Do Offspring of Insects Feeding on Defoliation-Resistant Trees Have Better Biological Performance When Exposed to Nutritionally-Imbalanced Food?
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Organism
2.2. Biological Parameters
2.3. Statistical Analyses
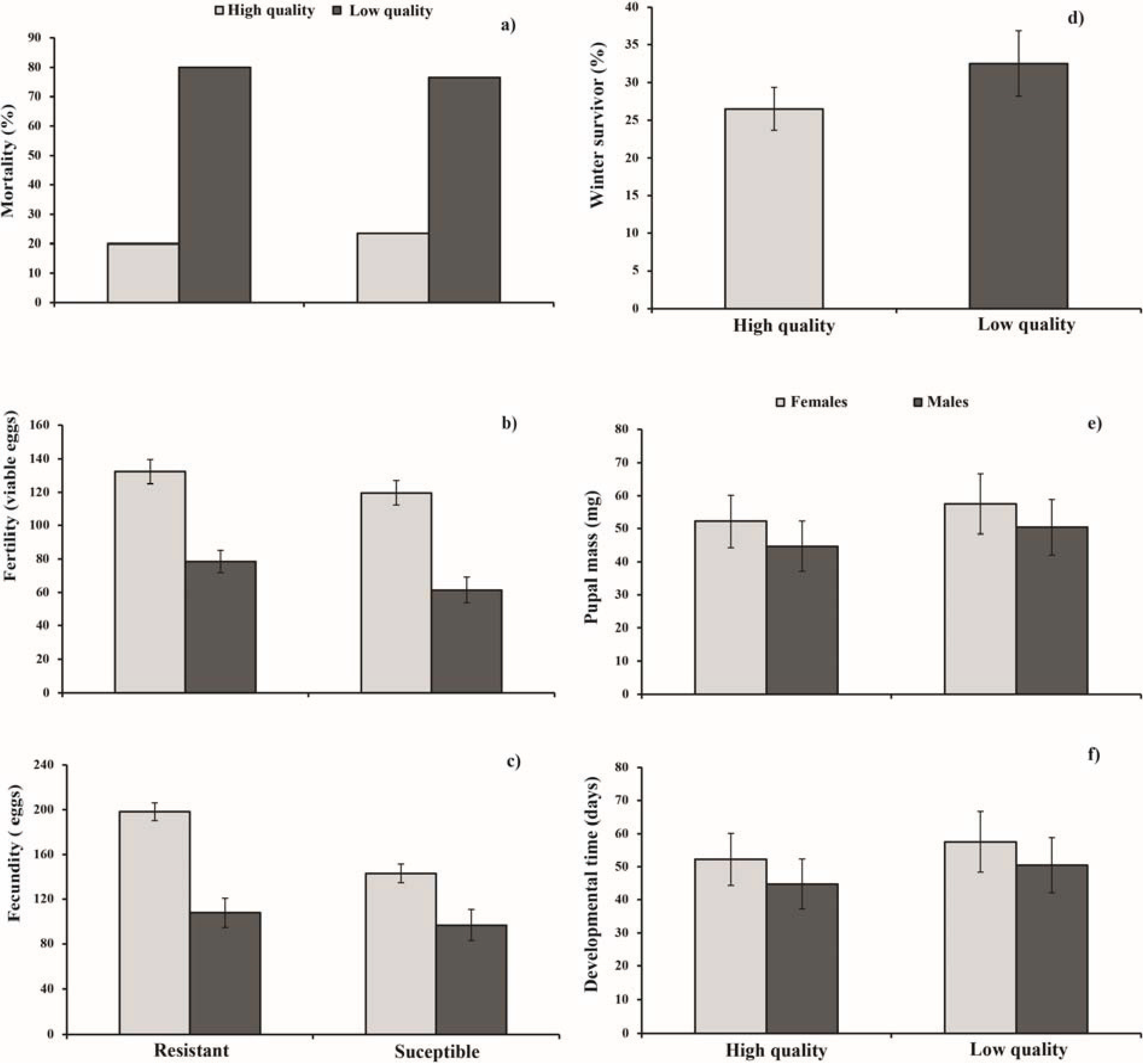
3. Results
3.1. Parental Generation
3.2. Offspring
| Fertility | Fecundity | Pupal Mass (Females) | Pupal Mass (Males) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source of variation | F | df | p | F | df | p | F | df | p | F | df | p |
| Tree phenotype | 0.06 | 1,351 | 0.8139 | 7.51 | 1,351 | 0.0064 | 36.93 | 1,347 | 0.0001 | 50.04 | 1,365 | 0.0001 |
| Fecundity | Pupal Mass (Females) | Developmental Time (Females) | Pupal Mass (Males) | Developmental Time (Males) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source of variation | F | df | p | F | df | p | F | df | p | F | df | p | F | df | p |
| Tree phenotype | 8.85 | 1,363 | 0.0031 | 2.05 | 1,363 | 0.1527 | 0.14 | 1,407 | 0.7085 | 2.72 | 1,362 | 1.000 | 0.25 | 1,447 | 0.6200 |
| Diet | 38.12 | 1,363 | 0.0001 | 137.04 | 1,363 | 0.0001 | 25.35 | 1,407 | 0.0001 | 112.40 | 1,362 | 0.0001 | 47.57 | 1,447 | 0.0001 |
| Tree phenotype * Diet | 3.95 | 1,363 | 0.0475 | 0.90 | 1,363 | 0.3438 | 1.39 | 1,407 | 0.2385 | 0.29 | 1,362 | 0.5900 | 0.00 | 1,447 | 0.9850 |
| Fertility | Winter Survival | Mortality * | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Source of variation | F | df | p | F | df | p | F | df | p |
| Tree phenotype | 4.05 | 1,353 | 0.0449 | 2.94 | 1,138 | 0.0885 | 12.95 | 1 | 0.0003 |
| Diet | 43.03 | 1,353 | 0.0001 | 7.41 | 1,138 | 0.0073 | 646.06 | 1 | 0.0001 |
| Tree phenotype * Diet | 0.92 | 1,353 | 0.3371 | 0.13 | 1,138 | 0.7169 | 0.17 | 1 | 0.6813 |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nuñez-Farfán, J.; Fornoni, J.; Valverde, P.L. The evolution of resistance and tolerance to herbivores. Ann. Rev. Ecol. Evol. Syst. 2007, 38, 541–566. [Google Scholar] [CrossRef]
- Ehrlich, P.R.; Raven, P.H. Butterflies and plants: A study in coevolution. Evolution 1964, 18, 586–608. [Google Scholar]
- Rhoades, D.F.; Cates, R.G. Towards a general theory of plant antiherbivore chemistry. Recent Adv. Phytochem. 1976, 10, 168–213. [Google Scholar]
- Fenny, P. Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 1970, 51, 565–581. [Google Scholar] [CrossRef]
- Southwood, T.R.E. Tactics, strategies and templets. Oikos 1988, 52, 3–18. [Google Scholar] [CrossRef]
- Blais, J.R. Some relationships of spruce budworm, Choristoneura fumiferana (Clem.) to black spruce, Picea mariana (Mill.) BSP. For. Chron. 1957, 33, 364–372. [Google Scholar] [CrossRef]
- Greenbank, D.O. Host species and the spruce budworm. In The Dynamics of Epidemic Spruce Budworm Populations; Entomological Society of Canada: Ottawa, ON, Canada, 1963; pp. 219–223. [Google Scholar]
- Thomas, A.W. Food consumption and utilization by 6th-instar larvae of spruce budworm, Choristoneura fumiferana: A comparison on three Picea (spruce) species. Entomol. Exp. Appl. 1989, 52, 205–214. [Google Scholar] [CrossRef]
- Fuentealba, A.; Bauce, É. Carry-over effect of host nutritional quality on performance of spruce budworm progeny. Bull. Entomol. Res. 2012, 102, 275–284. [Google Scholar] [CrossRef]
- Bauce, É.; Kumbasli, M. Natural resistance of fast growing white spruce, Picea glauca (Moench), trees against spruce budworm, Choristoneura fumiferana (Clem.). In Proceedings of the 150th Anniversary of Forestry Education in Turkey, Istanbul, Turkey, 17–19 October 2007.
- Daoust, S.; Mader, B.; Bauce, É.; Dussutour, A.; Despland, E.; Albert, P.J. Influence of epicuticular-wax composition on the feeding pattern of a phytophagous insect: Implications for host resistance. Can. Entomol. 2010, 142, 261–270. [Google Scholar] [CrossRef]
- Delvas, N.; Bauce, É.; Labbé, C.; Ollevier, T.; Bélanger, R. Phenolic compounds that confer resistance to spruce budworm. Entomol. Exp. Appl. 2011, 141, 34–44. [Google Scholar] [CrossRef]
- Mageroy, M.H.; Parent, G.; Germanos, G.; Giguère, I.; Delvas, N.; Maaroufi, H.; Bauce, É.; Bohlmann, J.; Mackay, J.J. Expression of the β-glucosidase gene Pgβglu-1 underpins natural resistance of white spruce against spruce budworm. Plant J. 2015, 81, 68–80. [Google Scholar]
- Miller, C.A. A technique for estimating the fecundity of natural population of the spruce budworm. Can. J. Zool. 1957, 35, 1–13. [Google Scholar] [CrossRef]
- Morris, R.F. Foliage depletion and spruce budworm. In The Dynamics of Epidemic Spruce Budworm Populations; Morris, R.F., Ed.; Entomological Society of Canada: Ottawa, ON, Canada, 1963. [Google Scholar]
- Carisey, N.; Bauce, É. Does nutrition-related stress carry over to spruce budworm, Choristoneura fumiferana (Lepidoptera: Tortricidae), progeny? Bull. Entomol. Res. 2002, 92, 101–108. [Google Scholar] [CrossRef]
- Blais, J.R. Effects of the destruction of the current year’s foliage of balsam fir on thfecundity and habits of flight of the spruce budworm. Can. Entomol. 1953, 85, 416–448. [Google Scholar] [CrossRef]
- Lorimer, N.; Bauer, L.S. Reproductive compatibility within and among spruce budworm (Lepidoptera: Tortricidae) populations. Great Lakes Entomol. 1983, 16, 149–152. [Google Scholar]
- Bidon, Y. Influence des sucres solubles et de l’azote sur la croissance, le développement et l’utilisation de la nourriture par la tordeuse des bourgeons de l’épinette, Choristoneura fumiferana (Clem). Master’s Thesis, Université Laval, Québec, Canada, 1993. [Google Scholar]
- Robertson, J.L. Handbook of Insect Rearing, volume II; Elsevier: Amsterdam, Netherland, 1985. [Google Scholar]
- McMorran, A. A synthetic diet for the spruce budworm, Choristoneura fumiferana (Clem.) (Lepidoptera: Tortricidae). Can. Entomol. 1965, 97, 58–62. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT User’s Guide, 9.1th ed.; SAS Institute Inc.: Cary, NC, USA, 2003. [Google Scholar]
- Frago, E.; Bauce, E. Life-history consequences of chronic nutritional stress in an outbreaking insect defoliator. PLOS ONE 2014, 9, e88039. [Google Scholar] [CrossRef] [PubMed]
- Colarsurdo, N.; Gélinas, Y.; Despland, E. Larval nutrition affects life history traits in a capital breeding moth. J. Exp. Biol. 2009, 212, 1794–1800. [Google Scholar] [CrossRef] [PubMed]
- Raubenheimer, D.; Simpson, S.J. Nutrient balancing in grasshoppers: Behavioural and physiological correlates of dietary breadth. J. Exp. Biol. 2003, 206, 1669–1681. [Google Scholar] [CrossRef] [PubMed]
- Quezada-Garcia, R.; Bauce, E. Heritability of life-history traits in the spruce budworm. Entomol. Sci. 2014, 17, 111–117. [Google Scholar] [CrossRef]
- Quezada-Garcia, R.; Seehausen, L.; Bauce, É. Adaptation of an outbreaking insect defoliator to chronic nutritional stress. J. Evol. Biol. 2015. [Google Scholar] [CrossRef]
- Régnière, J.; Nealis, V.G. The fine-scale population dynamics of spruce budworm: Survival of early instars related to forest condition. Ecol. Entomol. 2008, 33, 362–373. [Google Scholar] [CrossRef]
- Carisey, N.; Bauce, É. Impact of balsam fir foliage age on sixth-instar spruce budworm growth, development, and food utilization. Can. J. For. Res. 1997, 27, 257–264. [Google Scholar] [CrossRef]
- Awmack, C.S.; Leather, S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quezada-Garcia, R.; Fuentealba, A.; Nguyen, N.; Bauce, É. Do Offspring of Insects Feeding on Defoliation-Resistant Trees Have Better Biological Performance When Exposed to Nutritionally-Imbalanced Food? Insects 2015, 6, 112-121. https://doi.org/10.3390/insects6010112
Quezada-Garcia R, Fuentealba A, Nguyen N, Bauce É. Do Offspring of Insects Feeding on Defoliation-Resistant Trees Have Better Biological Performance When Exposed to Nutritionally-Imbalanced Food? Insects. 2015; 6(1):112-121. https://doi.org/10.3390/insects6010112
Chicago/Turabian StyleQuezada-Garcia, Roberto, Alvaro Fuentealba, Ngoc Nguyen, and Éric Bauce. 2015. "Do Offspring of Insects Feeding on Defoliation-Resistant Trees Have Better Biological Performance When Exposed to Nutritionally-Imbalanced Food?" Insects 6, no. 1: 112-121. https://doi.org/10.3390/insects6010112
APA StyleQuezada-Garcia, R., Fuentealba, A., Nguyen, N., & Bauce, É. (2015). Do Offspring of Insects Feeding on Defoliation-Resistant Trees Have Better Biological Performance When Exposed to Nutritionally-Imbalanced Food? Insects, 6(1), 112-121. https://doi.org/10.3390/insects6010112






