Tri-Tek (Petroleum Horticultural Oil) and Beauveria bassiana: Use in Eradication Strategies for Bemisia tabaci Mediterranean Species in UK Glasshouses
Abstract
:1. Introduction
2. Experimental Section
2.1. Products and Insect Cultures
2.2. Efficacy against Bemisia tabaci Mediterranean Eggs
2.3. Efficacy against Bemisia tabaci Mediterranean 2nd Instars
2.4. Analysis of Data
3. Results and Discussion
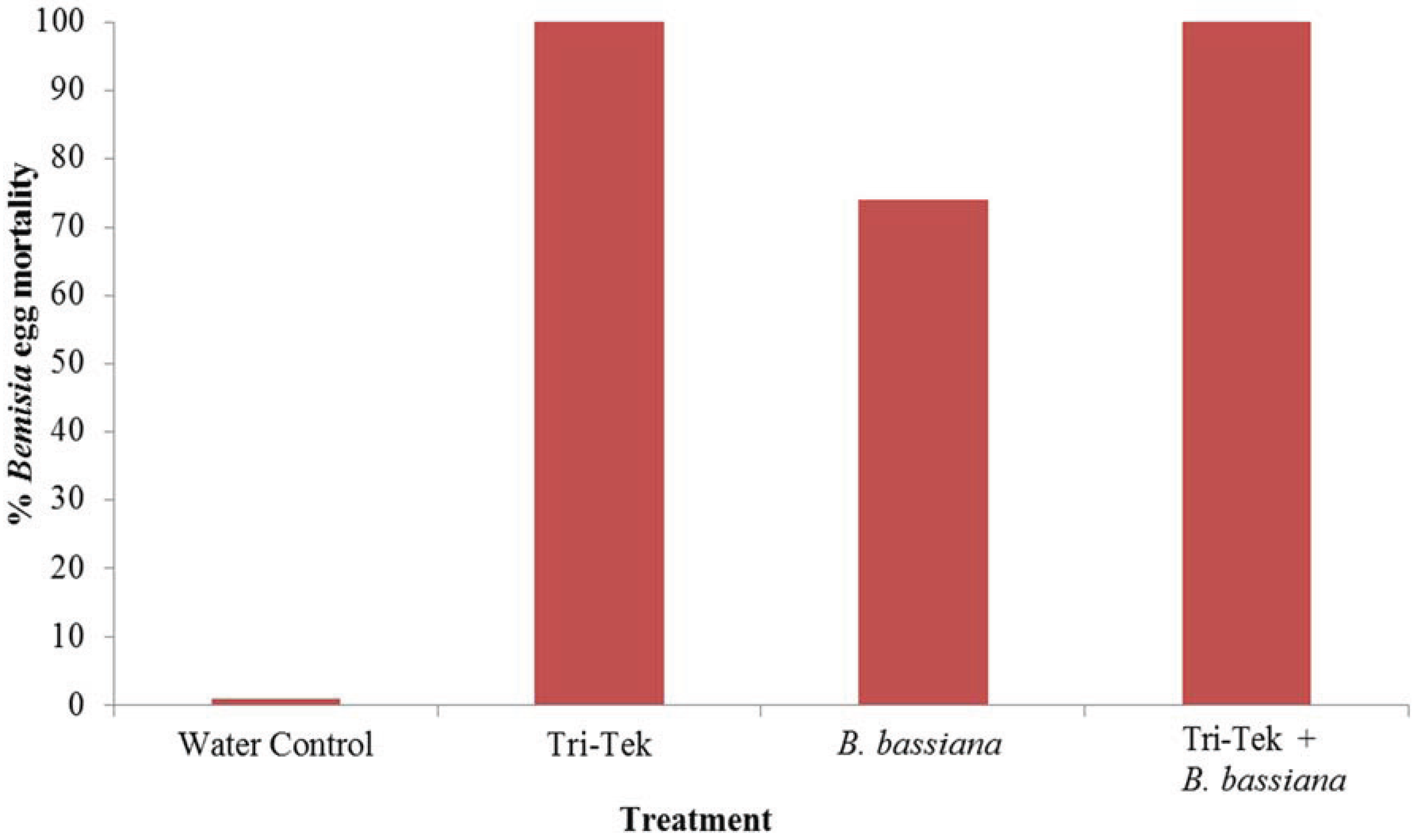

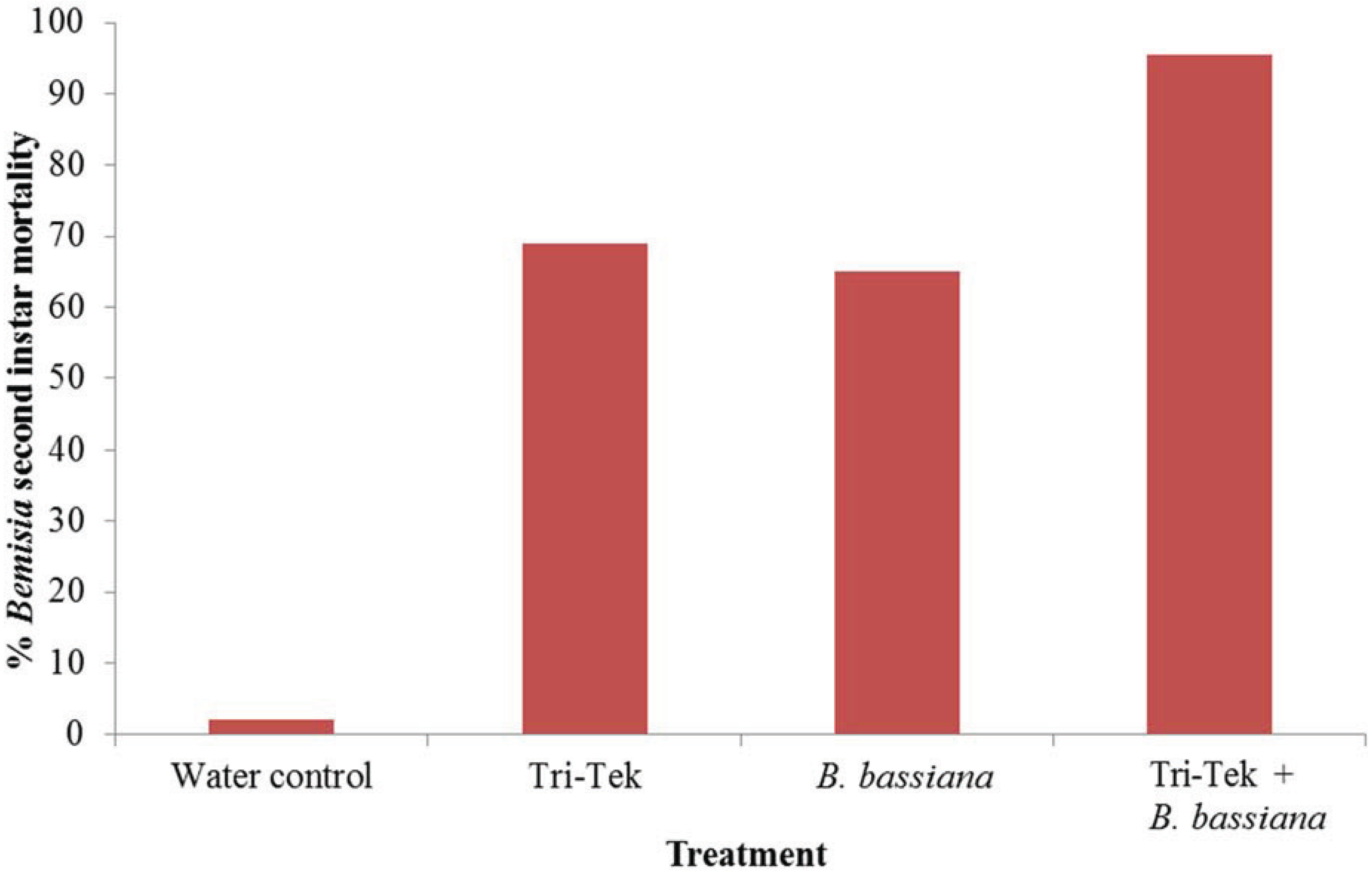
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Landa, Z.; Osborne, L.; Lopez, F.; Eyal, J. A bioassay for determining pathogenicity of entomogenous fungi on whiteflies. Biol. Control 1994, 4, 341–350. [Google Scholar] [CrossRef]
- Nomikou, M.; Janssen, A.; Schraag, R.; Sabelis, M.W. Phytoseiid predators as potential biological control agents for Bemisia tabaci. Exp. Appl. Acarol. 2001, 25, 271–291. [Google Scholar] [CrossRef] [PubMed]
- Gerling, D.; Motro, U.; Horowitz, R. Dynamics of Bemisia tabaci (Gennadius) (Homoptera: Aleyrodidae) attacking cotton in the coastal plain of Israel. Bull. Entomol. Res. 1980, 70, 213–219. [Google Scholar] [CrossRef]
- Osborne, L.S.; Landa, Z. Biological control of whiteflies with entomopathogenic fungi. Fla. Entomol. 1992, 75, 456–471. [Google Scholar]
- Gerling, D.; Mayer, R.T. (Eds.) Bemisia: Taxonomy, Biology, Damage, Control and Management; Intercept Limited: Andover, UK, 1996.
- Dinsdale, A.; Cook, L.; Riginos, C.; Buckley, Y.M.; De Barro, P. Refined global analysis of Bemisia tabaci (Hemiptera: Sternorrhyncha: Aleyrodoidea: Aleyrodidae) mitochondrial cytochrome oxidase 1 to identify species level genetic boundaries. Ann. Entomol. Soc. Am. 2010, 103, 196–208. [Google Scholar] [CrossRef]
- De Barro, P.J.; Liu, S.S.; Boykin, L.M.; Dinsdale, A.B. Bemisia tabaci: A statement of species status. Ann. Rev. Entomol. 2011, 56, 1–19. [Google Scholar]
- Alegbejo, M.D. Whitefly transmitted plant viruses in Nigeria. J. Sustain. Agric. 2000, 17, 99–109. [Google Scholar] [CrossRef]
- Cuthbertson, A.G.S.; Blackburn, L.F.; Eyre, D.P.; Cannon, R.J.C.; Millar, J.; Northing, P. Bemisia tabaci: The current situation in the UK and the prospect of developing strategies for eradication using entomopathogens. Insect Sci. 2011, 18, 1–10. [Google Scholar] [CrossRef]
- Cuthbertson, A.G.S. Update on the status of Bemisia tabaci in the UK and the use of entomopathogenic fungi within eradication programmes. Insects 2013, 4, 198–205. [Google Scholar] [CrossRef]
- Cuthbertson, A.G.S.; Buxton, J.H.; Blackburn, L.F.; Mathers, J.J.; Robinson, K.; Powell, M.E.; Fleming, D.A.; Bell, H.A. Eradicating Bemisia tabaci Q on poinsettia plants in the UK. Crop Prot. 2012, 42, 42–48. [Google Scholar] [CrossRef]
- Powell, M.E.; Cuthbertson, A.G.S.; Boonham, N.; Morris, J.; Bell, H.A.; Northing, P. First record of the Q Biotype of the sweetpotato whitefly, Bemisia tabaci, intercepted in the UK. Eur. J. Plant Pathol. 2012, 133, 797–801. [Google Scholar] [CrossRef]
- Prabhaker, N.; Coudriet, D.L.; Meyerdirk, D.E. Insecticide resistance in the sweetpotato whitefly, Bemisia tabaci (Homoptera: Aleyrodidae). J. Econ. Entomol. 1985, 78, 748–752. [Google Scholar] [CrossRef]
- Sharaf, N. Chemical control of Bemisia tabaci. Agric. Ecol. Environ. 1986, 17, 111–127. [Google Scholar] [CrossRef]
- Cahill, M.; Byrne, F.J.; Denholm, I.; Devonshire, A.L.; Gorman, K.J. Insecticide resistance in Bemisia tabaci. Pest Sci. 1994, 42, 137–139. [Google Scholar]
- Cahill, M.; Gorman, K.; Day, S.; Denholm, I.; Elbert, A.; Nauen, R. Baseline determination and detection of resistance to imidacloprid in Bemisia tabaci (Homoptera: Aleyrodidae). Bull. Entomol. Res. 1996, 86, 343–349. [Google Scholar] [CrossRef]
- Ahmad, M.; Arif, M.I.; Ahmad, Z.; Denholm, I. Cotton whitefly (Bemisia tabaci) resistance to organophosphate and pyrethroid insecticides in Pakistan. Pest Manag. Sci. 2002, 58, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Buxton, J.; Clarke, A. Evaluation of insecticide dips to control Bemisia tabaci on poinsettia cuttings. Pest Sci. 1994, 42, 141–142. [Google Scholar]
- Cheek, S.; Macdonald, O. Management of Bemisia tabaci. Pest Sci. 1994, 42, 135–137. [Google Scholar] [CrossRef]
- Cannon, R.J.C.; Eyre, D.; MacLeod, A.; Matthews, L.; Malumphy, C.; Cheek, S.; Bartlett, P.W. Interceptions and outbreaks of Bemisia tabaci in the UK. The BCPC International Congress—Crop Science and Technology 2005. Congress Proc. 2005, 2, 1007–1012. [Google Scholar]
- Cuthbertson, A.G.S.; Walters, K.F.A. Pathogenicity of the entomopathogenic fungus Lecanicillium muscarium against the sweetpotato whitefly Bemisia tabaci under laboratory and glasshouse conditions. Mycopathologia 2005, 160, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, A.G.S.; Head, J.; Walters, K.F.A.; Gregory, S.A. The efficacy of the entomopathogenic nematode Steinernema feltiae, against instars of Bemisia tabaci. J. Invertebr. Pathol. 2003, 83, 267–269. [Google Scholar] [CrossRef] [PubMed]
- MacGillivray, M.F.; Anderson, G.B. Three useful insect cages. Can. Entomol. 1957, 89, 43–46. [Google Scholar] [CrossRef]
- Cuthbertson, A.G.S.; Mathers, J.J.; Blackburn, L.F.; Korycinska, A.; Powell, M.E.; Luo, W.; Jacobson, R.J.; Northing, P. Population development of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) under simulated UK glasshouse conditions. Insects 2013, 4, 185–197. [Google Scholar] [CrossRef]
- Butler, G.D.; Henneberry, T.J.; Clayton, T.E. Bemisia tabaci (Homoptera: Aleyrodidae): Development, oviposition, and longevity in relation to temperature. Ann. Entomol. Soc. Am. 1983, 76, 310–313. [Google Scholar] [CrossRef]
- Bethke, J.A.; Paine, T.D.; Nuessly, G.S. Comparative biology, morphometrics and development of two populations of Bemisia tabaci (Homoptera: Aleyrodidae) on cotton and poinsettia. Ann. Entomol. Soc. Am. 1991, 84, 407–411. [Google Scholar] [CrossRef]
- Wang, K.H.; Tsai, J.H. Temperature effect on development and reproduction of silverleaf whitefly (Homoptera: Aleyrodidae). Ann. Entomol. Soc. Am. 1996, 89, 375–384. [Google Scholar] [CrossRef]
- Cuthbertson, A.G.S.; Walters, K.F.A.; Northing, P. Susceptibility of Bemisia tabaci immature stages to the entomopathogenic fungus Lecanicillium muscarium on tomato and verbena foliage. Mycopathologia 2005, 159, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Nauen, R. Insecticide resistance in European agriculture: Research instead of rumours. The BCPC International Congress—Crop Science & Technology 2005. Congress Proc. 2005, 1, 123–130. [Google Scholar]
- Fernandez, E.; Gravalos, C.; Haro, P.J.; Cifuentes, D.; Bielza, P. Insecticide resistance status of Bemisia tabaci Q-biotype in south-eastern Spain. Pest Manag. Sci. 2009, 65, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Dennehy, T.J.; Degain, B.A.; Harpold, V.S.; Zaborac, M.; Morin, S.; Fabrick, J.A.; Nichols, R.L.; Brown, J.K.; Bryne, F.J.; Li, X.-C. Extraordinary resistance to insecticides reveals exotic Q biotype of Bemisia tabaci in the new world. J. Econ. Entomol. 2010, 103, 2174–2186. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yan, H.; Yang, Y.; Wu, Y. Biotype and insecticide resistance status of the whitefly Bemisia tabaci in China. Pest Manag. Sci. 2010, 66, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Jones, C.M.; Devine, G.; Zhang, F.; Denholm, I.; Gorman, K. Insecticide resistance in Bemisia tabaci biotype Q (Hemiptera: Aleyrodidae) from China. Crop Prot. 2010, 29, 429–434. [Google Scholar] [CrossRef]
- Cuthbertson, A.G.S.; The Food and Environment Research Agency: Sand Hutton, York, UK. Personal Observation. 2014.
- McKenzie, C.; Kumar, V.; Palmer, C.L.; Oetting, R.D.; Osborne, L.S. Chemical class rotation for control of Bemisia tabaci (Hemiptera: Aleyrodidae) on poinsettia and their effect on cryptic species population composition. Pest Manag. Sci. 2014, 70, 1573–1587. [Google Scholar] [CrossRef] [PubMed]
- Najar-Rodriguez, A.J.; Lavidis, N.A.; Mensah, R.K.; Choy, P.T.; Walter, G.H. The toxicological effects of petroleum spray oils on insects - evidence for an alternative mode of action and possible new control options. Food Chem. Toxicol. 2008, 46, 3003–3014. [Google Scholar] [CrossRef] [PubMed]
- Powell, M.E.; Glover, R; Cuthbertson, A.G.S. Phylogenetic analysis of Bemisia tabaci, intercepted in the UK, based on Mitochondrial Cytochrome Oxidase 1. Bull. Entomol. Res. 2015, in press. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuthbertson, A.G.S.; Collins, D.A. Tri-Tek (Petroleum Horticultural Oil) and Beauveria bassiana: Use in Eradication Strategies for Bemisia tabaci Mediterranean Species in UK Glasshouses. Insects 2015, 6, 133-140. https://doi.org/10.3390/insects6010133
Cuthbertson AGS, Collins DA. Tri-Tek (Petroleum Horticultural Oil) and Beauveria bassiana: Use in Eradication Strategies for Bemisia tabaci Mediterranean Species in UK Glasshouses. Insects. 2015; 6(1):133-140. https://doi.org/10.3390/insects6010133
Chicago/Turabian StyleCuthbertson, Andrew G. S., and Debbie A. Collins. 2015. "Tri-Tek (Petroleum Horticultural Oil) and Beauveria bassiana: Use in Eradication Strategies for Bemisia tabaci Mediterranean Species in UK Glasshouses" Insects 6, no. 1: 133-140. https://doi.org/10.3390/insects6010133
APA StyleCuthbertson, A. G. S., & Collins, D. A. (2015). Tri-Tek (Petroleum Horticultural Oil) and Beauveria bassiana: Use in Eradication Strategies for Bemisia tabaci Mediterranean Species in UK Glasshouses. Insects, 6(1), 133-140. https://doi.org/10.3390/insects6010133




