Estimation of Median Lethal Concentration of Three Isolates of Beauveria bassiana for Control of Megacopta cribraria (Heteroptera: Plataspidae) Bioassayed on Solid Lygus spp. Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Colonies of Megacopta cribraria
2.2. Fungal Isolates and Identification of KUDSC
2.3. Production of KUDSC Spore Powder
2.4. Bioassay Procedure
2.5. Statistical Analysis
3. Results
3.1. Verification of Identity of Fungal Isolate KUDSC
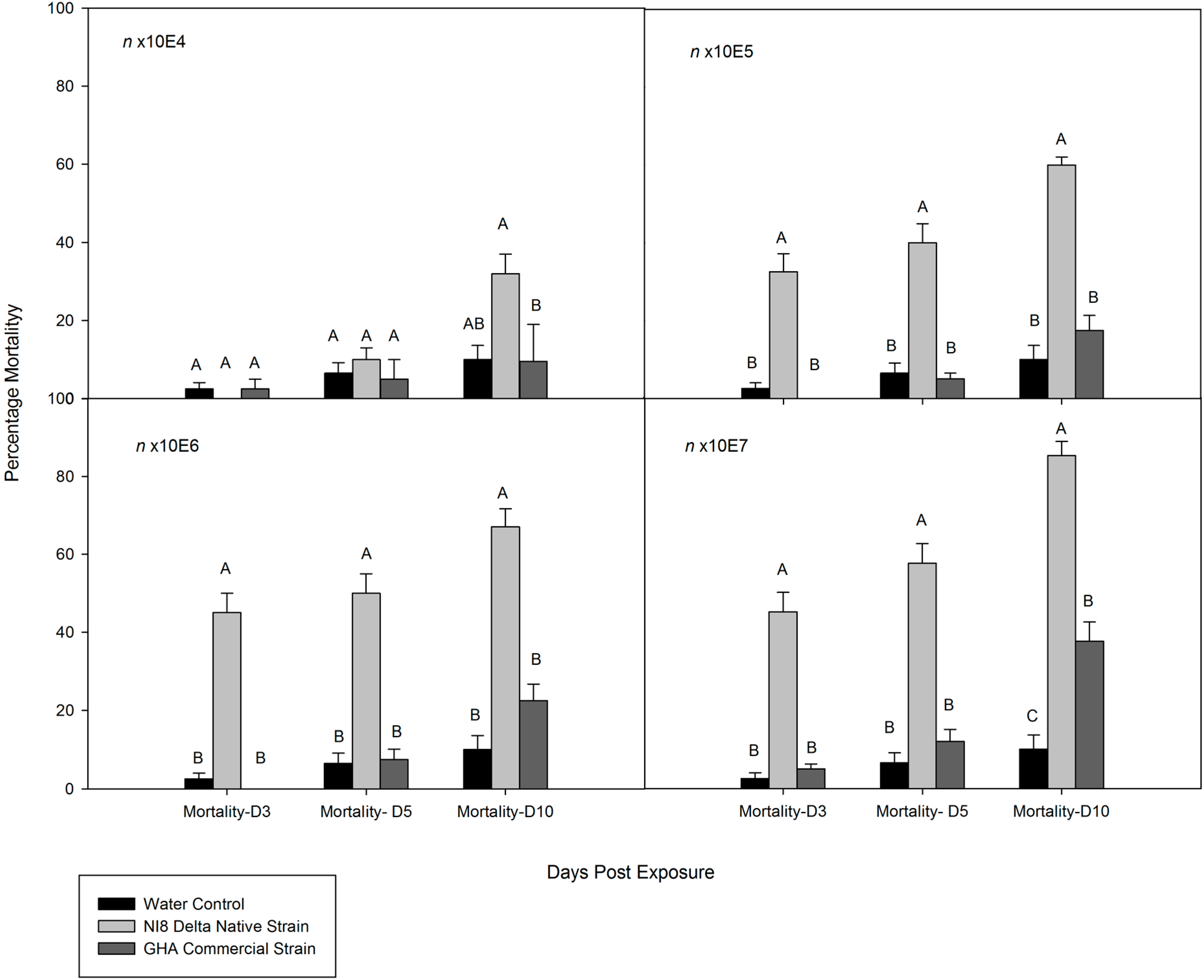
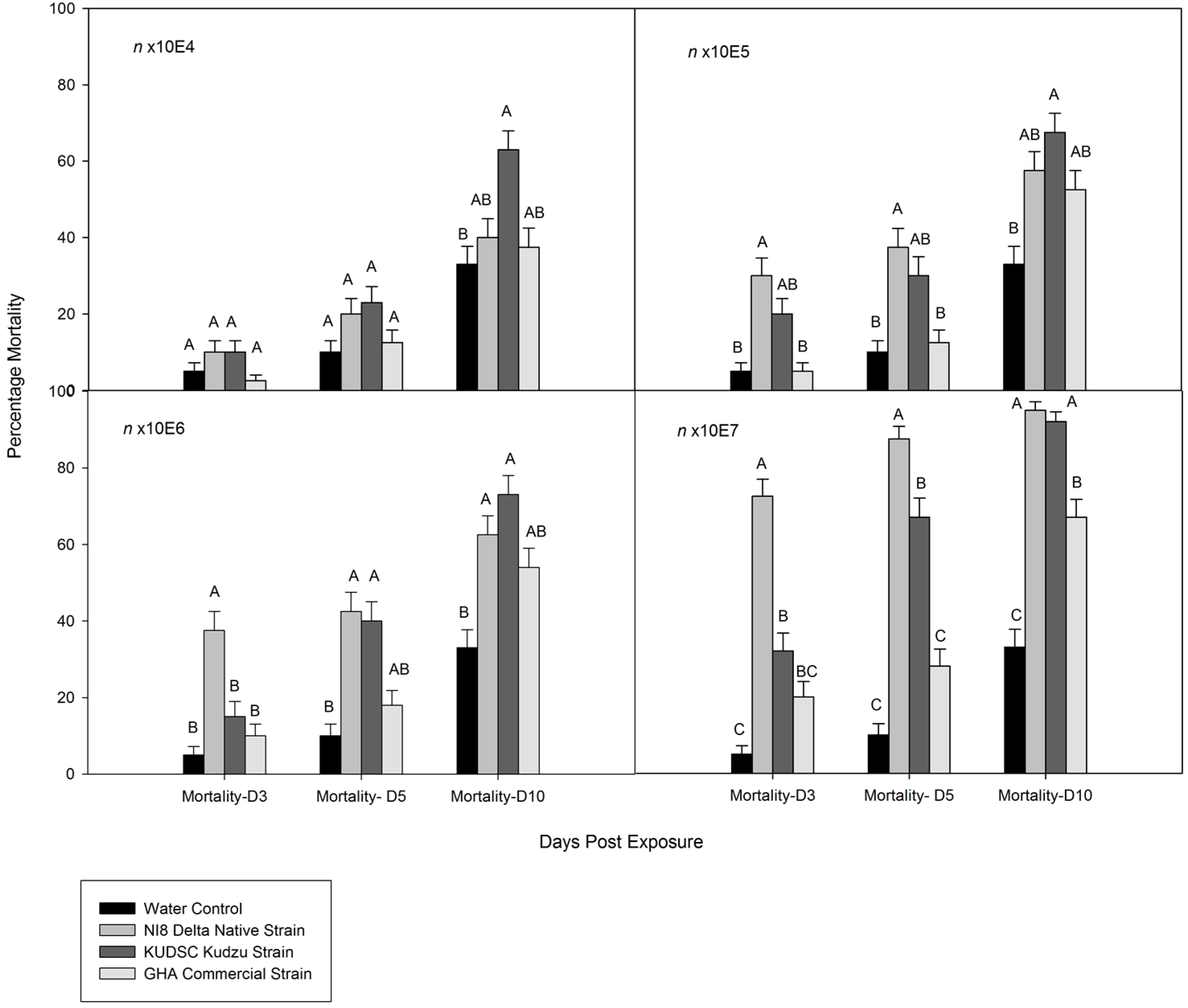
3.2. Dose-Mortality Response of M. cribraria to B. bassiana
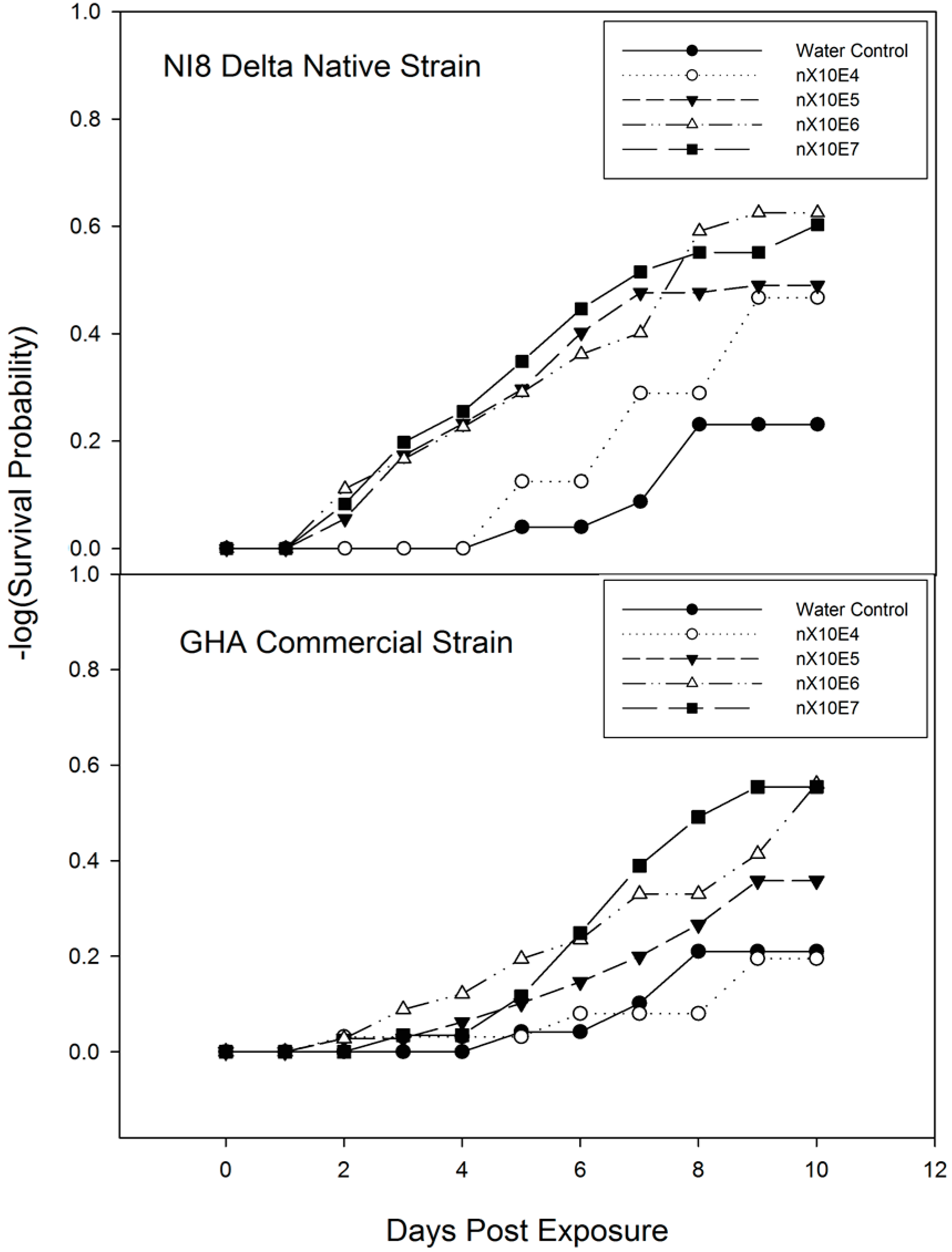
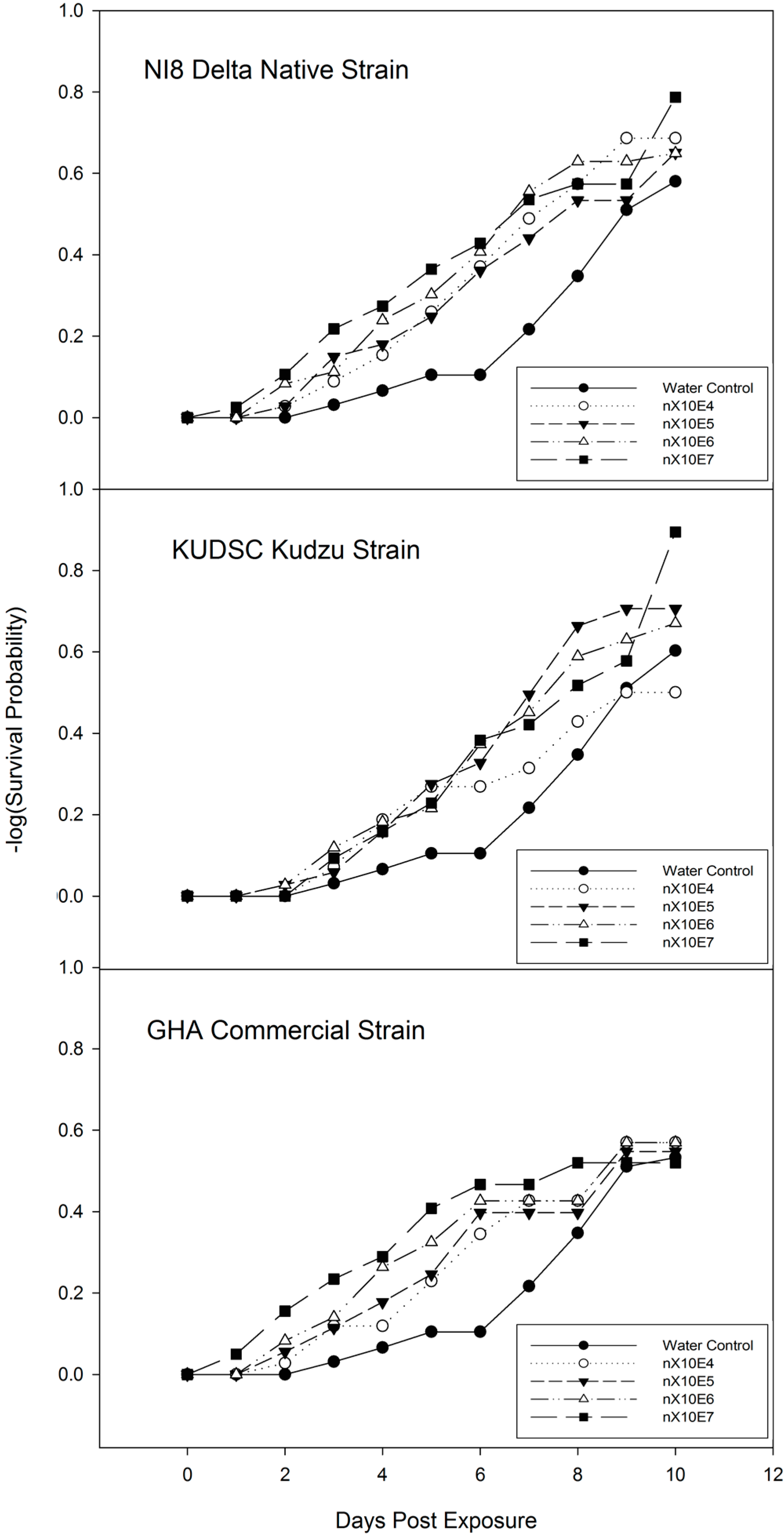
3.3. Time-Mortality Response of M. cribraria to B. bassiana
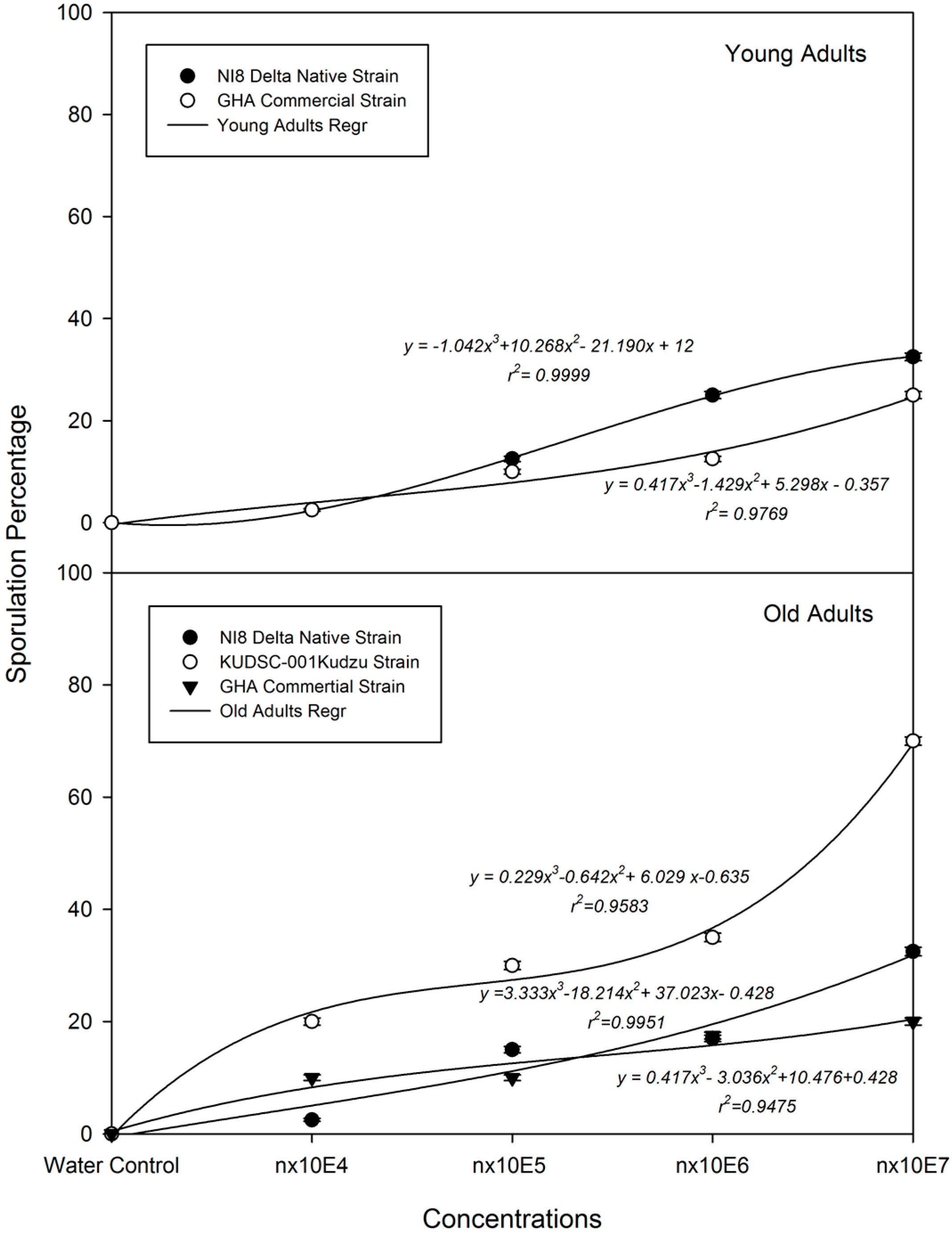
3.4. B. bassiana Dose-Sporulation response (LS50) on M. cribraria: Effects of Strain and Concentration
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Zhang, Y.; Hanula, J.L.; Horn, S. The biology and preliminary host range of Megacopta cribraria (Heteroptera: Plataspidae) and its impact on kudzu growth. Environ. Entomol. 2012, 41, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Eger, J.E., Jr.; Ames, L.M.; Suiter, D.R.; Jenkins, T.M.; Rider, D.A.; Halbert, S.E. Occurrence of the Old Word bug Megacopta cribraria (Fabricius) (Heteroptera: Plataspidae) in Georgia: A serious home invader and potential legume pest. Insecta Mundi 2010, 121, 1–11. [Google Scholar]
- Medal, J.; Halbert, S.; Santa Cruz, A. The bean plataspid, Megacopta cribraria (Hemiptera: Plataspidae), a new invader in Florida. Fla. Entomol. 2013, 96, 258–260. [Google Scholar] [CrossRef]
- Leslie, A.; Lamp, W.O. Maryland Kudzu Bug Survey. Available online: https://extension.umd.edu/news/photos/kudzu-bugs (accessed on 27 January 2014).
- Grant, J.; Alan, L.; Lamp, B. Kudzu bug, a potential soybean pest, survives the harsh winter. Available online: https://extension.umd.edu/sites/default/files/_docs/newsletters/Sept_16_2014.pdf (accessed on 27 March 2016).
- Wang, C.; Powell, J.E. Isolation and evaluation of Beauveria bassiana for control of Coptotermes formosanus and Reticulitermes flavipes (Isoptera: Rhinotermitidae). J. Sociobiol. 2002, 41, 1–13. [Google Scholar]
- Xing, G.N.; Zhao, T.J.; Gai, J.Y. Evaluation of soybean germplasm in resistance to globular stink bug [Megacopta cribraria (Fabricius)]. Acta Agronom. Sinica 2006, 32, 491–496. [Google Scholar]
- Seiter, N.J.; Grabke, A.; Greene, J.K.; Kerrigan, J.L.; Reay-Jones, F. Beauveria bassiana is a pathogen of Megacopta cribraria (Hemiptera: Plataspidae) in South Carolina. J. Entomol. Sci. 2014, 49, 326–330. [Google Scholar] [CrossRef]
- Seiter, N.J.; Benson, E.P.; Reay-Jones, F.P.F.; Greene, J.K.; Zungoli, P.A. Residual efficacy of insecticides applied to exterior building material surface for control of nuisance infestations of Megacoptera cribraria (Hemiptera: Plataspidae). J. Econ. Entomol. 2013, 106, 2448–2456. [Google Scholar] [CrossRef] [PubMed]
- Gardner, W.A.; Blount, J.L.; Golec, J.R.; Jones, W.A.; Hu, X.P.; Talamas, E.J.; Evans, R.M.; Dong, X.; Ray, C.H., Jr.; Buntin, G.D.; et al. Discovery of Paratelenomus saccharalis (Dodd) (Hymenoptera: Platygastridae), an egg parasitoid of Megacopta cribraria F. (Hemiptera: Plataspidae) in its expanded North American Range. J. Entomol. Sci. 2013, 48, 355–359. [Google Scholar] [CrossRef]
- Golec, J.R.; Hu, X.P.; Ray, C.; Woodley, N.E. Strongygaster triangulifera (Diptera: Tachinidae) as a parasitoid of adults of the invasive Megacopta cribraria (Heteroptera: Plataspidae) in Alabama. J. Entomol. Sci. 2013, 48, 352–354. [Google Scholar] [CrossRef]
- Stubbins, F.L.; Agudelo, P.; Reay-Jones, F.P.F.; Greene, J.K. First report of a mermithid nematode infecting the invasive Megacopta cribraria (Hemiptera: Plataspidae) in the United States. J. Invertebr. Pathol. 2015, 127, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Ruberson, J.R.; Takasu, K.; Buntin, G.D.; Eger, J.E., Jr.; Gardner, W.A.; Greene, J.K.; Jenkins, T.M.; Jones, W.A.; Olson, D.M.; Roberts, P.M.; et al. From Asia curiosity to eruptive American pest: Megacopta cribraria (Hemiptera: Plataspidae) and prospects for its biological control. Appl. Entomol. Zool. 2013, 48, 3–13. [Google Scholar] [CrossRef]
- Borah, B.K.; Dutta, S.K. Entomogenous fungus, Beauveria bassiana (Balsamo) Vuillemin: A natural biocontrol agent against Megacopta cribraria (Fab.). Insect Environ. 2002, 8, 7–8. [Google Scholar]
- Seiter, N.J.; Greene, J.K.; Reay-Jones, F.P.F. Reduction of soybean yield components by Megacopta cribraria (Hemiptera: Plataspidae). J. Econ. Entomol. 2013, 106, 1676–1683. [Google Scholar] [PubMed]
- Leland, J.E.; Sonodgrass, G.L. Prevalence of naturally occuring Beauveria bassiana in Lygus lineolaris (Heteroptera: Miridae) population from wild host plants of Mississippi. J. Agric. Urban Entomol. 2004, 21, 157–163. [Google Scholar]
- McGuire, M.R.; Ulloa, M.; Park, Y.H.; Hudson, N. Biological and molecular characteristics of Beauveria bassiana isolates from California Lygus hesperus (Hemiptera: Miridae) populations. Biol. Control 2005, 33, 307–314. [Google Scholar] [CrossRef]
- Portilla, M. Biological control as an alternative measure for TPB in Mississippi. Midsouth Entomol. 2014, 7, 38–46. [Google Scholar]
- Portilla, M.; Snodgrass, G.; Luttrell, R. A novel bioassay to evaluate the potential of Beauveria bassiana strain NI8 and the insect growth regulator novaluron against Lygus lineolaris on a non-autoclaved solid artificial diet. J. Insect. Sci. 2014, 14, 1–13. [Google Scholar]
- Portilla, M.; Snodgrass, G.; Luttrell, R. Effects of morning and night applications of Beauveria. bassiana strains NI8 and GHA against the tarnished plant bug in cotton. In Proceedings of Beltwide Cotton Conference, New Orleans, LA, USA, 5–8 January 2014; pp. 729–734.
- Leland, J.E.; Behle, R.W. Coating Beauveria bassiana, with lignin for protection for solar radiation and effects on pathogenicity to Lygus lineolaris. Biocontrol Sci. Technol. 2005, 15, 309–320. [Google Scholar]
- Leland, J.E.; McGuire, M.R.; Grace, J.A.; Jaronski, S.T.; Ulloa, M.; Park, Y.-H.; Plattner, R.D. Strain selection of a fungal entomopathogen, Beauveria bassiana, for control of plant bugs (Lygus spp.) (Heteroptera: Miridae). Biol. Control 2005, 35, 104–114. [Google Scholar] [CrossRef]
- Humber, R.A. Fungi-Identification. In Manuals of Techniques in Insect Pathology; Lacey, L.A., Ed.; Acad. Press: Cambridge, MA, USA, 1997; pp. 153–185. [Google Scholar]
- Rehner, S.A.; Buckley, E. A Beauveria Phylogeny inferred from nuclear ITS and EF1-alpha sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 2005, 97, 84–98. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute. SAS/STAT User’s Manual, version 9.4 ed.; SAS Institute: Cary, NC, USA, 2013. [Google Scholar]
- Velez, P.E.; Posada, F.J.; Marin, P.; Bustillo, A.; Gonzales, T.; Osorio, E. Tecnicas para el control de calidad de formulations de hongos entomopatogenos. Boletin Tecnico. Cenicafe 1997, 17, 37. (In Spanish) [Google Scholar]
- Fargues, J. Spécificité des chamgignons pathogenès imparfaits (Hyphomycètes) pour les larvas de Coléopterès (Scarabaeidae et Crysomelidae). Entomophaga 1976, 21, 313–323. (In French) [Google Scholar] [CrossRef]
- Ugine, T.A.; Wraight, S.P.; Brownbridge, M.; Sanderson, J.P. Development of a novel bioassay for estimation of median lethal concentrations (LC50) and doses (LD50) of the entomopathogenic fungus Beauveria bassiana, against wester flower thrips, Frankliniella occidentalis. J. Invertebr. Pathol. 2005, 89, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Portilla, M.; Jones, W.A.; Perera, O.P.; Seiter, N.; Greene, J.; Luttrell, R. Evaluation of Beauveria bassiana strains as potential agents for control of Megacopta cribraria (Heteroptera: Plataspidae). Available online: http://midsouthentomologist.org.msstate.edu/pdfs/Vol9_1/Vol9No1MEAAbstracts.pdf (accessed on 27 March 2016).
- Inglis, G.D.; Goettel, M.S.; Butt, T.M.; Strasser, H. Use of hyphomycetous fungi for managing insect pests. In Fungi as Biocontrol Agents Progress, Problems and Potential; Butt, T.M., Jackson, C., Magan, N., Eds.; CABI Publishing: Oxforshire, UK, 2001; pp. 23–69. [Google Scholar]
- Steinkraus, D.C. Control of tarnished plant bug with Beauveria bassiana and interactions with imidacloprid. Environ. Entomol. 1996, 2, 888–889. [Google Scholar]
- Noma, T.; Strickler, K. Factors affecting Beauveria bassiana for control of Lygus bug (Hemiptera: Miridae) in alfalfa seed fields. J. Agric. Urban Entomol. 1999, 16, 215–233. [Google Scholar]
- Noma, T.; Strickler, K. Effects of Beauveria bassiana on Lygus hesperus (Hemiptera: Miridae) feeding and oviposition. Environ. Entomol. 2000, 2, 394–402. [Google Scholar] [CrossRef]
- McGuire, M.R.; Leland, J.E.; Dara, S.; Park, Y.; Ulloa, M. Effect of different isolates of Beauveria bassiana on field population of Lygus hesperus. Biol. Control 2006, 38, 390–396. [Google Scholar] [CrossRef]
- Romana, C.A.; Fargues, J. Relative susceptibility of different stages of Rhodnus. prolixus to the entomopathogenic hyphomycete Beauveria bassiana. Mem. Inst. Oswaldo Cruz 1992, 87, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Todovora, S.I.; Cloutier, C.; Cote, J.C.; Coderre, D. Pathogenicity of six isolates of Beauveria bassiana (Balsamo) Vuillemin (Deuteromycotina, Hyphomycetes) to Perillus bioculatus (F.) (Hem.: Pentatomidae). J. Appl. Entomol. 2002, 126, 182–185. [Google Scholar]
- Feng, Z.; Carruthers, R.I.; Roberts, D.W.; Robson, D.S. Age-specific dose mortality effects of Beauveria bassiana (Deuteromycotina: Hyphomycetes) on the European corn borer, Ostrinia nubilalis (Lepidoptera: Pyralidae). J. Invertebr. Pathol. 1985, 46, 259–264. [Google Scholar] [CrossRef]
- Hu, W.J.; Hou, R.F.; Talekar, N.S. Pathogenicity of Beauveria bassiana to Riptortus linearis (Hemiptera: Coreidae), a pest of soybean. Appl. Entomol. Zool. 1996, 31, 187–194. [Google Scholar]
- Grimm, C.; Guharay, F. Potential of entomopathogenous fungi for the biological control of true bugs in Jatropha curcas L. plantations in Nicaragua. In Biofuels and Industrial Products from Jatropha curcas; Gübitz, G., Mittelbach, M., Trabi, M., Eds.; Dbv-Verlag: Graz, Austria, 1997; pp. 40–46. [Google Scholar]
- Vandenberg, J.D.; Ramos, M.; Altre, J.A. Dose-response and age- and temperature-related susceptibility of the diamondblack moth (Lepidoptera: Plutellidae) to two isolates of Beauveria bassiana (Hyphomycetes: Moniliaceae). Environ. Entomol. 1998, 27, 1017–1021. [Google Scholar] [CrossRef]
- Todovora, S.I.; Cote, J.-C.; Coderre, D. Evaluation of the effects of two Beauveria bassiana (Balsamo) Vuillemin strains on the development of Coleomegilla maculata lengi Timberlake (Col., Coccinellidae). J. Appl. Entomol. 1996, 120, 159–163. [Google Scholar]
- Al mazra’awi, M.S. Impact of the entomophatogenic fungus Beauveria bassiana on the honey bee, Apis mellifera (Hymenoptera: Apidae). J. Agric.Sci. 2007, 3, 7–11. [Google Scholar]
- Burdeos, A.T.; Gabriel, B.P. Virulence of different Metarhizium anisopliae (Metch.) Sorokin isolates against the rice bug, Leptocorisa oratorius Fabr. (Hemiptera: Alydidae). Philipp. Entomol. 1995, 9, 467–478. [Google Scholar]
- Leite, L.G.; Fraga, A.I.A.; Alves, S.B. Pathogenicidade de Beauveria bassiana (Bals.) Vuill e Paecilomyces sp. sobre Nezara viridula L. J. Ecossistema 1987, 12, 20–24. (In Portuguese) [Google Scholar]
| Strain | Spores/g | Spores/mL | Spores/mm2 (n ± SD) | |
|---|---|---|---|---|
| Total | Viable | |||
| NI8 Native Delta Strain | 1.20 × 1011 | 7.02 × 107 | 395 ± 107 a | 375 ± 100 a |
| GHA Commercial Strain | 1.18 × 1011 | 6.95 × 107 | 356 ± 74 a | 339 ± 69 a |
| KUDSC South Carolina Kudzu Strain | 1.19 × 1011 | 6.90 × 107 | 365 ± 97 a | 347 ± 91 a |
| Insect Age—Strains | Dose-Mortality Response (spores/mm2) | ||||||
|---|---|---|---|---|---|---|---|
| Slope ± SE | LC50 (95% CI) | Probit Trend | Dose-Ratio | ||||
| Test for Slope 1 | Test for GoF 2 | ||||||
| X2 | p > X2 | X2 | p > X2 | ||||
| Young—NI8 3 | 0.207 ± 0.044 | 4.989 (0.312—40.945) | 12.72 | 0.0004 | 22.08 | <0.0001 | 1 |
| Young—GHA 4 | 0.186 ± 0.069 | 4663 (427.812—5.885^10) | 7.27 | 0.0070 | 0.977 | 0.4652 | 934 |
| Old—NI8 | 0.241 ± 0.048 | 4.363 (0.404—26.356) | 25.61 | <0.0001 | 0.918 | 0.521 | 0.87 |
| Old—GHA | 0.119 ± 0.043 | 1.979 (0.0006—84.391) | 7.53 | 0.0061 | 0.131 | 0.9997 | 0.40 |
| Old—KUDSC-001 5 | 0.117 ± 0.041 | 0.830 (0.0001—32.827) | 7.81 | 0.0052 | 1.529 | 0.113 | 0.20 |
| Insect Age—Strains | Dose-Sporulation Response (spores/mm2) | ||||||
|---|---|---|---|---|---|---|---|
| Slope ± SE | LS50 (95% CI) | Probit Trend | Dose-Ratio | ||||
| Test for Slope 1 | Test for GoF 2 | ||||||
| X2 | p > X2 | X2 | p > X2 | ||||
| Young—NI8 3 | 0.301 ± 0.084 | 15338 (491.539–5230) | 12.72 | 0.0004 | 0.58 | 0.8468 | 1 |
| Young—GHA 4 | 0.282 ± 0.101 | 19020 (430.750–8.599^10) | 7.77 | 0.0053 | 1.618 | 0.0864 | 1.24 |
| Old—NI8 | 0.311 ± 0.089 | 1617 (141.446–3692) | 12.18 | 0.0005 | 0.629 | 0.0805 | 0.11 |
| Old—GHA | 0.124 ± 0.084 | 16780 (–) | 2.18 | 0.1394 | 1.657 | 0.0764 | 1.09 |
| Old—KUDSC-001 5 | 0.249 ± 0.056 | 64.3885(7.599–720.2412) | 19.66 | <0.0001 | 1.423 | 0.1545 | 0.004 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portilla, M.; Jones, W.; Perera, O.; Seiter, N.; Greene, J.; Luttrell, R. Estimation of Median Lethal Concentration of Three Isolates of Beauveria bassiana for Control of Megacopta cribraria (Heteroptera: Plataspidae) Bioassayed on Solid Lygus spp. Diet. Insects 2016, 7, 31. https://doi.org/10.3390/insects7030031
Portilla M, Jones W, Perera O, Seiter N, Greene J, Luttrell R. Estimation of Median Lethal Concentration of Three Isolates of Beauveria bassiana for Control of Megacopta cribraria (Heteroptera: Plataspidae) Bioassayed on Solid Lygus spp. Diet. Insects. 2016; 7(3):31. https://doi.org/10.3390/insects7030031
Chicago/Turabian StylePortilla, Maribel, Walker Jones, Omaththage Perera, Nick Seiter, Jeremy Greene, and Randall Luttrell. 2016. "Estimation of Median Lethal Concentration of Three Isolates of Beauveria bassiana for Control of Megacopta cribraria (Heteroptera: Plataspidae) Bioassayed on Solid Lygus spp. Diet" Insects 7, no. 3: 31. https://doi.org/10.3390/insects7030031
APA StylePortilla, M., Jones, W., Perera, O., Seiter, N., Greene, J., & Luttrell, R. (2016). Estimation of Median Lethal Concentration of Three Isolates of Beauveria bassiana for Control of Megacopta cribraria (Heteroptera: Plataspidae) Bioassayed on Solid Lygus spp. Diet. Insects, 7(3), 31. https://doi.org/10.3390/insects7030031








