Evolution and Structural Analyses of Glossina morsitans (Diptera; Glossinidae) Tetraspanins
Abstract
:1. Introduction
2. Experimental
2.1. Sequential Retrieval, Ortholog Search and Bioinformatic Analyses of Tetraspanins
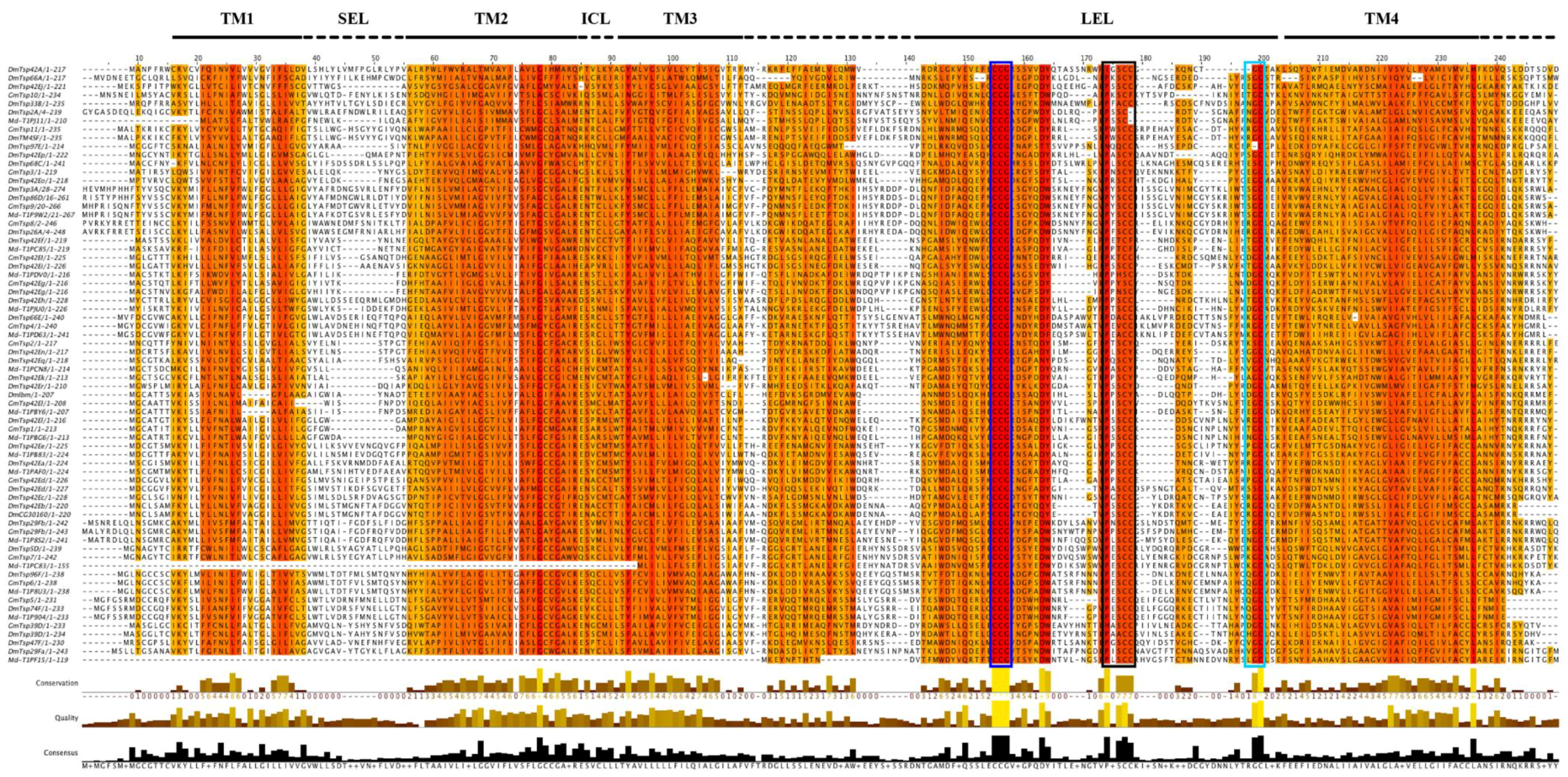
2.2. Multiple Sequence Alignment and Phylogenetic Analysis
2.3. Positive Selection Analysis
2.4. Protein Modeling
3. Results and Discussion
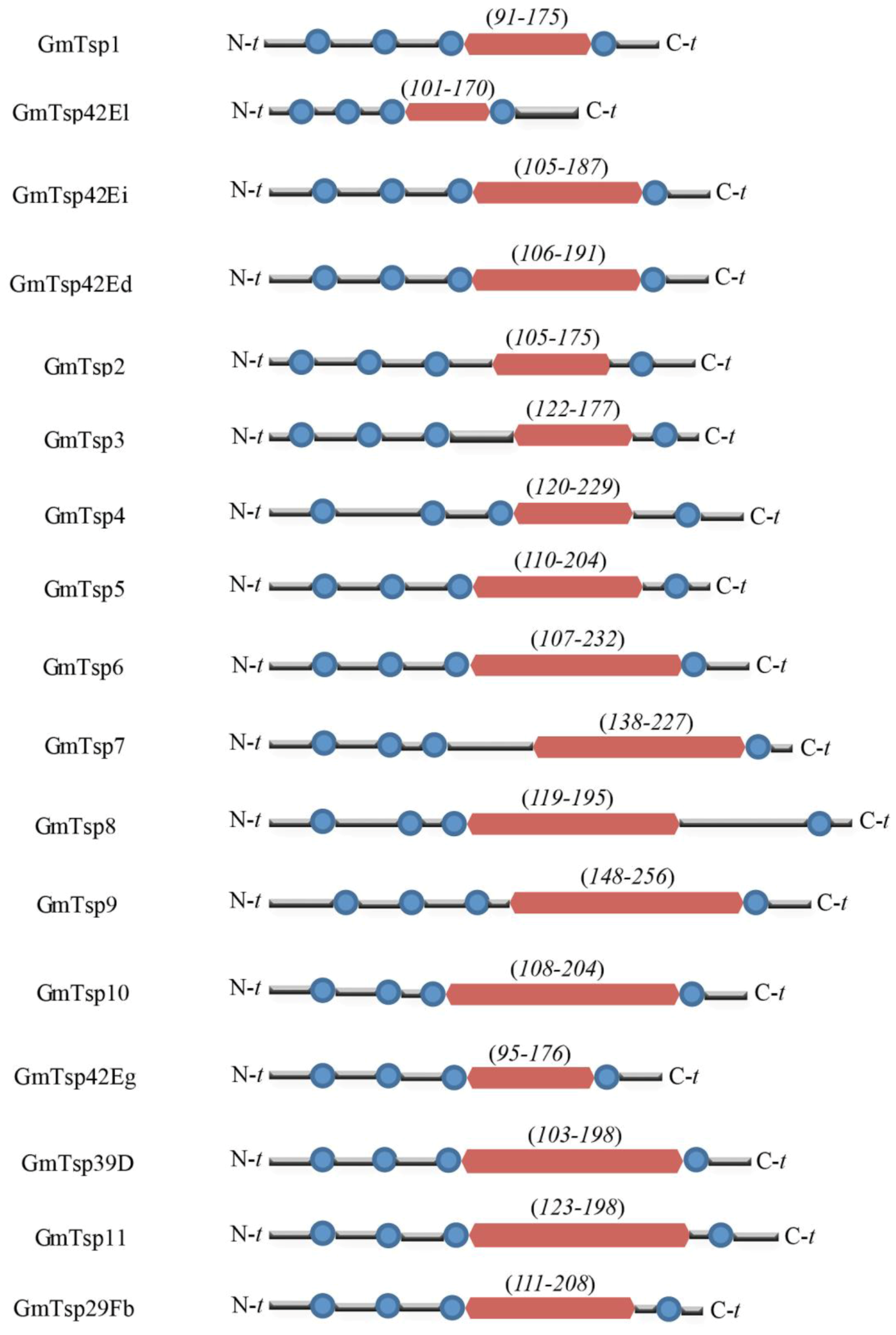
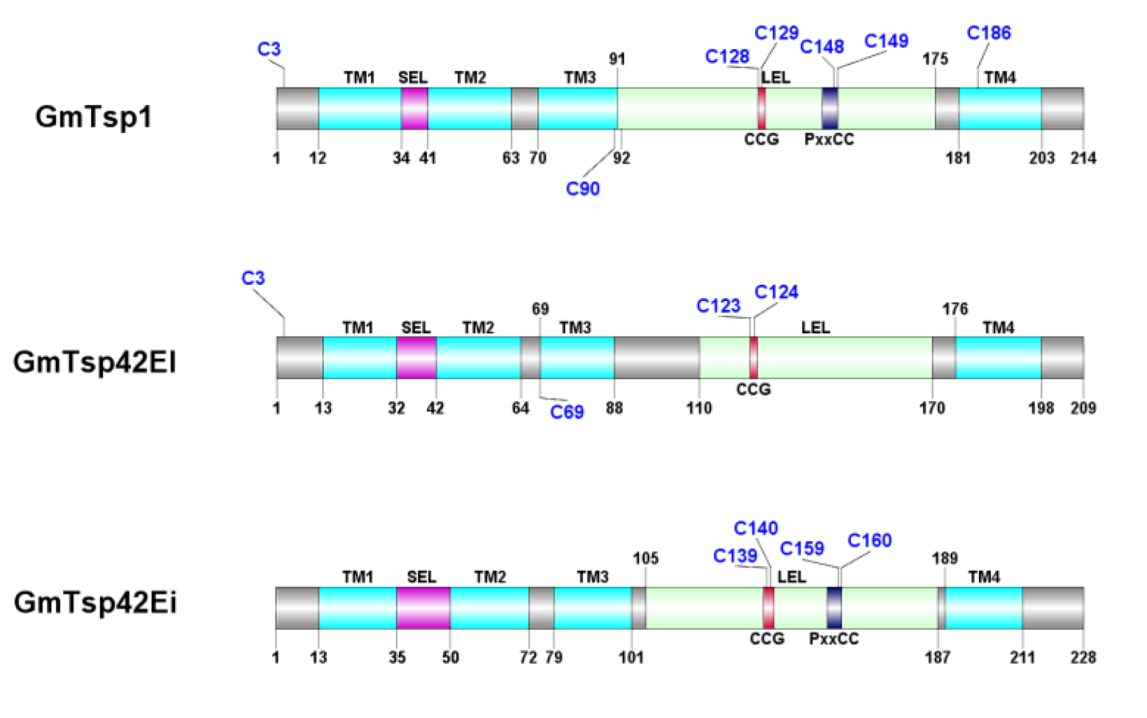
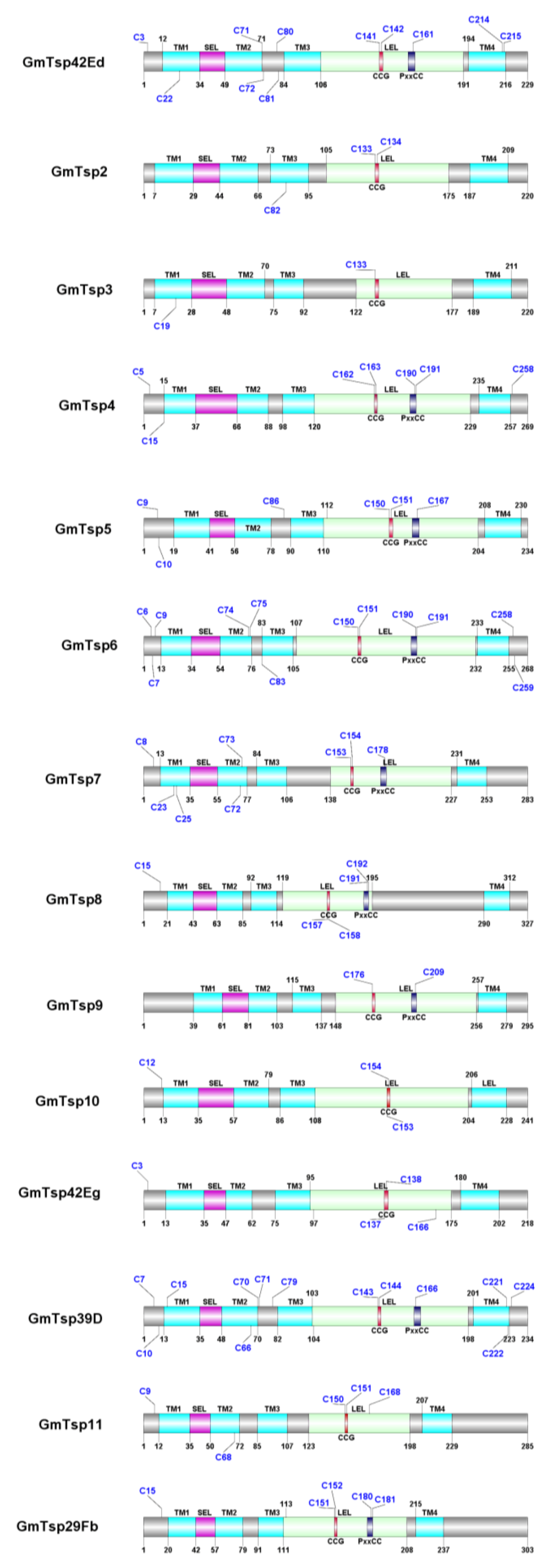
3.1. Glossina morsitans Putative Tetraspanins
| Protein Name (Description *) | UniProt/VectorBase ID | Length [aa] | LEL Domain Coordinates | TM Domain Coordinates |
|---|---|---|---|---|
| GmTsp1 (Protein late bloomer-like) | GMOY003645-PA ¥ | 214 | 91–175 | 12–34, 41–63, 70–92, 181–203 |
| GmTsp42El | D3TMF8 | 209 | 110–170 | 13–32, 42–64, 69-88, 176-198 |
| GmTsp 42Ei | D3TL43 | 228 | 105–187 | 13-35, 50–72, 79–101, 189–211 |
| GmTsp42Ed | D3TMA1 | 229 | 106–191 | 12–34, 49–71, 84–106, 194–216 |
| GmTsp2 (Tetraspanin EC2/Peripherin) | GMOY003647-PA ¥ | 220 | 105–175 | 7–29, 44–66, 73–95, 187–209 |
| GmTsp3 (Protein late bloomer-like) | GMOY003648-PA ¥ | 220 | 122–177 | 7–28, 48–70, 75–92, 189–211 |
| GmTsp4 (Tetraspanin, isoform A) | GMOY003747-PA ¥ | 269 | 120–229 | 15–37, 66–88, 98–120, 235–257 |
| GmTsp5 (CD151 antigen-like protein, isoform x2) | GMOY007608-PA ¥ | 234 | 110–204 | 19–41, 56–78, 90–112, 208–230 |
| GmTsp6 (Tetraspanin family integral membrane protein, isoform B) | D3TLU9 | 268 | 107–232 | 12–34, 54–76, 83–105, 233–255 |
| GmTsp7 (Tetraspanin, isoform C) | GMOY010508-PA ¥ | 283 | 138–227 | 13–35, 55–77, 84–106, 231–253 |
| GmTsp8 (Tetraspanin-5-like, isoform x1) | GMOY004352-PA ¥ | 327 | 119–195 | 21–43, 63–85, 92–114, 290–312 |
| GmTsp9 (Tetraspanin-33-like, isoform x1) | GMOY011478-PA ¥ | 295 | 148–256 | 39–61, 81–103, 115–137, 257–279 |
| GmTsp10 (CD63 antigen-like protein) | GMOY000619-PA ¥ | 241 | 108–204 | 13–35, 57–79, 86–108, 206–228 |
| GmTsp42Eg | D3TQ76 | 218 | 95–176 | 13–35, 40–62, 75–97, 180–202 |
| GmTsp39D | GMOY010261-PA ¥ | 234 | 103–198 | 13–35, 48–70, 82–104, 201–223 |
| GmTsp11 (Transmembrane 4 protein, isoform C) | GMOY009229-PA ¥ | 285 | 123–198 | 12–35, 50–72, 85–107, 207–229 |
| GmTsp29Fb | D3TQ22 | 303 | 111–208 | 20–42, 57–79, 91–113, 215–237 |
3.2. Evolutionary and Phylogenetic Analysis

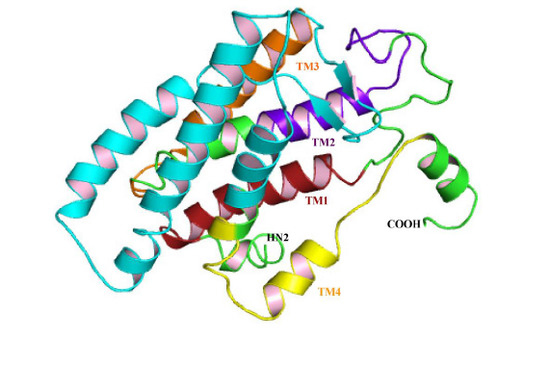
3.3. Structural Analysis
3.4. Positive Selection Analysis of G. morsitans Tetraspanins
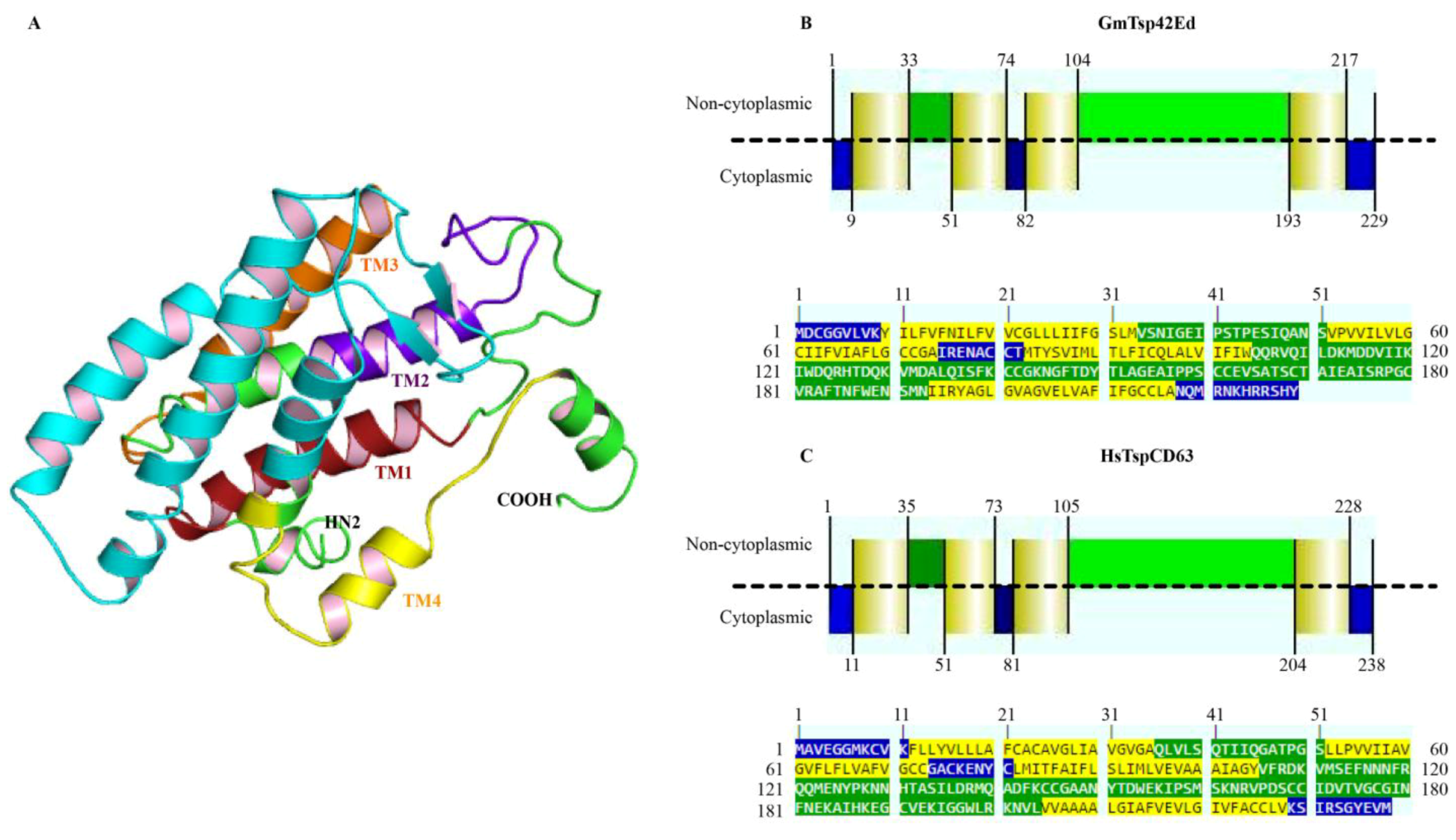
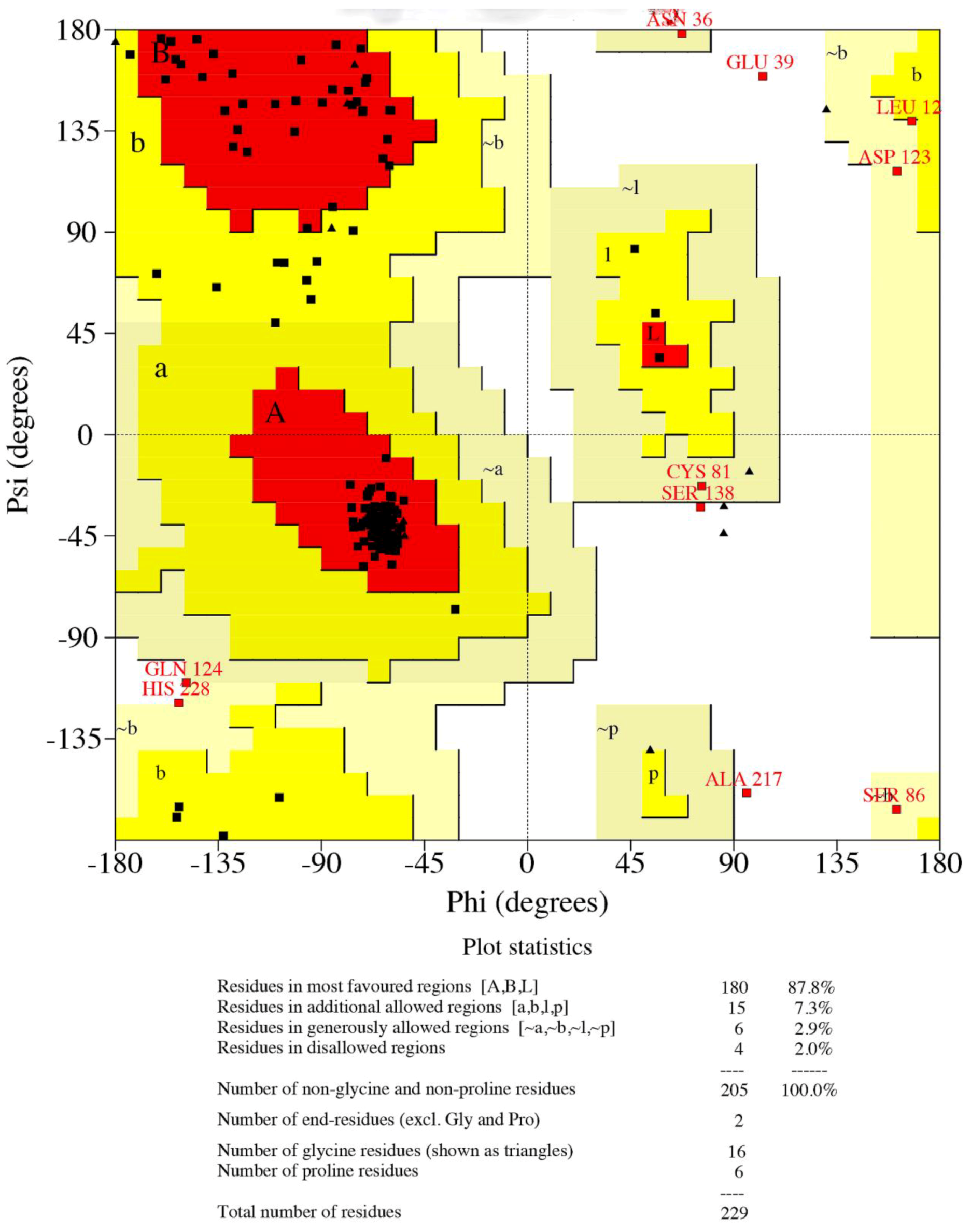
3.5. Targeting Tetraspanins as Potential Therapeutics against African Trypanosomes
4. Conclusions
Acknowledgments
Author Contributions
Appendix
Conflicts of Interest
References
- Maudlin, I. Transmission of African trypanosomiasis: Interactions among tsetse immune system, symbionts, and parasites. In Advances in Disease Vector Research, 7th ed.; Harris, K., Ed.; Springer: New York, NY, USA, 1991; pp. 117–148. [Google Scholar]
- Vreysen, M.J.B. Prospects for area-wide integrated control of tsetse flies (Diptera: Glossinidae) and trypanosomosis in sub-Saharan Africa. Rev. Soc. Entomol. Argent. 2006, 65, 1–21. [Google Scholar]
- Brun, R.; Blum, J.; Chappuis, F.; Burri, C. Human African trypanosomiasis. Lancet 2010, 375, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Chappuis, F.; Loutan, L.; Simarro, P.; Lejon, V.; Büscher, P. Options for field diagnosis of human African trypanosomiasis. Clin. Microbiol. Rev. 2005, 18, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Burri, C.; Nkunku, S.; Merolle, A.; Smith, T.; Blum, J.; Brun, R. Efficacy of new, concise schedule for melarsoprol in treatment of sleeping sickness caused by Trypanosoma brucei gambiense: A randomised trial. Lancet 2000, 355, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Burri, C. Chemotherapy against human African trypanosomiasis: Is there a road to success? Parasitology 2010, 137, 1987–1994. [Google Scholar] [CrossRef]
- Matovu, E.; Seebeck, T.; Enyaru, J.C.; Kaminsky, R. Drug resistance in Trypanosoma brucei spp., the causative agents of sleeping sickness in man and nagana in cattle. Microbes Infect. 2001, 3, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Brightwell, B.; Dransfield, R. Odour attractants for tsetse: Glossina austeni, G. brevipalpis and G. swynnertoni. Med. Vet. Entomol. 1997, 11, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Allsopp, R. Options for vector control against trypanosomiasis in Africa. Trends Parasitol. 2001, 17, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, G.J.; Leak, S.G.A.; Mulatu, W.; Nagda, S.M.; Wilson, A.; D’Ieteren, G.D.M. Use of deltamethrin “pour-on” insecticide for the control of cattle trypanosomosis in the presence of high tsetse invasion. Med. Vet. Entomol. 2001, 15, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Vreysen, M.J.; Saleh, K.M.; Ali, M.Y.; Abdulla, A.M.; Zhu, Z.R.; Juma, K.G.; Dyck, V.A.; Msangi, A.R.; Mkonyi, P.A.; Feldmann, H.U. Glossina austeni (Diptera: Glossinidae) eradicated on the island of Unguja, Zanzibar, using the sterile insect technique. J. Econ. Entomol. 2000, 93, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.P.; Vincent, I.M.; Burchmore, R.J.; Kazibwe, A.J.; Matovu, E. Drug resistance in human African trypanosomiasis. Future Microbiol. 2011, 6, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Geerts, S.; Holmes, P.H.; Eisler, M.C.; Diall, O. African bovine trypanosomiasis: The problem of drug resistance. Trends Parasitol. 2001, 17, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, R. Trypanosomiasis in Zululand and the control of tsetse flies by chemical means. Onderstepoort. J. Vet. Res. 1954, 26, 317–385. [Google Scholar]
- Hemler, M.E. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu. Rev. Cell Dev. Biol. 2003, 19, 397–422. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Tian, H.; Chen, Z.; Yu, T.; Xu, A. The evolution of vertebrate tetraspanins: gene loss, retention, and massive positive selection after whole genome duplications. BMC Evol. Biol. 2010, 10, e306. [Google Scholar] [CrossRef]
- Monk, P.N.; Partridge, L.J. Tetraspanins: Gateways for infection. Infect. Disord. Drug. Targets. 2012, 12, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Yunta, M.; Oliva, J.L.; Barcia, R.; Horejsi, V.; Angelisova, P.; Lazo, P.A. Transient activation of the c-Jun N-terminal kinase (JNK) activity by ligation of the tetraspan CD53 antigen in different cell types. Eur. J. Biochem. 2002, 269, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, B.B.; Kang, S.M.; Seo, G.W.; Lee, H.J.; Patnaik, H.H.; Jo, Y.H.; Tindwa, H.; Lee, Y.S.; Lee, B.L.; Kim, N.J.; et al. Molecular cloning, sequence characterization and expression analysis of a CD63 homologue from the coleopteran beetle, Tenebrio molitor. Int. J. Mol. Sci. 2013, 14, 20744–20767. [Google Scholar] [CrossRef] [PubMed]
- Boucheix, C.; Rubinstein, E. Tetraspanins. Cell Mol. Life. Sci. 2001, 58, 1189–1205. [Google Scholar] [CrossRef] [PubMed]
- Stipp, C.S.; Kolesnikova, T.V.; Hemler, M.E. Functional domains in tetraspanin proteins. Trends Biochem. Sci. 2003, 28, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, O.V.; Metcalf, D.G.; DeGrado, W.F.; Hemler, M.E. Structural organization and interactions of transmembrane domains in tetraspanin proteins. BMC Struct. Biol. 2005, 5, e11. [Google Scholar] [CrossRef]
- Charrin, S.; Jouannet, S.; Boucheix, C.; Rubinstein, E. Tetraspanins at a glance. J. Cell Sci. 2014, 127, 3641–3648. [Google Scholar] [CrossRef] [PubMed]
- Berditchevski, F. Complexes of tetraspanins with integrins: More than meets the eye. J. Cell Sci. 2001, 114, 4143–4151. [Google Scholar] [PubMed]
- Kovalenko, O.V.; Yang, X.; Kolesnikova, T.V.; Hemler, M.E. Evidence for specific tetraspanin homodimers: Inhibition of palmitoylation makes cysteine residues available for cross-linking. Biochem. J. 2004, 377, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Fradkin, L.G.; Kamphorst, J.T.; DiAntonio, A.; Goodman, C.S.; Noordermeer, J.N. Genome-wide analysis of the Drosophila tetraspanins reveals a subset with similar function in the formation of the embryonic synapse. Proc. Natl. Acad. Sci. USA 2002, 99, 13663–13668. [Google Scholar] [CrossRef] [PubMed]
- Charrin, S.; Le, N.F.; Oualid, M.; Billard, M.; Faure, G.; Hanash, S.M.; Boucheix, C.; Rubinstein, E. The major CD9 and CD81 molecular partner: identification and characterization of the complexes. J. Biol. Chem. 2001, 276, 14329–14337. [Google Scholar] [PubMed]
- Hemler, M.E. Tetraspanin functions and associated microdomains. Nat. Rev. Mol. Cell Biol. 2005, 6, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Hemler, M.E. Integrin associated proteins. Curr. Opin. Cell Biol. 1998, 10, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Stipp, C.S.; Orlicky, D.; Hemler, M.E. FPRP, a major, highly stoichiometric, highly specific. J. Biol. Chem. 2001, 276, 4853–4862. [Google Scholar] [CrossRef] [PubMed]
- Stipp, C.S.; Kolesnikova, T.V.; Hemler, M.E. EWI-2 is a major CD9 and CD81 partner and member of a novel Ig protein subfamily. J. Biol. Chem. 2001, 276, 40545–40554. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.; Shoham, T. Protein-protein interactions in the tetraspanin web. Physiology (Bethesda) 2005, 20, 218–224. [Google Scholar] [CrossRef]
- Lagaudrière-Gesbert, C.; Naour, F.L.; Lebel-Binay, S.; Billard, M.; Lemichez, E.; Boquet, P.; Boucheix, C.; Conjeaud, H.; Rubinstein, E. Functional analysis of four tetraspans, CD9, CD53, CD81, and CD82, suggests a common role in costimulation, cell adhesion, and migration: Only CD9 upregulates HB-EGF activity. Cell. Immunol. 1997, 182, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Kotha, J.; Jennings, L.K.; Zhang, X.A. Tetraspanins and vascular functions. Cardiovasc. Res. 2009, 83, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Charrin, S.; Le, N.F.; Silvie, O.; Milhiet, P.E.; Boucheix, C.; Rubinstein, E. Lateral organization of membrane proteins: Tetraspanins spin their web. Biochem. J. 2009, 420, 133–154. [Google Scholar] [PubMed]
- Tham, T.N.; Gouin, E.; Rubinstein, E.; Boucheix, C.; Cossart, P.; Pizarro-Cerda, J. Tetraspanin CD81 is required for Listeria monocytogenes invasion. Infect. Immun. 2010, 78, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Silvie, O.; Greco, C.; Franetich, J.F.; Dubart-Kupperschmitt, A.; Hannoun, L.; van Gemert, G.J.; Sauerwein, R.W.; Levy, S.; Boucheix, C.; Rubinstein, E.; et al. Expression of human CD81 differently affects host cell susceptibility to malaria sporozoites depending on the Plasmodium species. Cell Microbiol. 2006, 8, 1134–1146. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; He, M.; Cao, Y.; Xia, Y. The tetraspanin gene MaPls1 contributes to virulence by affecting germination, appressorial function and enzymes for cuticle degradation in the entomopathogenic fungus, Metarhizium acridum. Environ. Microbiol. 2013, 15, 2966–2979. [Google Scholar]
- Hassuna, N.; Monk, P.N.; Moseley, G.W.; Partridge, L.J. Strategies for targeting tetraspanin proteins: Potential therapeutic applications in microbial infections. BioDrugs 2009, 23, 341–359. [Google Scholar] [CrossRef] [PubMed]
- Van Spriel, A.B.; Figdor, C.G. The role of tetraspanins in the pathogenesis of infectious diseases. Microbes Infect. 2010, 12, 106–112. [Google Scholar] [CrossRef] [PubMed]
- The UniProt Consortium. The Universal Protein Resource (UniProt) 2009. Nucleic Acids Res. 2009, 37, D169–D174. [Google Scholar]
- Megy, K.; Emrich, S.J.; Lawson, D.; Campbell, D.; Dialynas, E.; Hughes, D.S.T.; Koscielny, G.; Louis, C.; MacCallum, R.M.; Redmond, S.N.; et al. VectorBase: improvements to a bioinformatics resource for invertebrate vector genomics. Nucleic Acids Res. 2012, 40, D729–D734. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.; Apweiler, R.; Attwood, T.K.; Bairoch, A.; Bateman, A.; Binns, D.; Bork, P.; Das, U.; Daugherty, L.; Duquenne, L.; et al. InterPro: The integrative protein signature database. Nucleic Acids Res. 2009, 37, D211–D215. [Google Scholar] [CrossRef] [PubMed]
- International Glossina Genome Initiative. Genome sequence of the tsetse fly (Glossina morsitans): Vector of African trypanosomiasis. Science 2014, 344, 380–386. [Google Scholar]
- Schultz, J.; Milpetz, F.; Bork, P.; Ponting, C.P. SMART, a simple modular architecture research tool: identification of signaling domains. Proc. Natl. Acad. Sci. USA 1998, 95, 5857–5864. [Google Scholar] [CrossRef] [PubMed]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L.L. Predicting transmembrane protein topology with a hidden markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, T.; Boon-Chieng, S.; Mitaku, S. SOSUI: Classification and secondary structure prediction system for membrane proteins. Bioinformatics 1998, 14, 378–379. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein identification and analysis tools on the ExPASy server. In The proteomics protocols handbook, 1st ed.; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Conesa, A.; Gotz, S.; Garcia-Gomez, J.M.; Terol, J.; Talon, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wen, L.; Gao, X.; Jin, C.; Xue, Y.; Yao, X. CSS-Palm 2.0: An updated software for palmitoylation sites prediction. Prot. Eng. Des. Sel. 2008, 21, 639–644. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic. Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.; Clamp, M.; Barton, G.J. Jalview Version 2: A multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef] [PubMed]
- Whelan, S.; Goldman, N. A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol. Biol. Evol. 2001, 18, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. Prot-Test 3: Fast selection of best-fit models of protein evolution. Bioinformatics 2011, 27, 1164–1165. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Delsuc, F.; Dufayard, J.F.; Gascuel, O. Estimating maximum likelihood phylogenies with PhyML. Methods Mol. Biol. 2009, 537, 113–137. [Google Scholar] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL): An online tool for phylogenetic tree display and annotation. Bioinformatics 2007, 23, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Delport, W.; Poon, A.F.; Frost, S.D.; Kosakovsky Pond, S.L. Datamonkey 2010: A suite of phylogenetic analysis tools for evolutionary biology. Bioinformatics 2010, 26, 2455–2457. [Google Scholar] [CrossRef]
- Kosakovsky Pond, S.L.; Frost, S.D.W. Not so different after all: A comparison of methods for detecting amino acid sites under selection. Mol. Biol. Evol. 2005, 22, 1208–1222. [Google Scholar] [CrossRef] [PubMed]
- Murrell, B.; Wertheim, J.O.; Moola, S.; Weighill, T.; Scheffler, K.; Kosakovsky Pond, S.L. Detecting individual sites subject to episodic diversifying selection. PLoS Genet. 2012, 8, e1002764. [Google Scholar] [CrossRef] [PubMed]
- Murrell, B.; Moola, S.; Mabona, A.; Weighill, T.; Sheward, D.; Kosakovsky Pond, S.L.; Scheffler, K. FUBAR: A fast, unconstrained Bayesian AppRoximation for inferring selection. Mol. Biol. Evol. 2013, 30, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Kosakovsky Pond, S.L.; Posada, D.; Gravenor, M.B.; Woelk, C.H.; Frost, S.D.W. GARD: A genetic algorithm for recombination detection. Bioinformatics 2006, 22, 3096–3098. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.A.; Sternberg, M.J. Protein structure prediction on the Web: A case study using the Phyre server. Nat. Protoc. 2009, 4, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Sali, A.; Blundell, T.L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Cryst. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Morris, A.L.; MacArthur, M.W.; Hutchinson, E.G.; Thornton, J.M. Stereochemical quality of protein structure coordinates. Proteins 1992, 12, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.; Luthy, R.; Bowie, J.U. VERIFY3D: Assessment of protein models with three-dimensional profiles. Methods. Enzymol. 1997, 277, 396–404. [Google Scholar] [PubMed]
- Colovos, C.; Yeates, T.O. Verification of protein structures: patterns of nonbonded atomic interactions. Protein Sci. 1993, 2, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Marchler-Bauer, A.; Zheng, C.; Chitsaz, F.; Derbyshire, M.K.; Geer, L.Y.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Lanczycki, C.J.; et al. CDD: Conserved domains and protein three-dimensional structure. Nucleic Acids Res. 2013, 41, D348–D352. [Google Scholar] [CrossRef] [PubMed]
- Adell, T.; Gamulin, V.; Perovic-Ottstadt, S.; Wiens, M.; Korzhev, M.; Muller, I.M.; Muller, W.E. Evolution of metazoan cell junction proteins: The scaffold protein MAGI and the transmembrane receptor tetraspanin in the demosponge Suberites domuncula. J. Mol. Evol. 2004, 59, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Todres, E.; Nardi, J.B.; Robertson, H.M. The tetraspanin superfamily in insects. Insect Mol. Biol. 2000, 9, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo-Gutierrez, G.; Rodrigues, J.; Ndikuyeze, G.; Povelones, M.; Molina-Cruz, A.; Barillas-Mury, C. Mosquito immune responses and compatibility between Plasmodium parasites and anopheline mosquitoes. BMC Microbiol. 2009, 9, e154. [Google Scholar] [CrossRef]
- Alves-Silva, J.; Ribeiro, J.M.C.; van den Abbeele, J.; Attardo, G.; Hao, Z.; Haines, L.R.; Soares, M.B.; Berriman, M.; Aksoy, S.; Lehane, M.J. An insight into the sialome of Glossina morsitans morsitans. BMC Genomics 2010, 11, e213. [Google Scholar] [CrossRef]
- Greaves, J.; Chamberlain, L.H. Palmitoylation-dependent protein sorting. J. Cell Biol. 2007, 176, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Linder, M.E.; Deschenes, R.J. Palmitoylation: Policing protein stability and traffic. Nat. Rev. Mol. Cell Biol. 2007, 8, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Berditchevski, F.; Odintsova, E.; Sawada, S.; Gilbert, E. Expression of the palmitoylation-deficient CD151 weakens the association of alpha 3 beta 1 integrin with the tetraspanin-enriched microdomains and affects integrin-dependent signaling. J. Biol. Chem. 2002, 277, 36991–37000. [Google Scholar] [CrossRef] [PubMed]
- Charrin, S.; Manie, S.; Oualid, M.; Billard, M.; Boucheix, C.; Rubinstein, E. Differential stability of tetraspanin/tetraspanin interactions: Role of palmitoylation. FEBS Lett. 2002, 516, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Claas, C.; Kraeft, S.K.; Chen, L.B.; Wang, Z.; Kreidberg, J.A.; Hemler, M.E. Palmitoylation of tetraspanin proteins: Modulation of CD151 lateral interactions, subcellular distribution, and integrin-dependent cell morphology. Mol. Biol. Cell 2002, 13, 767–781. [Google Scholar] [CrossRef] [PubMed]
- Hotta, H.; Ross, A.H.; Huebner, K.; Isobe, M.; Wendeborn, S.; Chao, M.V.; Ricciardi, R.P.; Tsujimoto, Y.; Croce, C.M.; Koprowski, H. Molecular cloning and characterization of an antigen associated with early stages of melanoma tumor progression. Cancer Res. 1988, 48, 2955–2962. [Google Scholar] [PubMed]
- Guindon, S.; Gascuel, O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Vandepoele, K.; van Lijsebettens, M. Tetraspanin genes in plants. Plant Sci. 2012, 190, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Espana, A.; Chung, P.J.; Sarkar, I.N.; Stiner, E.; Sun, T.T.; Desalle, R. Appearance of new tetraspanin genes during vertebrate evolution. Genomics 2008, 91, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Kariithi, H.M.; van Den, A.; Boeren, S.; Caljon, G.; İkbal, A.İ.; Murungi, K.E.; Abd-Alla, M.M.A.; Vlak, J.M.; Laboratory of Virology. Wageningen University: Wageningen, The Netherlands, Unpublished data. 2014.
- Seigneuret, M. Complete predicted three-dimensional structure of the facilitator transmembrane protein and hepatitis C virus receptor CD81: Conserved and variable structural domains in the tetraspanin superfamily. Biophys. J. 2006, 90, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Ch.S.; Vijayasarathy, K.; Srinivas, E.; Sastry, G.M.; Sastry, G.N. Homology modeling of membrane proteins: A critical assessment. Comput. Biol. Chem. 2006, 30, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T. Synonymous and nonsynonymous substitutions in mammalian genes and the nearly neutral theory. J. Mol. Evol. 1995, 40, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, R.; Yang, Z. Likelihood models for detecting positively selected amino acid sites and applications to the HIV-1 envelope gene. Genetics 1998, 148, 929–936. [Google Scholar] [PubMed]
- Kreitman, M.; Akashi, H. Molecular evidence for natural selection. Annu. Rev. Ecol. Syst. 1995, 26, 403–422. [Google Scholar] [CrossRef]
- Martin, F.; Roth, D.M.; Jans, D.A.; Pouton, C.W.; Partridge, L.J.; Monk, P.N.; Moseley, G.W. Tetraspanins in viral infections: A fundamental role in viral biology? J. Virol. 2005, 79, 10839–10851. [Google Scholar] [CrossRef]
- Van Compernolle, S.E.; Wiznycia, A.V.; Rush, J.R.; Dhanasekaran, M.; Baures, P.W.; Todd, S.C. Small molecule inhibition of hepatitis C virus E2 binding to CD81. Virology 2003, 314, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Spoden, G.; Freitag, K.; Husmann, M.; Boller, K.; Sapp, M.; Lambert, C.; Florin, L. Clathrin- and caveolin-independent entry of human papillomavirus type 16: Involvement of tetraspanin-enriched microdomains (TEMs). PLoS One 2008, 3, e3313. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.H.; Martin, F.; Higginbottom, A.; Partridge, L.J.; Parthasarathy, V.; Moseley, G.W.; Lopez, P.; Cheng-Mayer, C.; Monk, P.N. Recombinant extracellular domains of tetraspanin proteins are potent inhibitors of the infection of macrophages by human immunodeficiency virus type 1. J. Virol. 2006, 80, 6487–6496. [Google Scholar] [CrossRef] [PubMed]
- Nydegger, S.; Khurana, S.; Krementsov, D.N.; Foti, M.; Thali, M. Mapping of tetraspanin-enriched microdomains that can function as gateways for HIV-1. J. Cell Biol. 2006, 173, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Von Lindern, J.J.; Rojo, D.; Grovit-Ferbas, K.; Yeramian, C.; Deng, C.; Herbein, G.; Ferguson, M.R.; Pappas, T.C.; Decker, J.M.; Singh, A.; et al. Potential role for CD63 in CCR5-mediated human immunodeficiency virus type 1 infection of macrophages. J. Virol. 2003, 77, 3624–3633. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.H.; Pearson, M.S.; Bethony, J.M.; Smyth, D.J.; Jones, M.K.; Duke, M.; Don, T.A.; McManus, D.P.; Correa-Oliveira, R.; Loukas, A. Tetraspanins on the surface of Schistosoma mansoni are protective antigens against schistosomiasis. Nat. Med. 2006, 12, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.H.; Freitas, T.C.; Cooper, L.; Gaze, S.; Gatton, M.L.; Jones, M.K.; Lovas, E.; Pearce, E.J.; Loukas, A. Suppression of mRNAs encoding tegument tetraspanins from Schistosoma mansoni results in impaired tegument turnover. PLoS Pathog. 2010, 6, e1000840. [Google Scholar] [CrossRef] [PubMed]
- Dakshinamoorthy, G.; Munirathinam, G.; Stoicescu, K.; Reddy, M.V.; Kalyanasundaram, R. Large extracellular loop of tetraspanin as a potential vaccine candidate for filariasis. PLoS One. 2013, 8, e77394. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murungi, E.K.; Kariithi, H.M.; Adunga, V.; Obonyo, M.; Christoffels, A. Evolution and Structural Analyses of Glossina morsitans (Diptera; Glossinidae) Tetraspanins. Insects 2014, 5, 885-908. https://doi.org/10.3390/insects5040885
Murungi EK, Kariithi HM, Adunga V, Obonyo M, Christoffels A. Evolution and Structural Analyses of Glossina morsitans (Diptera; Glossinidae) Tetraspanins. Insects. 2014; 5(4):885-908. https://doi.org/10.3390/insects5040885
Chicago/Turabian StyleMurungi, Edwin K., Henry M. Kariithi, Vincent Adunga, Meshack Obonyo, and Alan Christoffels. 2014. "Evolution and Structural Analyses of Glossina morsitans (Diptera; Glossinidae) Tetraspanins" Insects 5, no. 4: 885-908. https://doi.org/10.3390/insects5040885
APA StyleMurungi, E. K., Kariithi, H. M., Adunga, V., Obonyo, M., & Christoffels, A. (2014). Evolution and Structural Analyses of Glossina morsitans (Diptera; Glossinidae) Tetraspanins. Insects, 5(4), 885-908. https://doi.org/10.3390/insects5040885