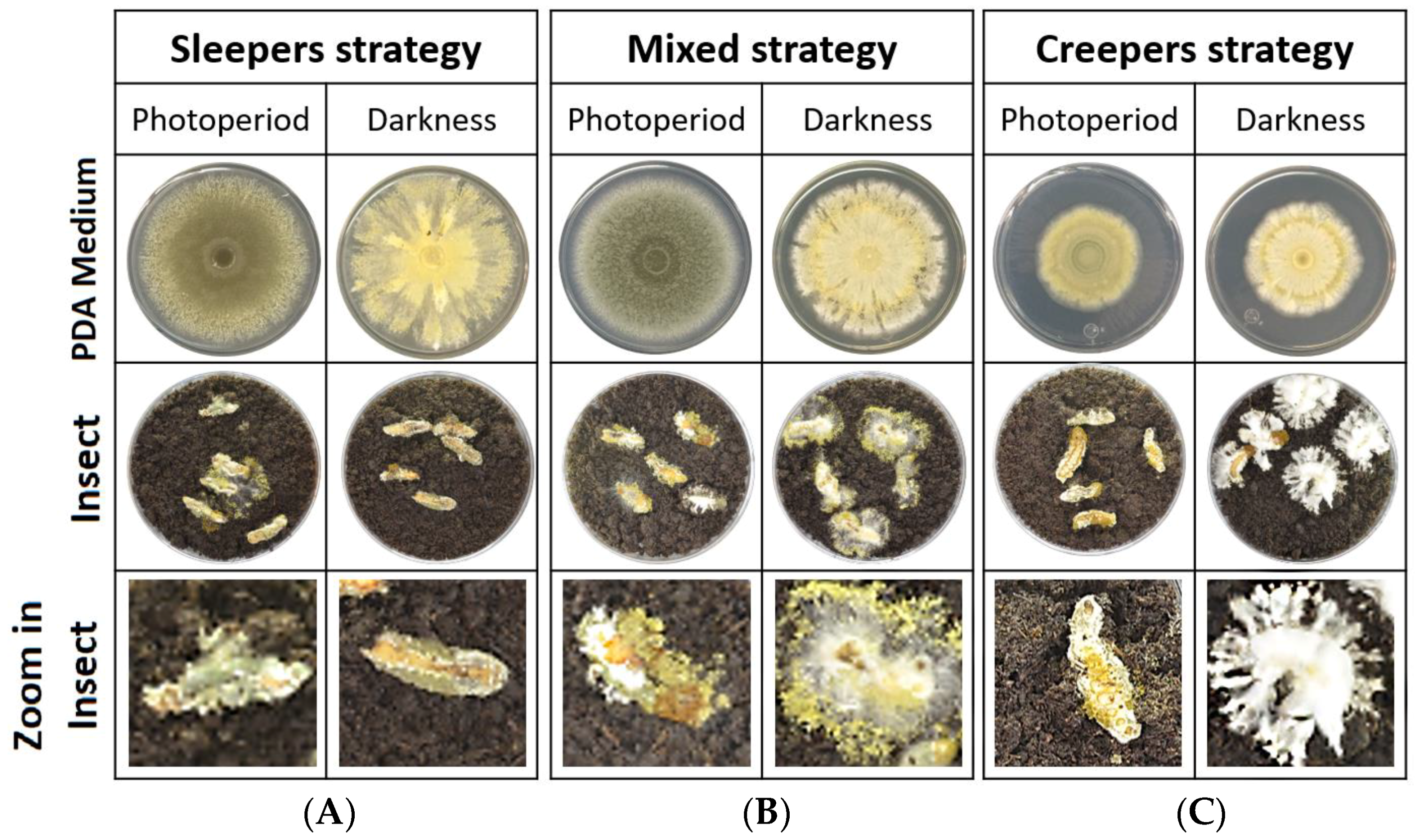

| Sleepers | Mixed Strategy | Creepers | |
|---|---|---|---|
| Isolates Canada | 3 (27.7%) | 4 (36.4%) | 4 (36.4%) |
| Isolates Mexico | 5 (41.6%) | 3 (25%) | 4 (33.3%) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelone, S.; Piña-Torres, I.H.; Padilla-Guerrero, I.E.; Bidochka, M.J. “Sleepers” and “Creepers”: A Theoretical Study of Colony Polymorphisms in the Fungus Metarhizium Related to Insect Pathogenicity and Plant Rhizosphere Colonization. Insects 2018, 9, 104. https://doi.org/10.3390/insects9030104
Angelone S, Piña-Torres IH, Padilla-Guerrero IE, Bidochka MJ. “Sleepers” and “Creepers”: A Theoretical Study of Colony Polymorphisms in the Fungus Metarhizium Related to Insect Pathogenicity and Plant Rhizosphere Colonization. Insects. 2018; 9(3):104. https://doi.org/10.3390/insects9030104
Chicago/Turabian StyleAngelone, Steven, Iván Horacio Piña-Torres, Israel Enrique Padilla-Guerrero, and Michael J. Bidochka. 2018. "“Sleepers” and “Creepers”: A Theoretical Study of Colony Polymorphisms in the Fungus Metarhizium Related to Insect Pathogenicity and Plant Rhizosphere Colonization" Insects 9, no. 3: 104. https://doi.org/10.3390/insects9030104
APA StyleAngelone, S., Piña-Torres, I. H., Padilla-Guerrero, I. E., & Bidochka, M. J. (2018). “Sleepers” and “Creepers”: A Theoretical Study of Colony Polymorphisms in the Fungus Metarhizium Related to Insect Pathogenicity and Plant Rhizosphere Colonization. Insects, 9(3), 104. https://doi.org/10.3390/insects9030104






