Climate-Driven Reshuffling of Species and Genes: Potential Conservation Roles for Species Translocations and Recombinant Hybrid Genotypes
Abstract
:1. Introduction

2. Biosystematic Levels (Bottom-Up Processes; Figure 1)
3. Ecosystems and Biodiversity Hotspots (Top Down Interpretations)
4. What Should Be Conserved in Conservation?
5. Latitudinal Gradients in Global Biodiversity (Macroecological Patterns)
6. An Experimental Evaluation: Do Specialists Retain the Capacity for Generalization (Host Shifts)?
7. Other Latitudinal Considerations
8. Plant Genetics and Phenotypic Induction of Resistance
9. Genetic Variation across Species Ranges (Central-Marginal Hypotheses)
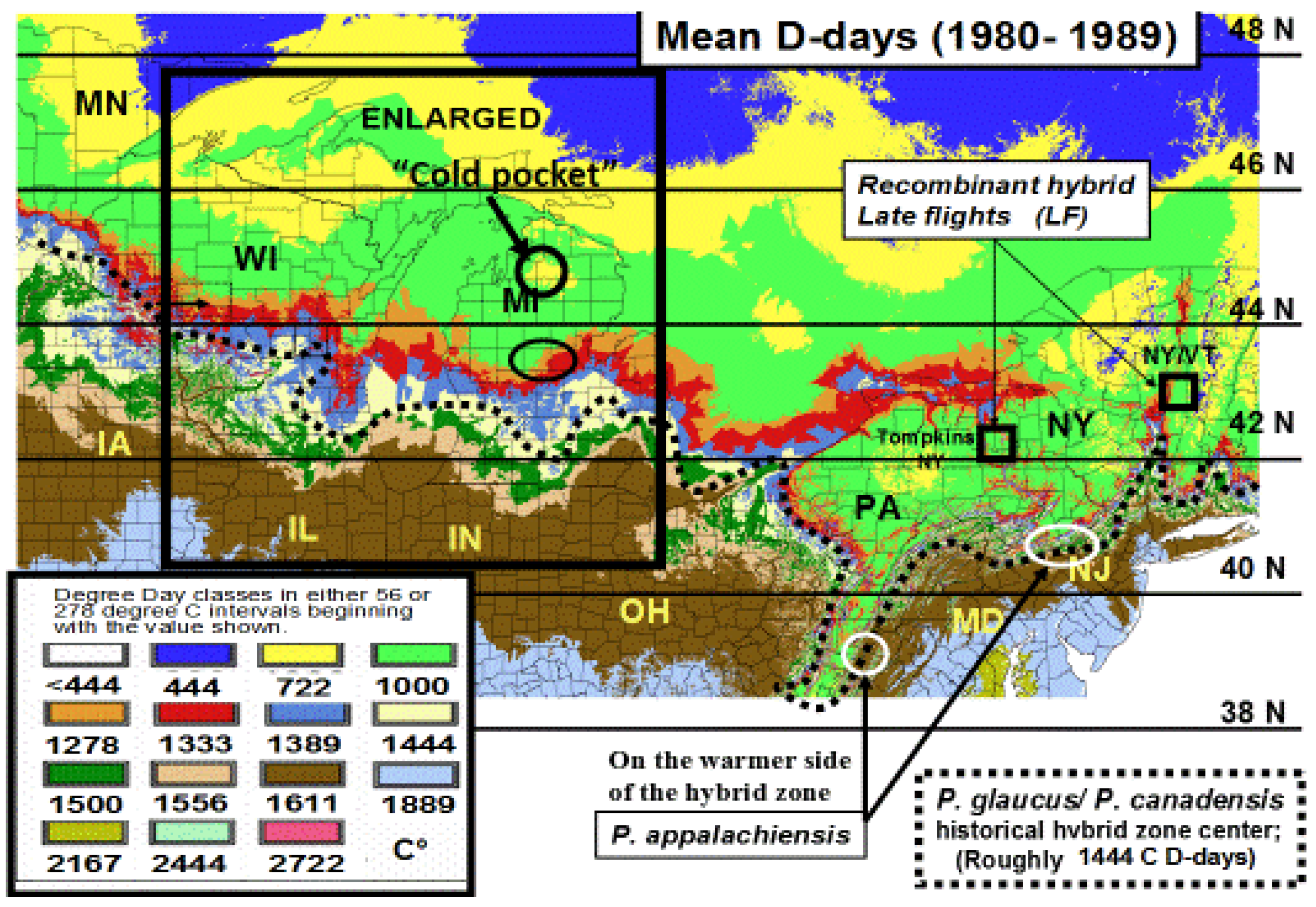
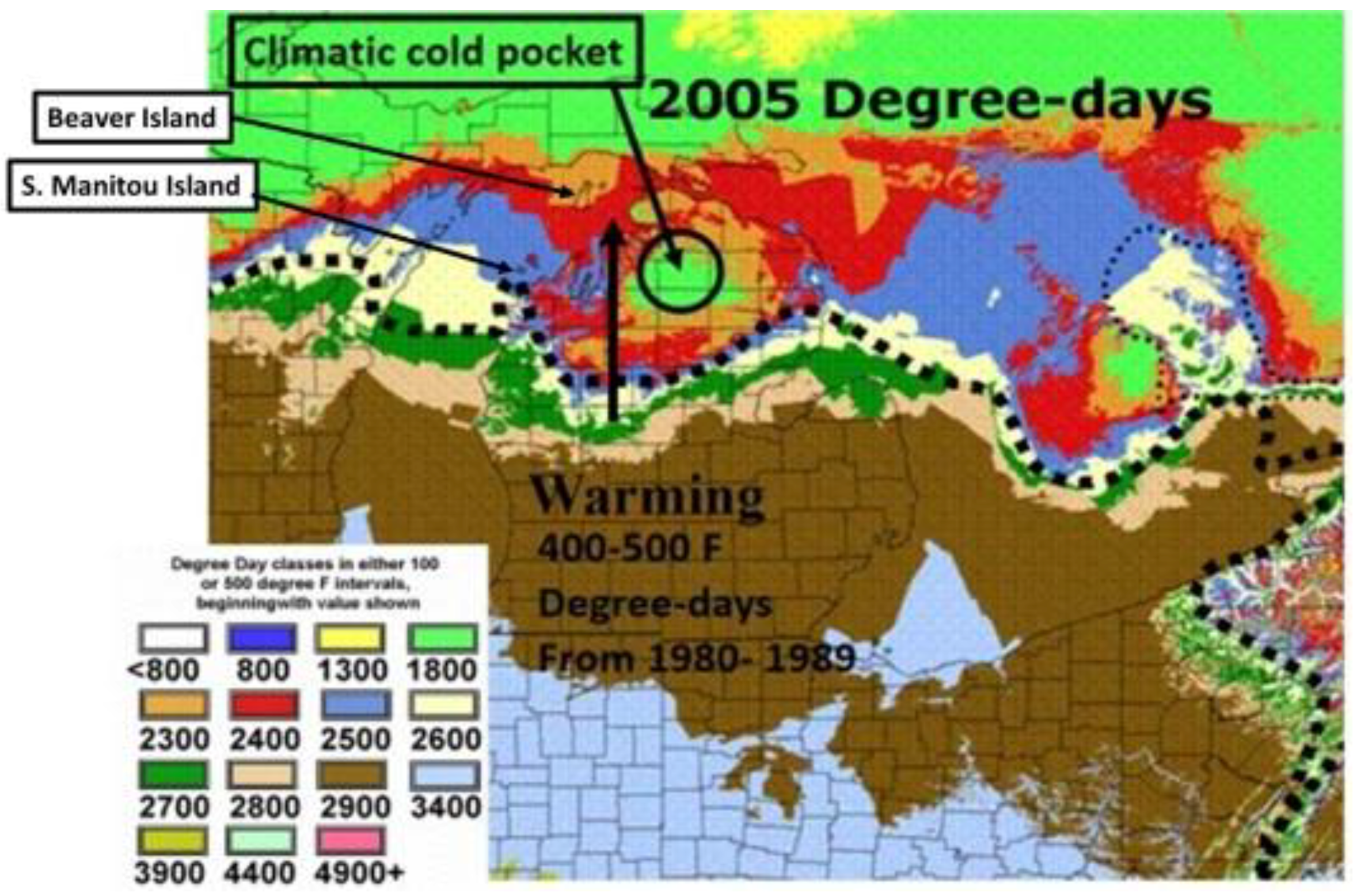
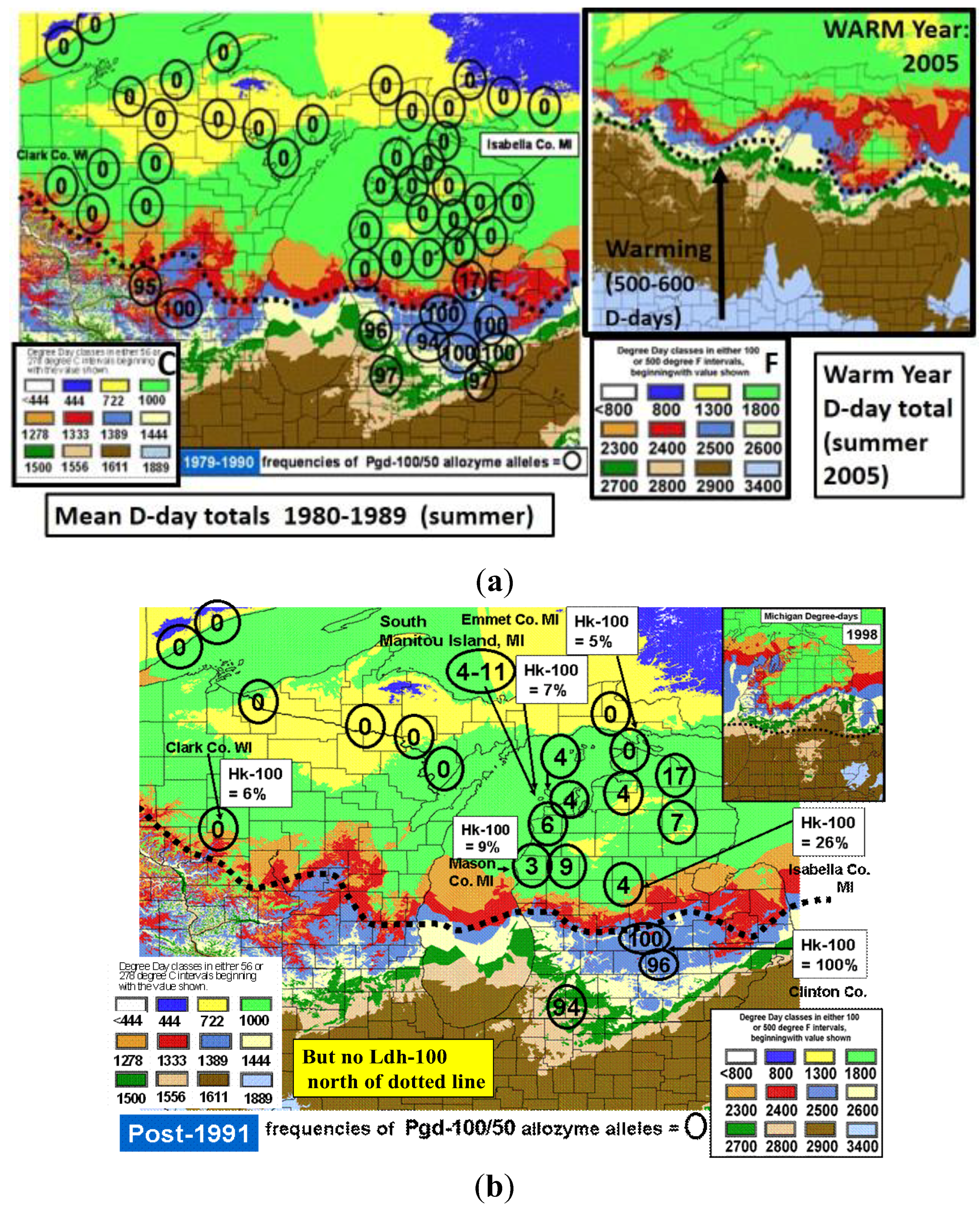
10. Climatic (Thermal) Extremes and Variability may Be More Important for Range Shifts than Mean Temperature Increases, (both in Winter and Summer)
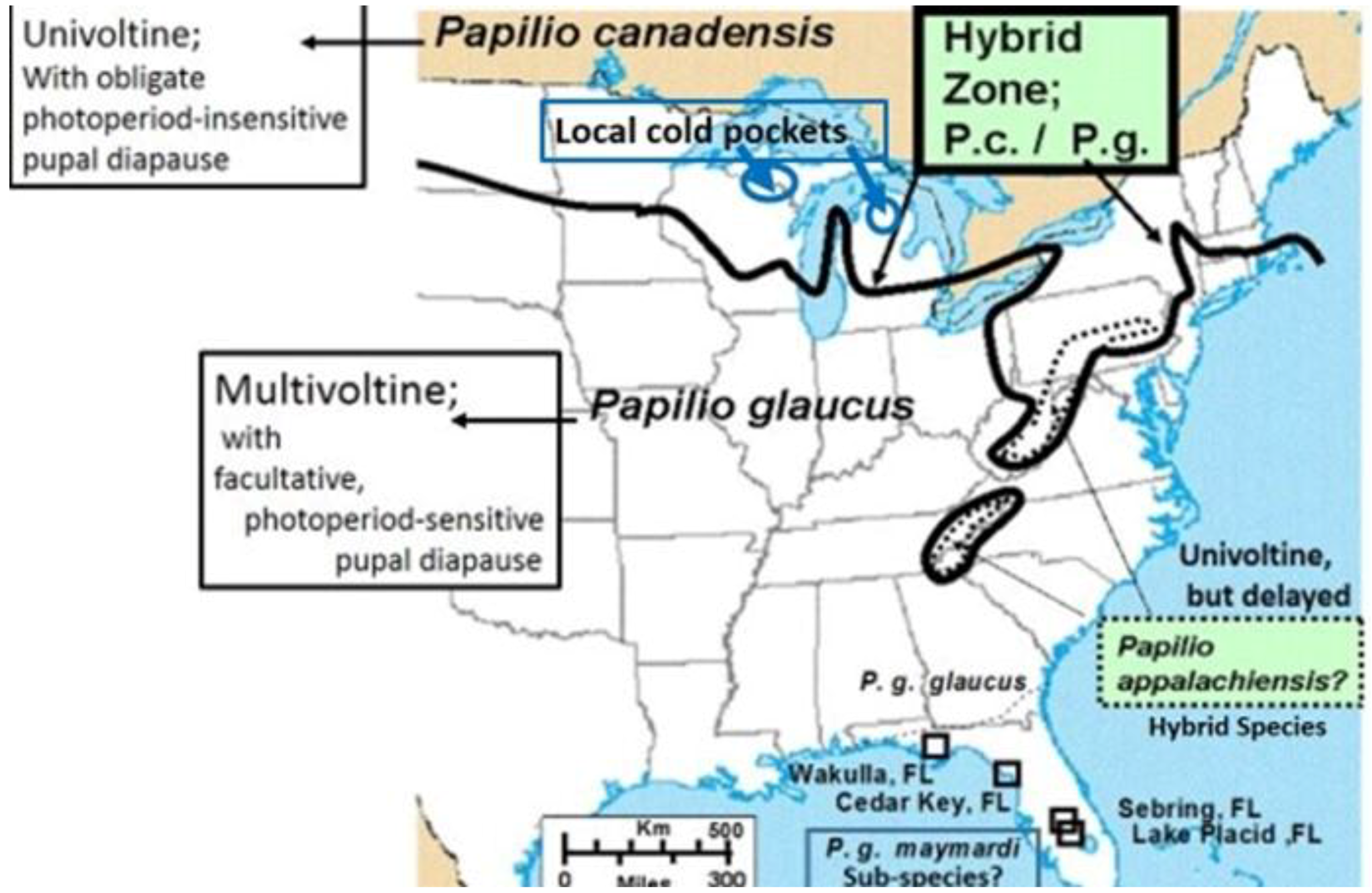
11. Background on Tiger Swallowtail Sister Species and Their Hybrids

12. Durations of Severe Mid-Winter Cold Stress
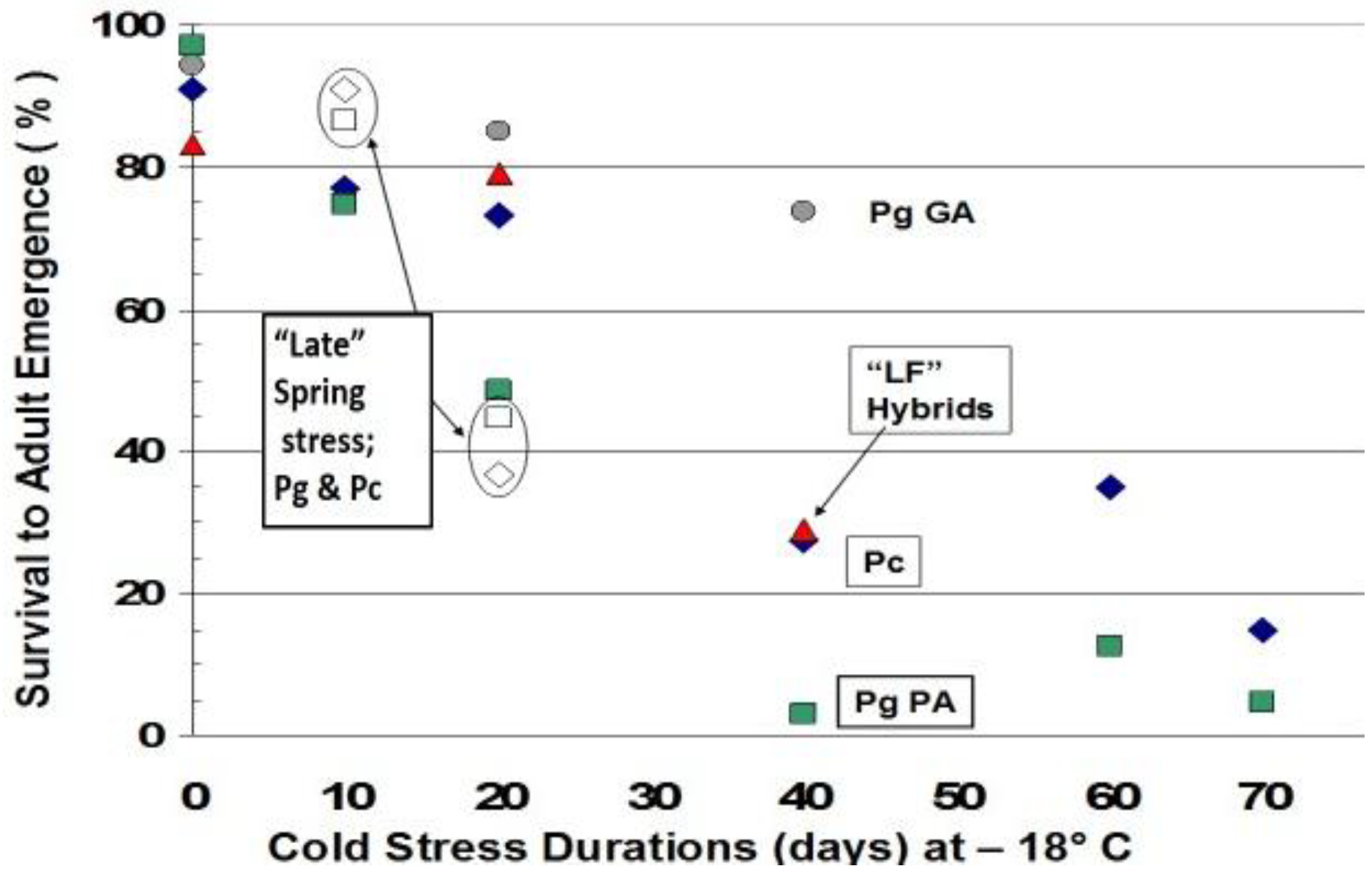
13. How Fast Do Insects Respond with Size Increases to Local Warming?
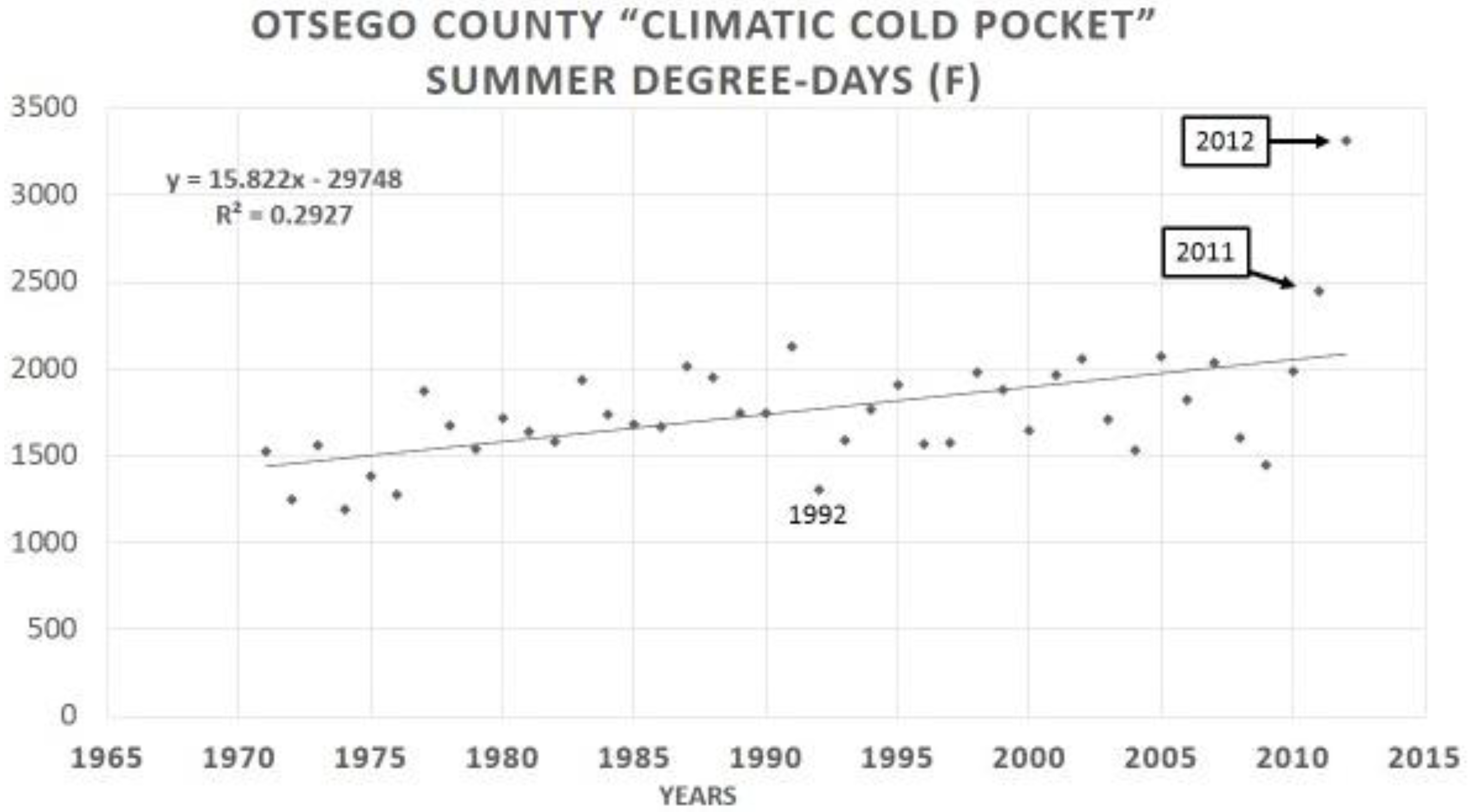
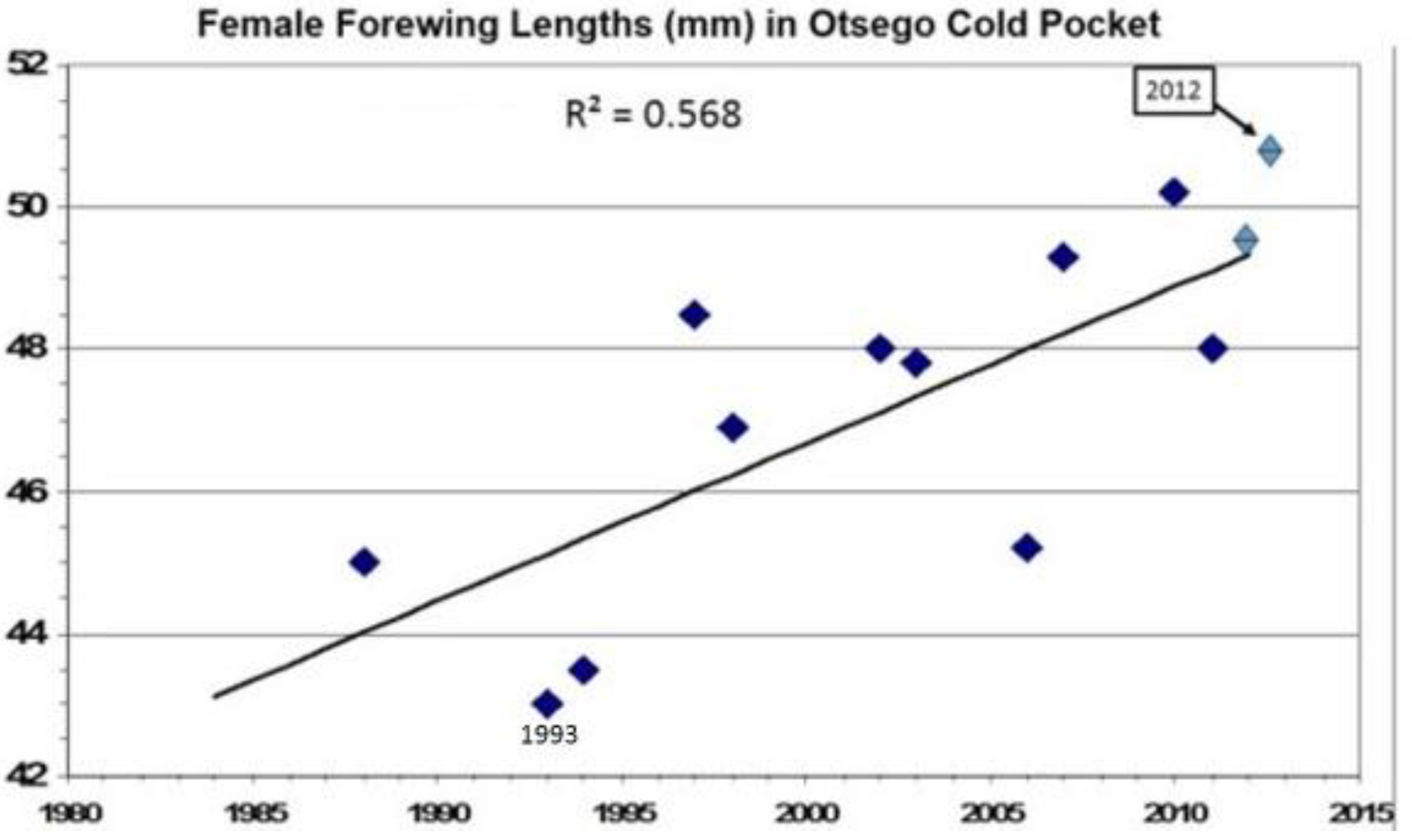
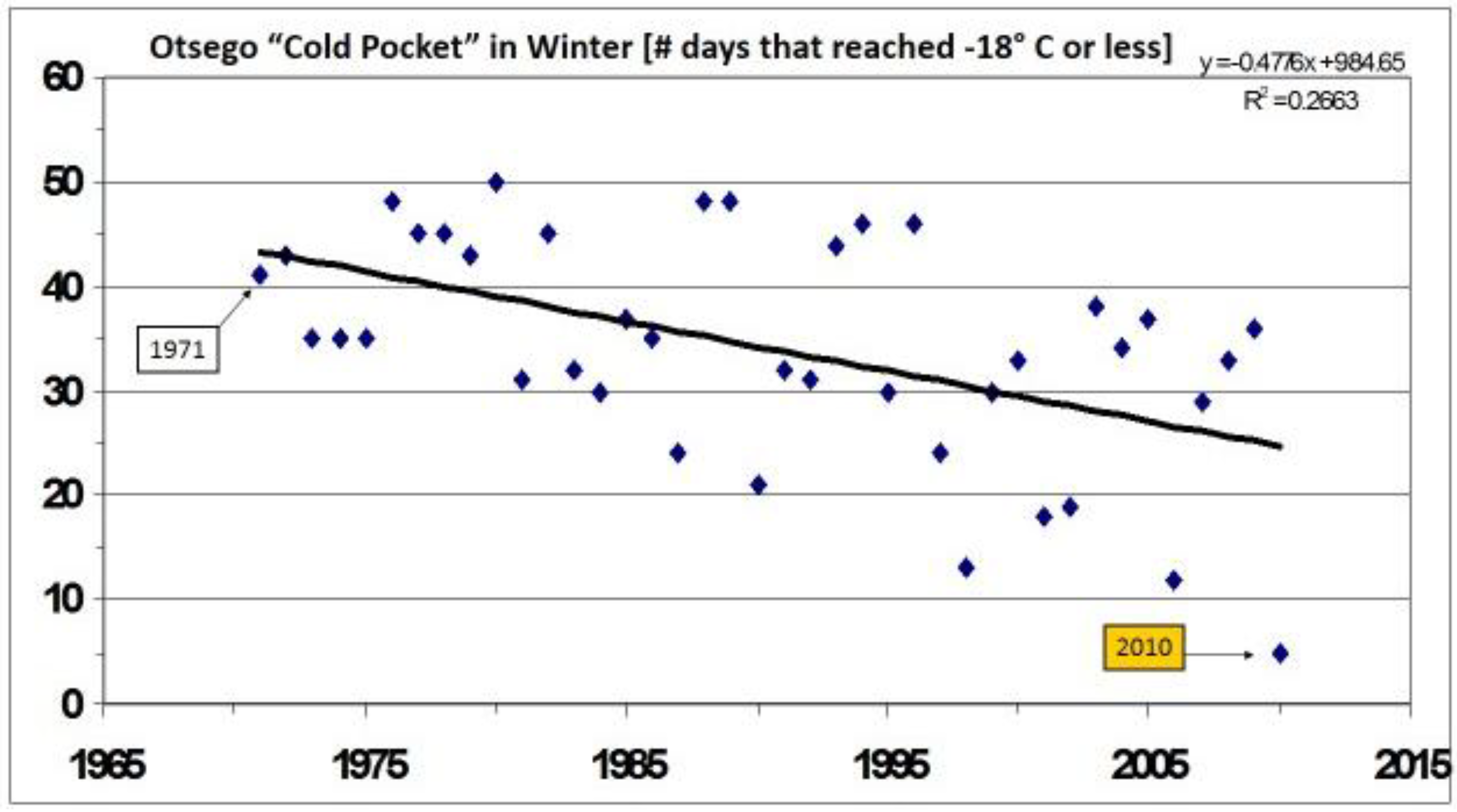
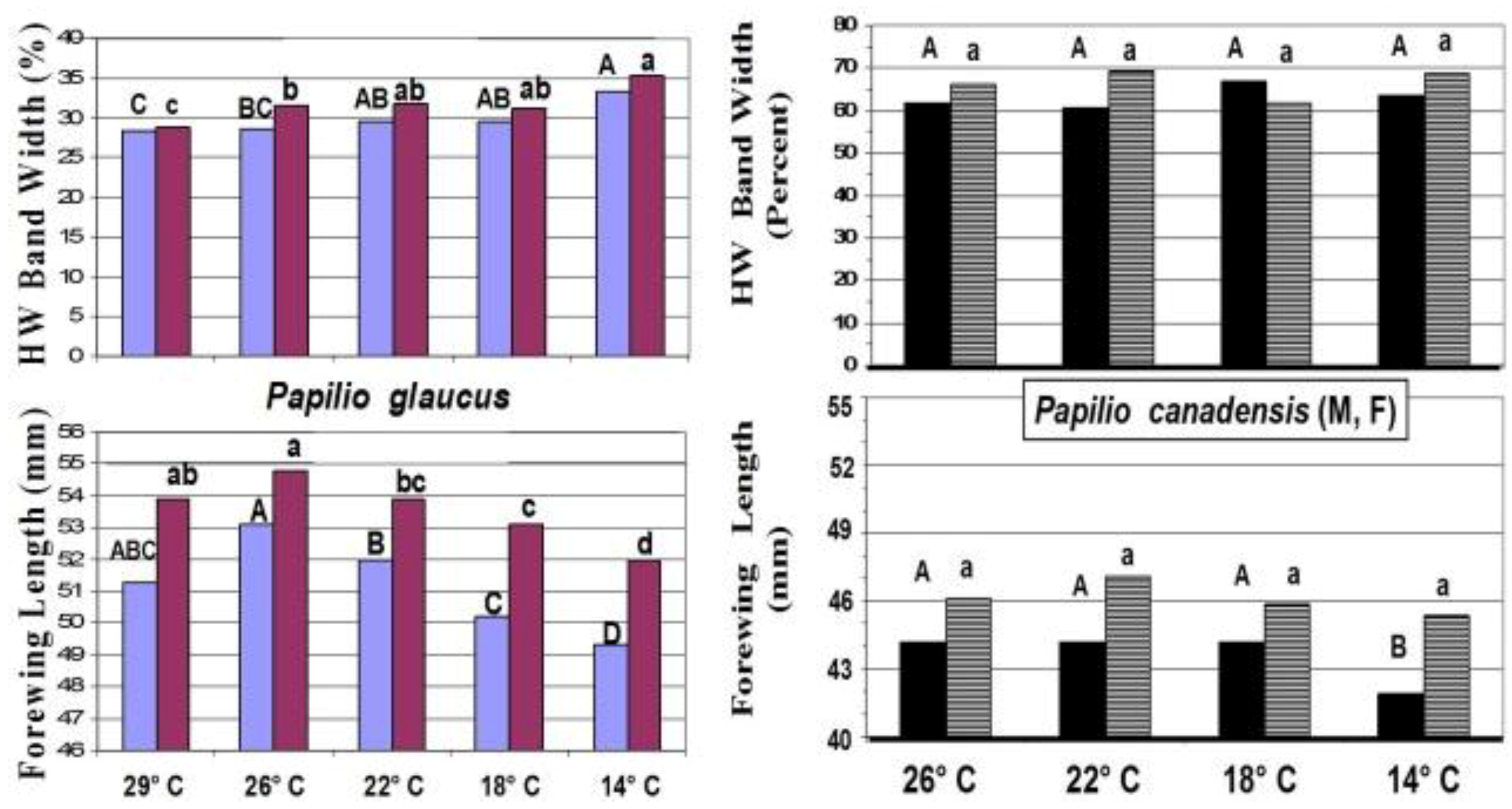
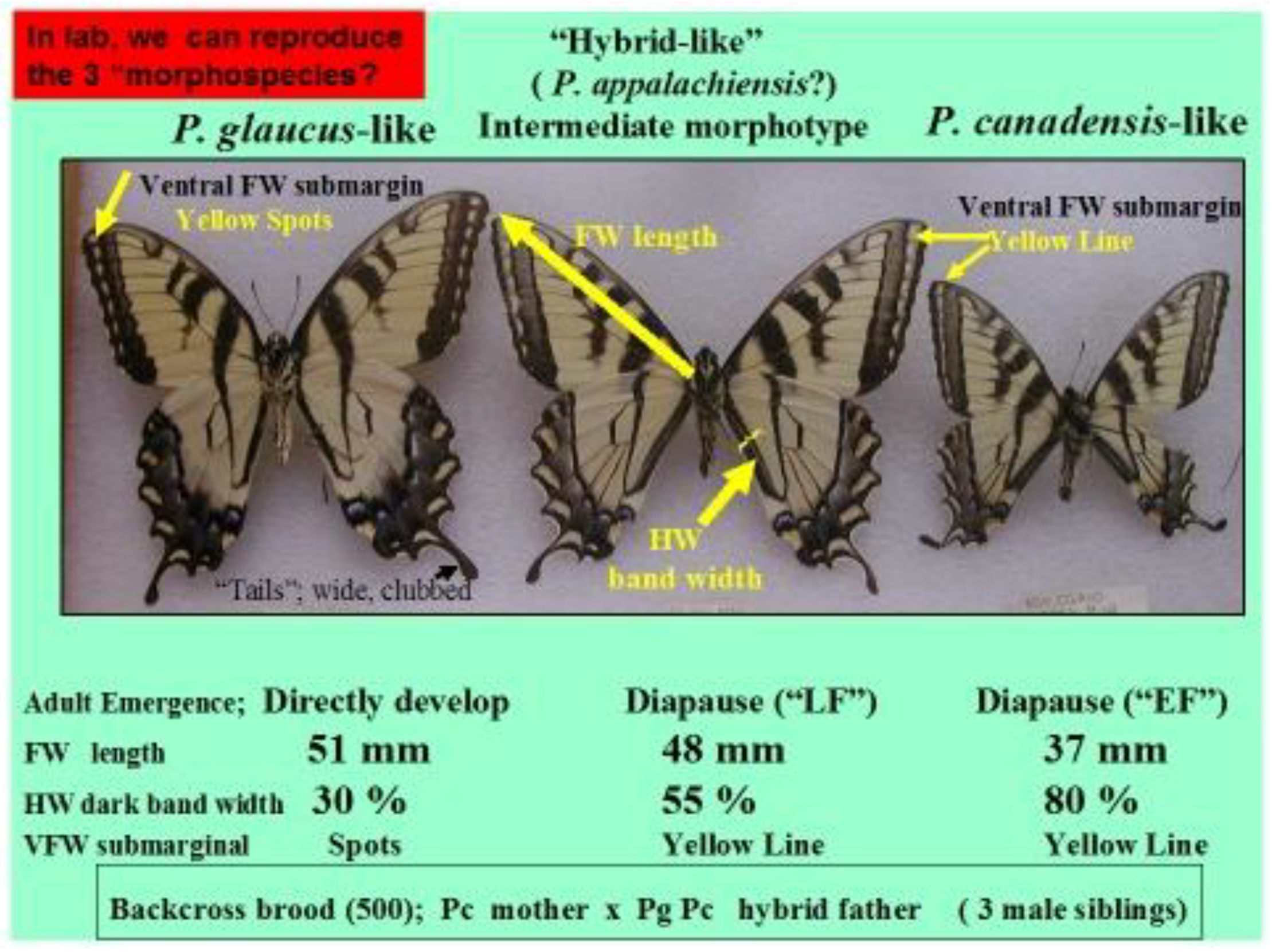
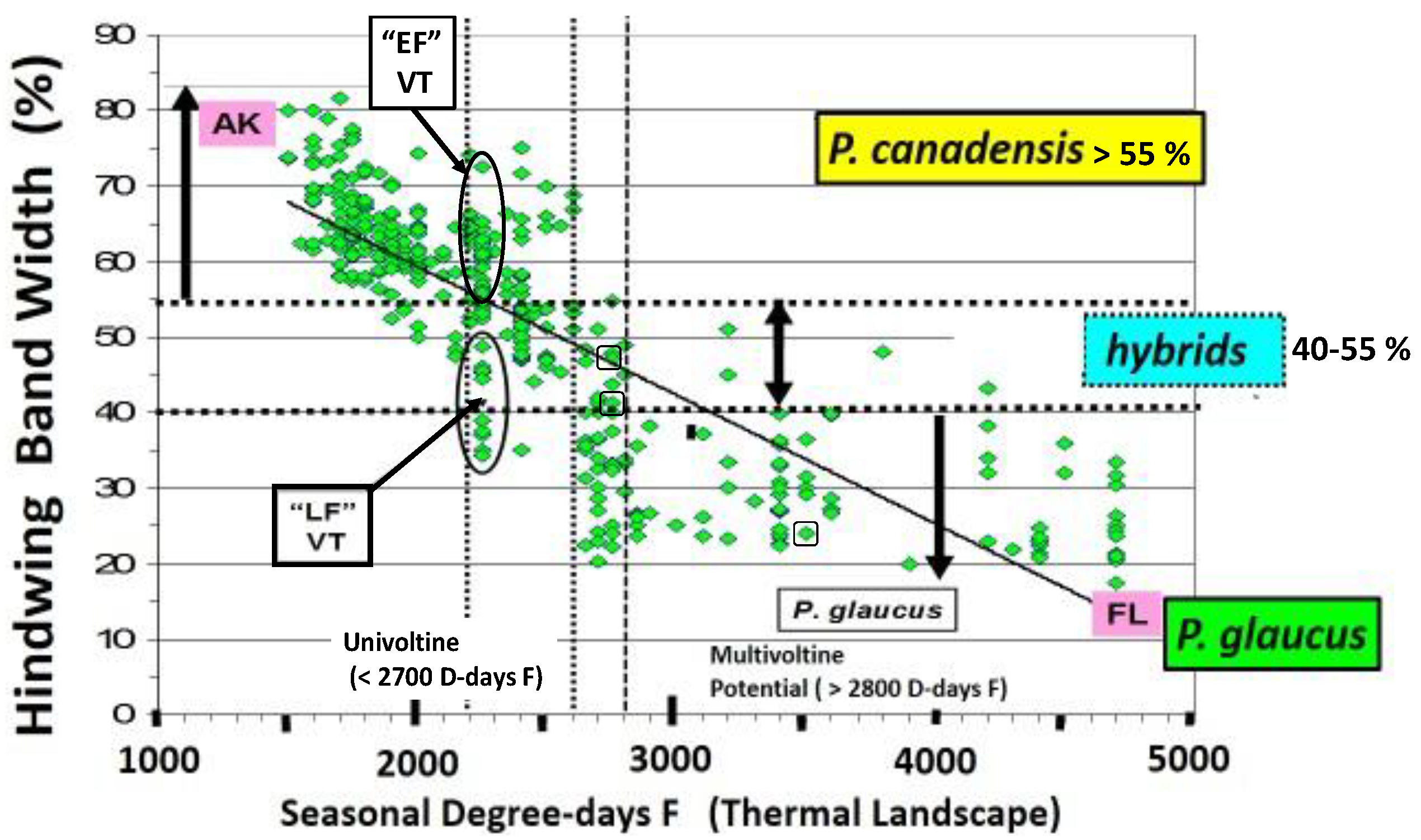
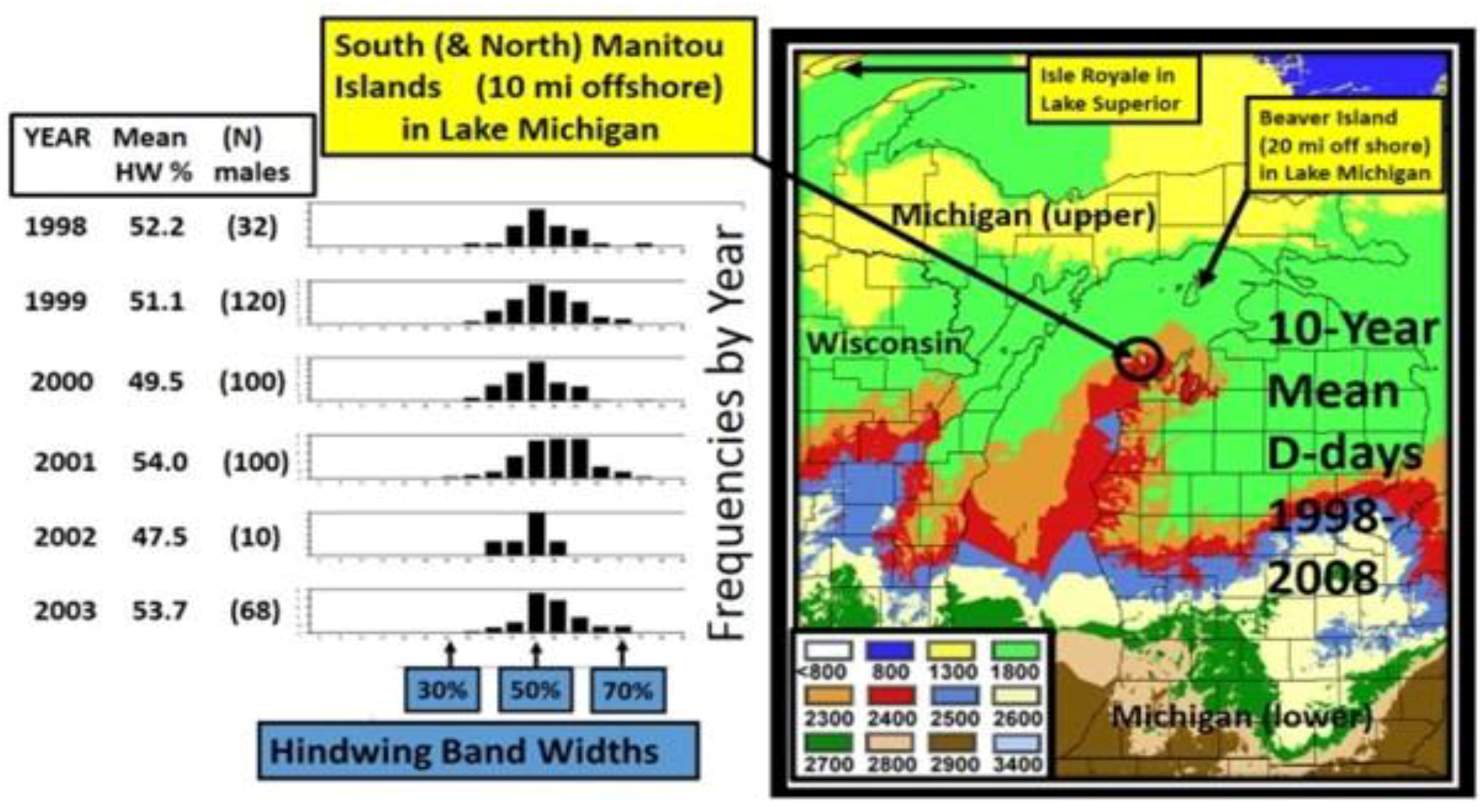
14. Species level “Invasive Genes” (Genetic Introgression)
15. The “Hybrids Are Bad” Concept
16. Plant Hybrids, Hybrid Zones, and Their Communities Should Be Protected (Community Genetics)
17. Global Change Impacts on Immune Functions and Disease Resistance (Are Animal Hybrids More Resistant to Disease?)
| Species/genotypes | Mothers | Mean total Eggs | Mean Viable Eggs,% | Total Duration neonate-pupal stage (days) | Mean Pupal Fresh Weight (mg) | Growth Rate Mg/day |
|---|---|---|---|---|---|---|
| Papilio glaucus | 246 | 110.5 b | 65.3 ab | 32.5 bc | 927 b | 28.5 c |
| P. c. × P. g. | 17 | 77.1 c | 68.0 a | 26.2 a | 1009 a | 38.5 a |
| P. g. × P. c. | 73 | 167.1 a | 62.3 b | 30.6 b | 1089 a | 35.6 b |
| Papilio canadensis | 305 | 53.5 d | 58.9 c | 34.1 c | 752 c | 22.0 d |
18. Translocations (and Assisted Migration) in Changing Environments for Maintaining Evolutionary Potential and “Genetic Rescue”
19. Are Locally-Adapted “Specialists” (or Local Endemics) More Vulnerable to Climate Change or Conservation Translocations (Assisted Colonization) than “Generalists”?
20. Rapid Genetic Changes (Evolution)
21. How Fast Can Animal Speciation Be?
22. Rapid Hybrid Speciation in Recombinant Papilio Hybrids Seems Feasible with Post-Diapause Developmental Delays and Temporal Reproductive Isolation


| Year/Date | n | Early Flight [May–June] | “EF” | n | Late Flight [Mid–July] | “LF” |
|---|---|---|---|---|---|---|
| HindWing Band (%) | Forewing Length (mm) | HindWing Band (%) | Forewing Length (mm) | |||
| 1984 | 52 | 72.0 | 44.9 | na | ||
| 1999 | 23 | 56.9 | 46.0 | 1 | 30 | 50 |
| 2000 | 125 | 54.7 | 47.1 | 35 | 39.4 | 50.6 |
| 2001 | 0 | x | x | 51 | 37.6 | 49.3 |
| 2002 | 154 | 56.1 | 48.9 | 13 | 34.3 | 50.6 |
| 2003 | 29 | 55.9 | 44.9 | 14 | 38.9 | 51.0 |
| 2004 | 205 | 63.3 | 47.0 | 12 | 48.8 | 51.4 |
| 2005 | 252 | 63.6 | 45.8 | 0 | X | X |
| 2006 | 116 | 64.3 | 46.6 | 75 | 46.3 | 50.1 |
| 2007 | 111 | 65.8 | 47.7 | 44 | 46.0 | 48.3 |
| 2008 | ||||||
| May25–June 2 | 138 | 63.9 | 46.7 | |||
| June 26–June 29 | 67 | 67.0 | 46.7 | |||
| 1–6 July | 77 | 45.3 | 47.6 | |||
| 9–16 July | 159 | 40.3 | 47.7 | |||
| 16–23 July | 51 | 42.4 | 48.3 | |||
| 27–31 July | 23 | 40.2 | 48.2 | |||
| 2009 | 142 | 64.4 | 47.0 | 36 | 45.7 | 47.3 |
| 2010 | 222 | 61.3 | 45.9 | 6 | 47.2 | 49.2 |
| 2011 | 241 | 65.3 | 46.1 | 179 | 44.4 | 49.5 |
23. Conclusions
Acknowledgments
Conflicts of Interest
References
- Diffenbaugh, N.S.; Field, C.B. Changes in ecologically critical terrestrial climate conditions. Science 2013, 341, 486–492. [Google Scholar] [CrossRef]
- Urban, M.C.; Tewksbury, J.J.; Sheldon, K.S. On a collision course: Competition and dispersal differences create no-analogue communities and cause extinctions during climate change. Proc. Roy. Soc. B 2012, 279, 2072–2080. [Google Scholar] [CrossRef]
- Van der Putten, W.H.; Macel, M.; Visser, M.E. Predicting species distribution and abundance responses to climate change: Why is it essential to include biotic interactions across trophic levels. Phil. Trans. R. Soc. B 2010, 365, 2025–2034. [Google Scholar] [CrossRef]
- Williams, J.W.; Jackson, S.T. Novel climates, no-analog communities, and ecosystem surprises. Front. Ecol. Environ. 2007, 5, 475. [Google Scholar] [CrossRef]
- Seehausen, O. Conditions when hybridization might predispose populations for adaptive radiation. J. Evol. Biol. 2013, 26, 279–281. [Google Scholar] [CrossRef]
- Blois, J.L.; Zarnetske, P.L.; Fitzpatrick, M.C.; Finnegan, S. Climate change and the past, present, and future of biotic interactions. Science 2013, 341, 499–504. [Google Scholar] [CrossRef]
- Engler, J.O.; Rodder, D.; Elle, O.; Hochkirch, A.; Second, J. Species distribution models contribute to determine the effect of climate and interspecific interactions in moving hybrid zones. J. Evol. Biol. 2013, 26, 2487–2496. [Google Scholar] [CrossRef]
- Ettinger, A.K.; Hillerislambers, J. Climate isn’t everything: Competitive interactions and variation by life stage will also affect range shifts in na warming world. Am. J. Bot. 2013, 100, 1344–1355. [Google Scholar] [CrossRef]
- Ordonez, A. Realized climate niche of North American plant taxa lagged behind climate during the end of the Pleistoicene. Am. J. Bot. 2013, 100, 1255–1265. [Google Scholar] [CrossRef]
- Hanski, I. Extinction debt at different spatial scales. Anim. Conserv. 2013, 16, 12–13. [Google Scholar] [CrossRef]
- Urban, M.C.; Zarneske, P.L.; Skelly, D.K. Moving forward: Dispersal and species interactioins determine biotic responses to climate change. Ann. N. Y. Acad. Sci. 2013, 1297, 44–60. [Google Scholar]
- Kanarek, A.; Webb, C.T. Allee effects, adaptive evolution, and invasion success. Evol. Appl. 2010, 3, 122–125. [Google Scholar] [CrossRef]
- Williams, S.E.; Shoo, L.P.; Isaac, J.L.; Hoffman, A.A.; Langham, G. Towards an integrated framework for assessing the vulnerability of specxies to climate change. PLoS Biol. 2008, 6, 2621–2626. [Google Scholar]
- McMahon, S.A.M.; Harrison, S.P.; Armbruster, W.S.; Bartein, P.J.; Beale, C.M.; Edwards, M.E.; Kattge, J.; Midgley, G.; Morin, X.; Prentice, I.C. Improving assessment of modelling of climate change impacts on global terrestrial biodiversity. Trends Ecol. Evol. 2011, 26, 249–259. [Google Scholar] [CrossRef]
- Franks, S.J.; Hoffman, A.A. Genetics of climate change adaptation. Annu. Rev. Genet. 2012, 46, 185–208. [Google Scholar] [CrossRef]
- Pauls, S.U.; Nowak, C.; Bálint, M.; Pfenninger, M. The impact of global climate change on genetic diversity within populations and species. Mol. Ecol. 2013, 22, 925–946. [Google Scholar] [CrossRef]
- Bálint, M.; Domisch, S.; Engelhardt, C.H.M.; Haase, P.; Lehrian, S.; Sauer, J.; Thessinger, K.; Pauls, S.U.; Nowak, C. Cryptic biodiversity loss linked to global climate change. Nat. Clim. Chang. 2011, 1, 313–318. [Google Scholar] [CrossRef]
- Seehausen, O. Hybridization and adaptive radiation. Trends Ecol. Evol. 2004, 19, 198–207. [Google Scholar] [CrossRef]
- Saccheri, I.J.; Brakefield, P.M. Rapid spread of immigrant genomes into inbred populations. Proc. R. Soc. B 2002, 269, 1073–1078. [Google Scholar] [CrossRef]
- Tallmon, D.A.; Luikart, G.; Waples, R.S. The alluring simplicity and complex reality of genetic rescue. Trends Ecol. Evol. 2004, 19, 489–496. [Google Scholar] [CrossRef]
- Abbott, R.; Albach, D.; Ansell, S.; Arntzen, J.W.; Baird, J.E.; Bierne, N.; Boughman, J.; Brelsford, A.; Buerkle, C.A.; Buggs, R.; et al. Hybridization and speciation. J. Evol. Biol. 2013, 26, 229–246. [Google Scholar] [CrossRef]
- Barton, N.H. Does hybridization influence speciation? J. Evol. Biol. 2013, 26, 267–269. [Google Scholar]
- Hedrick, P.W. Adaptive introgression in animals: Examples and comparison to new mutation and standing variation as sources of adaptive radiation. Mol. Ecol. 2013, 22, 4606–4618. [Google Scholar] [CrossRef]
- Scriber, J.M. Impacts of climate warming on hybrid zone movement; Geographically diffuse and biologically porous “species borders”. Insect Sci. 2011, 18, 121–159. [Google Scholar] [CrossRef]
- Putnam, A.S.; Scriber, J.M.; Andolfatto, P. Discordant divergence times among Z-chromosome regions between two ecologically distinct swallowtail butterfly species. Evolution 2007, 61, 912–927. [Google Scholar] [CrossRef]
- Kunte, K.; Shea, C.; Aardema, M.L.; Scriber, J.M.; Junger, T.E.; Gilbert, L.E.; Kronforst, M.R. Sex chromosome mosaicism and hybrid speciation among tiger swallowtail butterflies. PLoS Genet. 2011, 7, e1002274. [Google Scholar] [CrossRef]
- Zhang, W.; Kunte, K.; Kronforst, M.R. Genome-wide characterization of adaptation and speciation in tiger swallowtail butterflies using de novo transcriptome assemblies. Genome Biol. Evol. 2013, 5, 1233–1245. [Google Scholar] [CrossRef]
- Collins, N.M.; Smith, H.M. Threats and priorities in conserving swallowtails. In Swallowtail Butterflies, Their Ecology and Evolutionary Biology; Scriber, J.M., Tsubaki, Y., Lederhouse, R.C., Eds.; Scienctifc Publishers: Gainesville, FL, USA, 1995; pp. 345–353. [Google Scholar]
- Bagley, K. Climate change mix-up. Audubon 2013, 115, 00977136. [Google Scholar]
- Braby, M.F.; Eastwood, R.; Murray, N. The subspecies concept in butterflies: Has its application in taxonomy and conservation outlived its usefulness? Biol. J. Linn. Soc. 2012, 106, 699–716. [Google Scholar] [CrossRef]
- Whitham, T.G.; Bailey, J.K.; Schweitzer, J.A.; Shuster, S.M.; Bangert, R.K.; LeRoy, C.J.; Lonsdorf, E.V.; Allan, G.J.; DiFazio, S.P.; Potts, B.M.; et al. A framework for community and ecosystem genetics: From genes to ecosystems. Nat. Rev. Genet. 2006, 7, 510–523. [Google Scholar] [CrossRef]
- Kiritani, K. Different effects of climate change on population dynamics of insects. Appl. Ent. Zool. 2013, 48, 97–104. [Google Scholar] [CrossRef]
- Sgrò, C.M.; Lowe, A.J.; Hoffmann, A.A. Building evolutionary resilience for conserving biodiversity under climate change. Evol. Appl. 2011, 4, 326–337. [Google Scholar] [CrossRef]
- Wagner, H.H.; Fortin, M.-J. A conceptual framework for spatial analysis of landscape genetic data. Conserv. Genet. 2013, 14, 253–261. [Google Scholar] [CrossRef]
- Sommer, S.; McDevitt, A.D.; Balkenhol, N. Landscape genetic approaches in conservation biology and management. Conserv. Genet. 2013, 14, 249–251. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Sgró, C.M. Climate change and evolutionary adaptation. Nature 2011, 470, 479–485. [Google Scholar] [CrossRef]
- Barbash, D.; Siino, D.; Tarone, A.; Roote, J. A rapidly evolving MYB-related protein causes species isolation in Drosophila. Proc. Natl. Acad. Sci. USA 2003, 100, 5302–5307. [Google Scholar] [CrossRef]
- Kahilainen, K.; Ostbye, K.; Harrod, C.; Shikano, T.; Malinen, T.; Merila, J. Species introduction promotes hybridization and introgression in Coregonus: Is there sign of selection against hybrids? Mol. Ecol. 2011, 20, 3838–3855. [Google Scholar] [CrossRef]
- Finger, A.; Kettle, C.J.; Kaiser-Bunbury, C.N.; Valentin, T.; Doudee, D.; Matatiken, D.; Ghazoul, J. Back from the brink: Potential for genetic rescue in a critically endangered tree. Mol. Ecol. 2003, 20, 3773–3784. [Google Scholar]
- Prendergast, J.R.; Quinn, R.; Lawton, J.H.; Eversham, B.C.; Gibbons, D.W. Rare species, the coincidence of diversity hotspots and conservation strategies. Nature 1993, 365, 335–337. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.B.; deFonseca, G.A.B.; Kent, J. Hodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar]
- Carnaval, A.C.; Moritz, C. Historical climate change predicts current biodiversity patterns in the Brazilian Atlantic Rainforest. J. Biogeogr. 2008, 35, 1187–1201. [Google Scholar] [CrossRef]
- Malcolm, J.R.; Liu, C.; Neilson, R.R.; Hansen, L.; Hannah, L. Global warming and extinctions of endemic species from biodiversity hotspots. Conserv. Biol. 2006, 20, 538–548. [Google Scholar] [CrossRef]
- Carnaval, A.C.; Hickerson, M.J.; Rodrigues, M.T.; Haddad, C.F.B.; Moritz, C. Stability predicts genetic diversity in the Brazilian Atlantic Rainforest hotspot. Science 2009, 323, 785–789. [Google Scholar] [CrossRef]
- Fritz, S.A.; Schnitzler, J.; Eronen, J.T.; Hof, C.; Böhning-Gaese, K.; Graham, C.H. Diversity in time and space: Wanted dead and alive. Trends Ecol. Evol. 2013, 28, 509–516. [Google Scholar] [CrossRef]
- Diniz-Filho, J.A.F.; Telles, M.P.C. Optimization procedures for establishing reserve networks for biodiversity conservation taking into account population genetic structure. Genet. Mol. Biol. 2006, 29, 207–214. [Google Scholar] [CrossRef]
- Chapin, F.S.; Marek, A.F.; Mitchell, R.A.; Dickinson, K.J.M. Design principles for socio-ecological transformation toward sustainability: Lessons from New Zealand sense of place. Ecosphere 2012, 3. article40. [Google Scholar]
- Hitzhusen, G.E.; Tucker, M.E. The potential of religion for earth stewardship. Front. Ecol. Environ. 2013, 11, 368–376. [Google Scholar] [CrossRef]
- Fisher, J.; Dyball, R.; Fazey, I.; Gross, C.; Dovers, S.; Ehrlich, P.R.; Brulle, R.J.; Christensen, C.; Borden, R.J. Human behavior and sustainability. Front. Ecol. Environ. 2012, 10, 153–160. [Google Scholar] [CrossRef]
- Reiners, D.S.; Reiners, W.A.; Lockwood, J.A. The relationship between environmental advocacy, values, and science: A survey of ecological scientist’s attitudes. Ecol. Appl. 2013, 23, 1226–1242. [Google Scholar] [CrossRef]
- Santamaria, L.; Méndez, P.F. Evolution in biodiversity policy—Current gaps and future needs. Evol. Appl. 2012, 5, 202–218. [Google Scholar] [CrossRef] [Green Version]
- Tobin, P.C.; Bai, B.B.; Eggen, D.A.; Leonard, D.S. The ecology, geopolitics, and economics of managing Lymantira dispar in the United States. Intern. J. Pest Manag. 2012, 58, 195–210. [Google Scholar] [CrossRef]
- Rosauer, D.F.; Mooers, A.O. Nurturing the use of evolutionary diversity in nature conservation. Trends Ecol. Evol. 2013, 28, 322–323. [Google Scholar] [CrossRef]
- Winter, M.; Devictor, V.; Schweiger, O. Phylogenetic diversity and nature conservation: Where are we? Trends Ecol. Evol. 2013, 28, 199–204. [Google Scholar] [CrossRef]
- Gompert, Z.; Lucas, L.K.; Nice, C.C.; Fordyce, J.A.; Forister, M.L.; Buerkle, C.A. Genomic regions with a history of divergent selection affect fitness of hybrids between two butterfly species. Evolution 2012, 66, 2167–2181. [Google Scholar] [CrossRef]
- Gompert, Z.; Parchman, T.L.; Buerkle, C.A. Genomics of isolation in hybrids. Phil. Trans. R. Soc. B 2012, 367, 439–450. [Google Scholar] [CrossRef]
- Carstens, B.C.; Pelletier, T.A.; Reid, N.M.; Satler, J.D. How to fail at species delimitation. Mol. Ecol. 2013, 22, 4369–4383. [Google Scholar] [CrossRef]
- Reusch, T.B.H.; Ehlers, A.; Hammerli, A.; Worm, B. Ecosystem recovery after climatic extremes enhanced by genotypic diversity. Proc. Natl. Acad. Sci. USA 2005, 102, 2826–2831. [Google Scholar] [CrossRef]
- Palkovacs, E.P.; Kinnison, M.T.; Correa, C.; Dalton, C.M.; Hendry, A.P. Fates beyond traits: Ecological consequences of human-induced trait change. Evol. Appl. 2012, 5, 183–191. [Google Scholar] [CrossRef]
- Armbruster, P.; Reed, D.H. Inbreeding depression in benign and stressful environments. Heredity 2005, 95, 235–242. [Google Scholar] [CrossRef]
- Bijlsma, R.; Loeschcke, V. Genetic erosion impedes adaptive responses to stressful environments. Evol. Appl. 2012, 5, 117–129. [Google Scholar] [CrossRef]
- Reed, D.H.; Fox, C.W.; Enders, L.S. Inbreeding-stress-interactions: Evolutionary conservation consequences. Ann. N. Y. Acad. Sci. 2012, 1256, 33–48. [Google Scholar] [CrossRef]
- Behm, J.E.; Ives, A.R.; Boughman, J.W. Breakdown in post-mating isolation and collapse of a species pair through hybridization. Am. Nat. 2010, 175, 11–26. [Google Scholar] [CrossRef]
- Seehausen, O.; Takimoto, G.; Roy, D.; Jokela, J. Speciation reversal and biodiversity dynamics with hybridization in changing environments. Mol. Ecol. 2007, 17, 30–44. [Google Scholar] [CrossRef]
- McKinnon, J.S.; Taylor, E.B. Biodiversity: Species choked and blended. Nature 2012, 482, 313–314. [Google Scholar] [CrossRef]
- Arnold, M.L.; Martin, N.H. Hybrid fitness across time and habitats. Trends Ecol. Evol. 2010, 25, 530–536. [Google Scholar] [CrossRef]
- Collins, M.M. Genetics and ecology of a hybrid zone in Hyalophora (Lepidoptera: Saturniidae). Univ. Calif. Publ. Entomol. 1984, 104, 1–93. [Google Scholar]
- Gompert, Z.; Fordyce, J.A.; Forister, M.; Shapiro, A.M.; Nice, C.C. Homoploid hybrid speciation in an extreme habitat. Science 2006, 314, 1923–1925. [Google Scholar] [CrossRef]
- Nice, C.C.; Gompert, Z.; Fordyce, J.A.; Forister, M.L.; Lucas, L.K. Hybrid speciation and independent evolution in lineages of alpine butterflies. Evolution 2013, 67, 1055–1068. [Google Scholar] [CrossRef]
- Mouquet, N.; deVictor, V.; Meynard, C.N.; Munoz, F.; Bersier, L.-F.; Chave, J.; Couteron, P.C.; Daleckky, A.; Fontane, C.; Gravel, D.; et al. Ecophylogenetics: Advances and perspectives. Biol. Rev. 2012, 87, 769–785. [Google Scholar] [CrossRef]
- Bracken, M.E.S.; Low, N.H.N. Realistic losses of rare species disproportionately impact higher trophic levels. Ecol. Lett. 2012, 15, 461–467. [Google Scholar] [CrossRef]
- Williams, S.E.; Williams, Y.M.; vanderWal, J.; Shoo, L.P.; Johnson, C.N. Ecological specialization and population size in a biodiversity hotspot: How rare species avoid extinction. Proc. Natl. Acad. Sci. USA 2009, 106, 19737–19741. [Google Scholar] [CrossRef]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Syst. 2006, 37, 637–669. [Google Scholar]
- Thomas, C.D.; Bulman, C.R.; Wilson, R.J. Where within a species geographical range do species survive best? A matter of scale. Insect Conserv. Divers. 2008, 1, 2–8. [Google Scholar] [CrossRef]
- Daniels, J.C. Cooperative conservation efforts to help recover an endangered south Florida butterfly. Insect Conserv. Divers. 2009, 2, 62–64. [Google Scholar] [CrossRef]
- Roy, H.E.; Hails, R.S.; Hesketh, H.; Roy, D.B.; Pell, J.K. Beyond biological control: Non-pest insects and their pathogens in a changing world. Insect Conserv. Divers. 2009, 2, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Tyliankis, J.M. Warming up the food webs. Science 2009, 323, 1300–1301. [Google Scholar] [CrossRef]
- Didham, R.K.; Basset, Y.; Leather, S.R. Research needs in insect conservation and diversity. Insect Conserv. Divers. 2010, 3, 1–4. [Google Scholar] [CrossRef]
- Massad, T.J.; Dyer, L.A. A meta-analysis of the effects of global environment change on plant-herbivore interactions. Arthropod Plant Int. 2010, 4, 181–188. [Google Scholar] [CrossRef]
- Zvereva, E.L.; Kozlov, M.V.; Hilker, M. Evolutionary variations on a theme: Host plant specialization in five geographical populations of the leaf beetle Chrysomela lapponica. Popul. Ecol. 2010, 52, 389–396. [Google Scholar] [CrossRef]
- Isaacs, R.; Tuell, J.; Fiedler, A.; Gardner, M.; Landis, D. Maximizing arthropod-mediated ecosystem services in agricultural landscapes: The role of native plants. Front. Ecol. Environ. 2009, 7. [Google Scholar] [CrossRef]
- Chown, S.L.; Hioffmann, A.A.; Kristensen, T.N.; Angilletta, M.J.; Stenseth, N.C.; Pertoldi, C. Adapting to climate change: A perspective from evolutionary physiology. Clim. Res. 2010, 43, 3–15. [Google Scholar] [CrossRef]
- Gilman, S.E.; Urban, M.C.; Tewksbury, J.; Gilchrist, G.W.; Holt, R.D. A framework for community interactions under climate change. Trends Ecol. Evol. 2010, 25, 325–331. [Google Scholar] [CrossRef]
- Gilman, R.T.; Fabina, N.S.; Abbott, K.C.; Rafferty, N.E. Evolution of plant-pollinator mutualisms in response to climate change. Evol. Appl. 2012, 5, 2–16. [Google Scholar] [CrossRef]
- Magurran, A.E.; Baillie, S.R.; Buckland, S.T.; Dick, J.M.; Elston, D.A.; Scott, E.M.; Somerville, P.J.; Watt, A.D. Long-term datasets in biodiversity research and monitoring: Assessing change in ecological communities through time. Trends Ecol. Evol. 2010, 25, 574–582. [Google Scholar] [CrossRef]
- Sutherst, R.W.; Constable, F.; Finlay, K.J.; Harringtoin, R.; Luck, J.; Zalucki, M.P. Adapting to crop pest and pathogen risks under a changing climate. Clim. Chang 2011, 2, 220–237. [Google Scholar]
- Juroszek, P.; von Tiedmann, A. Plant pathogens, insect pests and weeds in a changing global climate: A review of approaches, challenges, research gaps, key studies and concepts. J. Agric. Sci. 2013, 151, 163–185. [Google Scholar] [CrossRef]
- Burkle, L.A.; Martin, J.C.; Knight, T.M. Plant-pollinator interactions over 120 years: Loss of species, co-occurrence, and function. Science 2013, 339, 1611–1615. [Google Scholar] [CrossRef]
- Hunter, M.C.; Hunter, M.D. Designing for conservation in the built environment. Insect Conserv. Divers. 2008, 1, 189–196. [Google Scholar]
- Haaland, C.; Naisbit, R.E.; Bersier, L.-F. Sown wildflower strips for insect conservation: A review. Insect Conserv. Divers. 2011, 4, 60–80. [Google Scholar] [CrossRef]
- Lankau, R.A.; Strauss, S.Y. Newly rare or newly common: Evolutionary feedbacks through changes in population density and relative species abundance, and their management implications. Evol. Appl. 2011, 4, 338–353. [Google Scholar] [CrossRef]
- Gillson, L.; Dawson, T.P.; Jack, S.; McGeoch, M.A. Accommodating climate change contingencies in conservation strategy. Trends Ecol. Evol. 2013, 28, 135–142. [Google Scholar] [CrossRef]
- Simberloff, D.; Martin, J.-L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; Garcia-Berthou, E.; Pascal, M.; et al. Combined effects of global pressures on animal-mediated pollination. Trends Ecol. Evol. 2013, 28, 524–530. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Varo, J.P.; Biesmeijer, J.C.; Bommarco, R.; Potts, S.G.; Schweiger, O.; Smith, H.G.; Steffan-Dewenter, I.; Szengyörgyi, H.; Woyciechowski, M.; Vilà, M. Combined effects of global pressures on animal-mediated pollination. Trends Ecol. Evol. 2013, 28, 524–530. [Google Scholar] [CrossRef] [Green Version]
- Culumber, Z.W.; Shepard, D.B.; Coleman, S.W.; Rosenthal, G.G.; Tobler, M. Physiological adaptation along environmental gradients and replicated hybrid zone structure in swordtails (Teleostei: Xiphorus). J. Evol. Biol. 2012, 25, 1800–1814. [Google Scholar] [CrossRef]
- Scriber, J.M. Integrating ancient patterns and current dynamics of insect-plant interactions: Taxonomic and geographic variation in herbivore specialization. Insect Sci. 2010, 17, 471–507. [Google Scholar] [CrossRef]
- Kendra, P.A.; Montgomery, W.S.; Niogret, J.; Epsky, N.D. An uncertain future for American Lauraceae: A lethal threat from Redbay Ambrosia beetlke and Laurel Wilt Disease. Am. J. Plant Sci. 2013, 4, 727–738. [Google Scholar] [CrossRef]
- Pavulaan, H.; Wright, D.M. Pterourus appalachiensis (Papilionidae: Papilioninae), a new swallowtail butterfly from the Appalachian region of the United States. Taxon. Rept. 2002, 3, 1–20. [Google Scholar]
- Scriber, J.M.; Ording, G.L. Ecological speciation without host plant specialization: Possible origins of a recently described cryptic Papilio species (Lepidoptera: Papilionidae). Ent. Exp. Appl. 2005, 115, 247–263. [Google Scholar] [CrossRef]
- Ording, G.J.; Mercade, R.J.; Aardema, M.L.; Scriber, J.M. Allochronic isolation and incipient hybrid speciation in tiger swallowtail butterflies. Oecologia 2010, 162, 523–531. [Google Scholar] [CrossRef]
- Seehausen, O. Progressive levels of trait divergence along a “speciation transect” in the lake victoria cichlid fish Pundamila. In Speciation and Patterns of Diversity; Butlin, R., Bridle, R., Schluter, D., Eds.; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Feder, J.L.; Egan, S.P.; Nosil, P. The genomics of speciation-with-gene-flow. Trends Genet. 2012, 28, 342–350. [Google Scholar] [CrossRef]
- Harrison, R.G. The language of speciation. Evolution 2011, 66, 3643–3657. [Google Scholar] [CrossRef]
- Webb, C.O.; Losos, J.B.; Agrawal, A.A. Integrating phylogenies into community ecology. Ecology 2006, 87, 51–52. [Google Scholar]
- Raffa, K.F.; Aukema, B.H.; Bentz, B.J.; Carroll, A.L.; Hicke, J.A.; Turner, M.G.; Romme, W.H. Cross-scale drivers of natural disturbances prone to anthropogenic amplification: The dynamics of bark beetle eruptions. BioScience 2008, 58, 501–517. [Google Scholar] [CrossRef]
- Bailey, J.K.; Schweitzer, J.A.; Ubeda, F.; Koricheva, J.; LeRoy, C.J.; Madrich, M.D.; Rehill, B.J.; Bangert, R.KI.; Fischer, D.G.; Allan, G.J.; et al. From genes to ecosystems: A synthesis of the effects of plant genetic factors across levels of organization. Phil. Trans. R. Soc. B 2009, 364, 1607–1616. [Google Scholar] [CrossRef]
- Norberg, J.; Urban, M.C.; Klausmeier, C.A.; Loeuille, N. Eco-evolutionary responses of biodiversity to climate change. Nat. Clim. Chang 2012, 2, 747–751. [Google Scholar] [CrossRef]
- Segelbacher, G.; Cushman, S.A.; Epperson, B.K.; Fortin, M.-J.; Francois, O.; Handy, O.J.; Holderegger, R.; Manel, S. Applications of landscape genetics in conservation biology: Concepts and challenges. Conserv. Genet. 2010, 11, 375–385. [Google Scholar] [CrossRef]
- Holderegger, R.; Wagner, H.H. Landscape genetics. Bioscience 2008, 58, 199–207. [Google Scholar] [CrossRef]
- Storfer, A.; Murphy, M.A.; Spear, S.F.; Holderegger, R.; Waits, L.P. Landscape genetics: Where are we now? Mol. Ecol. 2010, 19, 3496–3514. [Google Scholar] [CrossRef]
- Sork, V.L.; Waits, L. Contributions of landscape genetics—Approaches, insights, and future potential. Mol. Ecol. 2010, 19, 3489–3495. [Google Scholar] [CrossRef]
- Storfer, A.; Murphy, M.A.; Evans, J.S.; Goldberg, C.S.; Robinson, S.; Spear, S.F.; Dezzani, R.; Delmelle, E.; Vierling, L.; Waits, L.P. Putting the “landscape” in landscape genetics. Heredity 2007, 98, 128–142. [Google Scholar] [CrossRef]
- Clarke, K.E.; Rinderer, T.E.; Franck, P.; Quezada-Euan, J.G.; Oldroyd, B.P. The Africanization of honeybees (Apis mellifera L.) of the Yucatan: A study of a massive hybridization event across time. Evolution 2002, 56, 1462–1474. [Google Scholar]
- Manel, S.; Joost, S.; Epperson, B.K.; Holderegger, R.; Storfer, A.; Rosenberg, M.S.; Scribner, K.T.; Bonin, A.; Fortin, M.J. Perspectives on the use of landscape genetics to detect genetic adaptive variation in the field. Mol. Ecol. 2010, 19, 3760–3772. [Google Scholar] [CrossRef]
- Spear, S.F.; Balkenhol, N.; Fortin, M.J.; McRae, B.H.; Scribner, K.T. Use of resistance surfaces for landscape genetics studies: Consideration for parameterization and analysis. Mol. Ecol. 2010, 19, 3576–3591. [Google Scholar] [CrossRef]
- Aardema, M.L.; Scriber, J.M.; Hellmann, J.J. Considering local adaptation in issues of Lepidopteran conservation—A review and recommendations. Am. Midl. Nuturalist 2011, 165, 294–303. [Google Scholar] [CrossRef]
- Evans, L.M.; Allan, G.J.; Shuster, S.M.; Woolbright, S.A.; Whitham, T.G. Tree hybridization and genotypic variation drive cryptic speciation of a specialist mite herbivore. Evolution 2008, 62, 3027–3040. [Google Scholar] [CrossRef]
- Dowling, T.E.; Secor, C.L. The role of hybridization and introgression in the diversification of animals. Annu. Rev. Ecol. Syst. 1997, 28, 593–620. [Google Scholar] [CrossRef]
- Mallet, J. Hybrid speciation. Nature 2007, 446, 279–283. [Google Scholar] [CrossRef]
- Mavarez, J.; Linares, M. Homoploid hybrid speciation in animals. Mol. Ecol. 2008, 17, 4181–4185. [Google Scholar] [CrossRef]
- Dittrich-Reed, D.R.; Fitzpatrick, B.M. Transgressive hybrids as hopeful monsters. Evol. Biol. 2012, 40, 310–315. [Google Scholar] [CrossRef]
- Rhymer, J.M.; Simberloff, D. Extinction by hybridization and introgression. Annu. Rev. Ecol. Syst. 1996, 27, 83–109. [Google Scholar] [CrossRef]
- Allendorf, F.W.; Leary, R.F.; Spruell, P.; Wenberg, J.K. The problems with hybrids: Setting conservation guidelines. Trends Ecol. Evol. 2001, 16, 613–622. [Google Scholar] [CrossRef]
- Wirtz, P. Mother species-father species: Unidirectional hybridization in animals with female choice. Anim. Behav. 1999, 58, 1–12. [Google Scholar] [CrossRef]
- Andolfatto, P.; Scriber, J.M.; Charlesworth, B. No association between mitochondrial DNA haplotypes and a female-limited mimicry phenotype in Papilio glaucus. Evolution 2003, 57, 305–316. [Google Scholar]
- Qvarnström, A.; Bailey, R.I. Speciation through evolution of sex-linked genes. Heredity 2009, 102, 4–15. [Google Scholar] [CrossRef]
- Joost, S.; Vuilleumire, S.; Jensen, J.D.; Schoville, S.; Leempoel, K.; Stuki, S.; Widmer, I.; Melodelima, C.; Rolland, J.; Manel, S. Uncovering the genetic basis of adaptive change: On the intersection of landscape genomics and theoretical population genetics. Mol. Ecol. 2013, 22, 3659–3665. [Google Scholar] [CrossRef]
- Stockwell, C.A.; Hendry, A.P.; Kinnison, M.T. Contemporary evolution meets conservation biology. Trends Ecol. Evol. 2003, 18, 94–101. [Google Scholar] [CrossRef]
- Hannah, L.E.E. Climate change, connectivity, and conservation success. Conserv. Biol. 2011, 25, 1139–1142. [Google Scholar] [CrossRef]
- Hill, J.K.; Griffiths, H.M.; Thomas, C.D. Climate change and evolutionary adaptations at species’ ranges. Annu. Rev. Entomol. 2011, 56, 143–159. [Google Scholar] [CrossRef]
- Dawson, T.P.; Jackson, S.T.; House, J.I.; Prentice, I.C.; Mace, G.M. Beyond predictions: Biodiversity conservation in a changing climate. Science 2011, 332, 53–58. [Google Scholar] [CrossRef]
- Singer, M.C.; Parmesan, C. Phenological asychrony between herbivorous insects and their hosts: Signal of climate change or pre-existing adaptive strategy? Phil. Trans. R. Soc. B 2010, 365, 3161–3176. [Google Scholar] [CrossRef]
- Koricheva, J.; Larsson, S. Insect performance on experimentally stressed woody plants: A meta-analysis. Annu. Rev. Entomol. 1998, 43, 195–216. [Google Scholar] [CrossRef]
- Kocmankova, E.; Trnka, M.; Eitzinger, J.; Dubrovsky, M.; Stepanek, P.; Semeradova, D.; Balek, J.; Skalak, P.; Farda, A.; Juroch, J.; et al. Estimating the impact of climate change on the occurrence of selected pests at high spatial resolution: A novel approach. J. Agric. Sci. 2011, 149, 185–195. [Google Scholar] [CrossRef]
- Pöyry, J.; Leinonen, R.; Söderman, G.; Nieminen, M.; Heikkinen, R.K.; Carter, T.R. Climate-induced increase of moth multivoltinism in boreal regions. Glob. Ecol. Biogeogr. 2011, 20, 289–298. [Google Scholar] [CrossRef]
- Corbet, P.S.; Suhling, F.; Soendgerath, D. Voltinism in Odonata: A review. Intern. J. Odonatol. 2006, 9, 1–44. [Google Scholar] [CrossRef]
- Altermatt, F. Climatic warming increases voltinism in European butterflies and moths. Proc. R. Soc. Lon. B 2010, 277, 1281–1287. [Google Scholar] [CrossRef]
- Chen, I.-C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Carroll, S.P. Conciliation biology: The eco-evolutionary management of permanently invaded biotic systems. Evol. Appl. 2011, 4, 184–199. [Google Scholar] [CrossRef]
- Roderick, G.K.; Navajas, M. Genes in new environments: Genetics and evolution in biological control. Nat. Rev. Genet. 2003, 4, 883–899. [Google Scholar]
- Thomas, C.D. Translocation of species, climate change, and the end of trying to recreate past ecological communities. Trends Ecol. Evol. 2011, 26, 216–221. [Google Scholar] [CrossRef]
- Benton, M.J. Diversification and extinction in the history of life. Science 1995, 268, 52–58. [Google Scholar]
- Loxdale, H.D. Rapid genetic changes in natural insect populations. Ecol. Entomol. 2010, 35, 155–164. [Google Scholar] [CrossRef]
- Feder, J.L.; Flaxmann, S.M.; Egan, S.P.; Nosil, P. Hybridization and the build-up of genomic divergence during speciation. J. Evol. Biol. 2013, 26, 261–266. [Google Scholar] [CrossRef]
- Arnold, M.L.; Arnold, M.L. Natural Hybridization and Evolution; Oxford University Press: New York, NY, USA, 1997; p. 232. [Google Scholar]
- Gavrilets, S.; Li, H.; Vose, M.D. Rapid parapatric speciation on holey adaptive landscapes. Proc. R. Soc. Lond. B 1998, 265, 1483–1489. [Google Scholar] [CrossRef]
- Thompson, J.N. The Geographic Mosaic of Coevolution; University Chicago Press: Chicago, IL, USA, 2005. [Google Scholar]
- Ehrlich, P.R.; Raven, P.H. Butterflies and plants: A study in coevolution. Evolution 1964, 18, 586–608. [Google Scholar] [CrossRef]
- Scriber, J.M. Evolution of insect-plant relationships: Chemical constraints, coadaptation, and concordance of insect/plant traits. Entomol. Exp. Appl. 2002, 104, 217–235. [Google Scholar]
- Berenbaum, M.R.; Feeny, P.P. Chemical mediation of host-plant specialization: The Papilio paradigm. In Specialization, Speciation, and Radiation: The Evolutionary Biology of Herbivorous Insects; Tilmon, K.J., Ed.; California University Press: Berkeley, CA, USA, 2008; pp. 3–19. [Google Scholar]
- Futuyma, D.J.; Agrawal, A.A. Macroevolution and biological diversity of plants and herbivores. Proc. Natl. Acad. Sci. USA 2009, 106, 18054–18061. [Google Scholar] [CrossRef]
- Nyman, T. To speciate, or not to speciate? Resource heterogeneity, the subjectivity of similarity, and the macroevolutionary consequences of niche-width shifts in plant-feeding insects. Biol. Rev. 2010, 85, 393–411. [Google Scholar] [CrossRef]
- Janz, N. Ehrlich and Raven revisited: Mechanisms underlying codiversification of plants and enemies. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 71–89. [Google Scholar] [CrossRef]
- Forister, M.L.; Dyer, L.A.; Singer, M.S.; Stireman, J.O.; Lill, J.T. Revisiting the evolution of ecological specialization, with emphasis on insect-plant interactions. Ecology 2012, 93, 981–991. [Google Scholar] [CrossRef]
- Janz, N.; Nylin, S. The oscillation hypothesis of host-plant range and speciation. In Speciation, Specialization, and Radiation: The Evolutionary Biology of Herbivorous Insects; Tilmon, K.J., Ed.; University California Press: Berkeley, CA, USA, 2008; pp. 203–215. [Google Scholar]
- Nylin, S.; Janz, N. Butterfly host plant range: An example of plasticity as a promoter of speciation? Evol. Ecol. 2009, 23, 137–146. [Google Scholar] [CrossRef]
- Fordyce, J.A. Host shifts and evolutionary radiations of butterflies. Proc. R. Soc. B 2010, 277, 3735–3743. [Google Scholar] [CrossRef]
- Dennis, R.L.H.; Dapporto, L.; Fattorini, S.; Cook, L.M. The generalism-specialism debate: The role of generalists in the life and death of species. Biol. J. Linn. Soc. 2011, 104, 725–737. [Google Scholar] [CrossRef]
- Loxdale, H.D.; Lushai, G.; Harvey, J.A. The evolutionary improbability of “generalism” in nature, with special reference to insects. Biol. J. Linn. Soc. 2011, 103, 1–18. [Google Scholar] [CrossRef]
- Hodkinson, I.D. Terrestrial insects along elevation gradients: Species and community responses to altitude. Biol. Rev. 2005, 80, 489–513. [Google Scholar] [CrossRef]
- Wilson, R.J.; Gutierrez, D.; Martinez, J.; Aguto, R.; Monserrat, V.J. Changes to the elevational limits and extent of species ranges associated with climate change. Ecol. Lett. 2005, 8, 1138–1146. [Google Scholar] [CrossRef]
- Franco, A.M.A.; Hill, J.K.; Kitschke, C.; Collinham, Y.C.; Roy, D.B.; Fox, R.; Huntley, B.; Thomas, C.D. Impacts of climate warming and habitat loss on extinctions at species’ low-latitude range boundaries. Glob. Chang Biol. 2006, 12, 1545–1553. [Google Scholar] [CrossRef]
- Lewinsohn, R.C.; Roslin, T. Four ways towards tropical herbivore mega-diversity. Ecol. Lett. 2008, 11, 398–416. [Google Scholar] [CrossRef]
- Lomilino, M.V.; Riddle, B.R.; Whittaker, R.J.; Brown, J.H. Biogeography, 4th ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2010. [Google Scholar]
- Gaston, K.J. Global patterns in biodiversity. Nature 2000, 40, 220–227. [Google Scholar] [CrossRef]
- Willig, M.R.; Kaufmann, D.M.; Stevens, R.D. Latitudinal gradients of biodiversity: Pattern, process, scale, and synthesis. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 273–309. [Google Scholar] [CrossRef]
- Case, T.J.; Holt, R.D.; McPeek, M.A.; Keitt, T.H. The community context of species’ borders: Ecological and evolutionary perspectives. Oikos 2005, 108, 28–40. [Google Scholar] [CrossRef]
- Diniz-Filho, J.A.F.; deMarco, P.; Hawkins, B.A. Defying the curse of ignorance: Perspectives in insect macroecology and conservation biology. Insect Conserv. Divers. 2010, 3, 172–179. [Google Scholar]
- Hawkins, B.A. Multiregional comparisons of the ecological and phylogenetic structure of butterfly species richness gradients. J. Biogeogr. 2010, 37, 647–656. [Google Scholar] [CrossRef]
- Hawkins, B.A.; DeVries, P.J. Tropical niche conservatism and species richness in North American butterflies. J. Biogeogr. 2009, 36, 1698–1711. [Google Scholar] [CrossRef]
- Parmesan, C.; Gaines, S.; Gonzalez, L.; Kaufman, D.M.; Kingslover, J.; Peterson, A.T.; Sagarin, R. Empirical perspectives on species borders: From traditional biogeography to global change. Oikos 2005, 108, 58–75. [Google Scholar] [CrossRef]
- Leather, S.R.; Bassett, Y.; Hawkins, B.A. Insect conservation: Finding the way forward. Insect Conserv. Divers. 2008, 1, 67–69. [Google Scholar] [CrossRef]
- Janzen, D.H. Why mountain passes are higher in the tropics. Am. Nat. 1967, 101, 233–249. [Google Scholar]
- Deutsch, C.A.; Tewksbury, J.J.; Huey, R.B.; Sheldon, K.S.; Ghalambor, C.K.; Haak, D.C.; Martin, P.R. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA 2008, 105, 6668–6672. [Google Scholar]
- Ghalambor, C.K.; Huey, R.B.; Martin, P.R.; Tewksbury, J.J.; Wang, G. Are mountain passes higher in the tropics? Janzen’s hypothesis revisited. Integr. Comp. Biol. 2006, 46, 5–17. [Google Scholar] [CrossRef]
- Huey, R.B.; Tewksbury, J.J. Can behavior douse the fire of climate warming? Proc. Natl. Acad. Sci. USA 2009, 106, 3647–3648. [Google Scholar] [CrossRef]
- Merila, J. Genetic constraints on adaptation. Science 2009, 325, 1212–1213. [Google Scholar] [CrossRef]
- Keller, I.; Seehausen, O. Thermal adaptation and ecological speciation. Mol. Ecol. 2012, 21, 782–799. [Google Scholar] [CrossRef]
- Bonebrake, T.C.; Deutsch, C.A. Climate heterogeneity modulates impact of warming on tropical insects. Ecology 2012, 93, 449–455. [Google Scholar] [CrossRef]
- Kellermann, V.; von Heerwaarden, B.; Sgrò, C.M.; Hoffmann, A.A. Fundamantal evolutionary limits in ecological traits drive Drosophila species distributions. Science 2009, 325, 1244–1246. [Google Scholar] [CrossRef]
- Gienapp, P.; Teplitsky, C.; Alho, J.A.; Mills, A.; Merila, J. Climate change and evolution: Disentangling environmental and genetic responses. Mol. Ecol. 2008, 17, 167–178. [Google Scholar] [CrossRef]
- Buckley, L.B.; Tewksbury, J.J.; Deutsch, C.A. Can terrestrial ectotherms escape the heat of climate change by moving? Proc. R. Soc. B Biol. Sci. 2013, 280. [Google Scholar] [CrossRef]
- Sheldon, K.S.; Yang, S.; Tewksbury, J.J. Climate change and community disassembly: Impacts of warming on tropical and temperate montane community structure. Ecol. Lett. 2011, 14, 1191–1200. [Google Scholar] [CrossRef]
- Corlett, R.T. Climate change in the tropics: The end of the world as we know it? Biol. Conserv. 2012, 151, 22–25. [Google Scholar] [CrossRef]
- Diamond, S.E.; Sorger, D.M.; Huler, J.; Pelini, S.L.; DelToro, I.; Hirsch, C.; Oberg, E.; Dunn, R. Who likes it hot? A global analysis of climate, ecological, and evolutionary determinants of warming tolerance in ants. Glob. Chang. Biol. 2012, 18, 448–456. [Google Scholar] [CrossRef]
- Schemske, D.W.; Mittelbach, G.C.; Cornell, H.V.; Sobel, J.M.; Roy, K. Is there a latitudinal gradient in the importance of biotic interactions? Annu. Rev. Ecol. Syst. 2009, 40, 245–269. [Google Scholar]
- Mittlebach, G.C.; Schemske, D.W.; Cornell, H.V.; Allen, A.P.; Brown, J.M. Evolution and latitudinal diversity gradient: Speciation, extinction, and biogeography. Ecol. Lett. 2007, 10, 315–331. [Google Scholar] [CrossRef]
- Jablonski, D.; Roy, K.; Valentine, J.W. Out of the tropics: Evolutionary dynamics of the latitudinal diversity gradient. Science 2006, 314, 102–106. [Google Scholar]
- Novotny, V.; Drozd, D.; Miller, S.E.; Kulfan, M.; Janda, M.; Basset, Y.; Weiblen, G.O. Why are there so many species of herbivorous insects in tropical rainforests? Science 2006, 313, 1115–1118. [Google Scholar] [CrossRef]
- Becerra, J.X.; Venable, D.L. Macroevolution of insect-plant associations: The relevance of host biogeography to host affiliation. Proc. Natl. Acad. Sci. USA 1999, 96, 12625–12631. [Google Scholar]
- Winkler, I.S.; Mitter, C.; Scheffer, S.J. Repeated climate-linked host shifts have promoted diversification in a temperate clade of leaf-mining flies. Proc. Natl. Acad. Sci. USA 2009, 106, 18103–18108. [Google Scholar] [CrossRef]
- Condamine, F.L.; Sperling, F.A.X.; Wahlberg, N.; Rasplus, J.-Y.; Kergoat, G.J. What causes latitudinal gradients in species diversity? Evolutionary processes and ecological constraints on swallowtail biodiversity. Ecol. Lett. 2012, 15, 267–277. [Google Scholar] [CrossRef]
- Slansky, F., Jr. Latitudinal gradients in species diversity of the new world swallowtail butterflies. J. Res. Lepidopt. 1972, 11, 201–207. [Google Scholar]
- Scriber, J.M. Latitudinal gradients in larval feeding specialization of the world Papilionidae. Psyche 1973, 80, 355–373. [Google Scholar] [CrossRef]
- Scriber, J.M. Larval foodplant utilization by the world Papilionidae (Lep.): Latitudinal gradients reappraised. Tokurana (Acta Rhopalocerol.) 1984, 6/7, 1–50. [Google Scholar]
- Dyer, L.A.; Singer, M.S.; Lill, J.T.; Stireman, J.O.; Gentry, G.L.; Marquis, R.J.; Ricklefs, R.E.; Greeney, H.F.; Wagner, D.L.; Morais, H.C.; et al. Host specificity of Lepidoptera in tropical and temperate forests. Nature 2007, 448, 696–700. [Google Scholar] [CrossRef]
- Simonson, T.J.; Zakharov, E.V.; Djernaes, M.; Cotton, A.M.; Vane-Wright, R.I.; Sperling, F.A.H. Phylogenies and divergence times of Papilioninae (Lepidoptera) with special reference to the enigmatic genera Teinopalpus and Meandrusa. Cladistics 2011, 27, 113–137. [Google Scholar] [CrossRef]
- Collins, N.M.; Morris, M.G. Threatened Swallowtail Butterflies of the World: The IUCN Red Data Book; International Union for Conservation of Nature and Natural Resources: Cambridge, UK, Gland, Switzerland, 1985. [Google Scholar]
- Scriber, J.M.; Lederhouse, R.C.; Hagen, R.H. Foodplants and evolution within the Papilio glaucus and Papilio troilus species groups (Lepidoptera: Papilionidae). In Plant-Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions; Price, P.W., Lewinsohn, T.M., Fernades, G.W., Benson, W.W., Eds.; John Wiley: New York, NY, USA, 1991; pp. 341–373. [Google Scholar]
- Lehnert, M.; Scriber, J.M. Salicaceae detoxification abilities in Florida swallowtail butterflies (Papilio glaucus maynardi Gauthier): Novel ability or Pleistocene holdover? Insect Sci. 2012, 19, 337–345. [Google Scholar] [CrossRef]
- Zakharov, E.V.; Caterino, M.S.; Sperling, F.A.H. Molecular phylogeny, historical biogeography, and divergence times estimates for swallowtail butterflies of the genus Papilio (Lepidoptera: Papilionidae). Syst. Biol. 2004, 53, 193–215. [Google Scholar] [CrossRef]
- Nylin, S.; Wahlberg, N. Does plasticity drive speciation? Host-plant shifts and diversification in nymphaline butterflies (Lepidoptera: Nymphalidae) during the tertiary. Biol. J. Linn. Soc. 2008, 94, 115–130. [Google Scholar] [CrossRef]
- Scriber, J.M.; Larsen, M.L.; Allen, G.R.; Walker, P.W.; Zalucki, M.P. Interactions between Papilionidae and ancient Australian Angiosperms: Evolutionary specialization or ecological monophagy in the Papilionidae? Ent. Expt. Appl. 2008, 128, 230–239. [Google Scholar] [CrossRef]
- Nosil, P. Transition rates between specialization and generalization on phytophagous insects. Evolution 2002, 56, 1701–1706. [Google Scholar]
- Pateman, R.M.; Hill, J.K.; Roy, D.B.; Fox, R.; Thomas, C.D. Temperature-dependent alterations in host use drive rapid range expansion in a butterfly. Science 2012, 336, 1028–1030. [Google Scholar] [CrossRef]
- Kelly, S.T.; Farrel, D.B. Is specialization a dead end? The phylogeny of host use in Dendroctonus bark beetles (Scolytidae). Evolution 1998, 52, 1731–1743. [Google Scholar] [CrossRef]
- Pelini, S.L.; Keppel, J.A.; Kelly, A.E.; Hellmann, J.J. Adaptation to host plants may prevent rapid insect responses to climate change. Glob. Chang. Biol. 2010, 16, 2923–2929. [Google Scholar]
- Nitao, J.K.; Ayres, M.P.; Lederhouse, R.C.; Scriber, J.M. Larval adaptation to lauraceous hosts: Geographic divergence in the spicebush swallowtail butterflies. Ecology 1991, 72, 1428–1435. [Google Scholar] [CrossRef]
- Lederhouse, R.C.; Ayres, M.P.; Nitao, J.K.; Scriber, J.M. Differential use of lauraceous hosts by swallowtail butterflies, Papilio troilus and P. palamedes (Papilionidae). Oikos 1992, 63, 244–252. [Google Scholar] [CrossRef]
- Li, W.; Schuler, M.A.; Berenbaum, M.R. Diversification of furanocoumarin-metabolizing cytochrome P450 monooxygenases in two papilionids: Specificity and substrate encounter rate. Proc. Natl. Acad. Sci. USA 2003, 100, 14593–14595. [Google Scholar] [CrossRef]
- Cohen, M.B.; Schuler, M.A.; Berenbaum, M.R. Host-inducible cytochrome P450 from a host-specific caterpillar: Molecular cloning and evolution. Proc. Natl. Acad. Sci. USA 1992, 89, 10920–10924. [Google Scholar] [CrossRef]
- Saikkonen, K.; Tauavuori, K.; Hyvönen, T.; Gundel, P.E.; Hamilton, C.E.; Vänninen, I.; Nissinen, A.; Helander, M. Climate change-driven species’ range shifts filtered by photoperiodism. Nat. Clim. Chang 2012, 2, 239–242. [Google Scholar] [CrossRef]
- Dewar, R.C.; Watt, A.D. Predicting changes in the synchrony of larval emergence and budburst under climatic warming. Oecologia 1992, 89, 557–559. [Google Scholar]
- Franks, S.J.; Sim, S.; Weis, A.E. Rapid evolution of flowering time by an annual plant in response to climate fluctuation. Proc. Natl. Acad. Sci. USA 2007, 104, 1278–1282. [Google Scholar] [CrossRef]
- Niemelä, P.; Mattson, W.J. Invasion of North American forests by European phytophagous insects. Bioscience 1996, 46, 741–753. [Google Scholar] [CrossRef]
- Bradshaw, W.E.; Holzapfel, C.M. Evolutionary response to rapid climate change. Science 2006, 312, 1477–1478. [Google Scholar] [CrossRef]
- Bradshaw, W.E.; Holzapfel, C.M. Genetic response to rapid climate change: It’s seasonal timing that matters. Mol. Ecol. 2008, 17, 157–166. [Google Scholar] [CrossRef]
- Scriber, J.M. Latitudinal and local geographic mosaics in host plant preferences as shaped by thermal units and voltinism in Papilio spp. (Lepidoptera). Eur. J. Entomol. 2002, 99, 225–239. [Google Scholar]
- Best, A.S.; Johst, K.; Munkemuller, T.; Travis, J.M.J. Which species will successfully track climate change? The influence of intraspecific competition and density dependent dispersal on range shifting dynamics. Oikos 2007, 116, 1531–1539. [Google Scholar]
- Urban, M.C.; deMeester, L.; Vellend, M.; Stoks, R.; Vanoverbeke, J. A critical step toward realism: Responses to climate change from an evolving metacommunity perspective. Evol. Appl. 2012, 5, 154–167. [Google Scholar] [CrossRef]
- Cahill, A.E.; Aiello-Lammens, M.E.; Fisher-Reid, M.C.; Hua, X.; Karanewsky, C.J.; Ryu, H.Y.; Sbeglia, G.C.; Spagnolo, F.; Waldron, J.B.; Warsi, O.; et al. How does climate change cause extinction? Proc. R. Soc. B 2013, 280. [Google Scholar] [CrossRef]
- Marsico, T.D.; Burt, J.W.; Espeland, E.K.; Gilchrist, G.W.; Jamieson, M.A.; Lindström, L.; Roderick, G.K.; Swope, S.; Szücs, M.; Tsutsui, N.D. Underutilized resources for studying the evolution of invasive species during their introduction, establishment, and lag phases. Evol. Appl. 2010, 3, 203–219. [Google Scholar] [CrossRef]
- Shea, K.; Chesson, P. Community ecology theory as a framework for biological invasions. Trends Ecol. Evol. 2002, 17, 170–176. [Google Scholar] [CrossRef]
- Scriber, J.M. Non-target impacts of forest defoliator management options: Decision for no spraying may have worse impacts on non-target Lepidoptera than Bacillus thuringiensis insecticides. J. Insect Conserv. 2004, 8, 241–261. [Google Scholar] [CrossRef]
- Strauss, S.Y.; Lau, J.A.; Carroll, S.P. Evolutionary responses of natives to introduced species: What do introductions tell us about natural communities? Ecol. Lett. 2006, 9, 354–371. [Google Scholar]
- Gandhi, K.J.K.; Herms, D.A. Direct and indirect effects of alien insect herbivores on ecological processes and interactions in forests of eastern North America. Biol. Invas. 2009, 20. [Google Scholar] [CrossRef]
- Both, C.; van Asch, M.; Bijlsma, R.G.; van den Burg, A.B.; Visser, M.E. Climate change and unequal phenological changes across four trophic levels: Constrants and adaptations. J. Anim. Ecol. 2009, 78, 73–83. [Google Scholar] [CrossRef]
- Thomson, L.J.; MacFadgen, S.; Hoffmann, A.A. Predicting the effects of climate change on natural enemies of agricultural pests. Biol. Contr. 2010, 52, 296–306. [Google Scholar] [CrossRef]
- Moorcroft, P.R.; Pacala, S.W.; Lewis, M.A. Potential role of natural enemies during tree range expansions following climate change. J. Theor. Biol. 2006, 241, 601–616. [Google Scholar] [CrossRef]
- Menendez, R.; González-Megías, A.; Lewis, O.T.; Shaw, M.R.; Thomas, C.D. Escape from natural enemies during climate-driven range expansions: A case study. Ecol. Entomol. 2008, 33, 413–421. [Google Scholar] [CrossRef]
- Schierenbeck, K.A.; Ellstrand, N.C. Hybridization and the evolution of invasiveness in plants and other organisms. Biol. Invas. 2009, 11, 1093–1105. [Google Scholar]
- Becks, L.; Ellner, S.P.; Jones, L.E.; Hairston, N.G. Reduction of adaptive genetic diversity radically alters eco-evolutionary community dynamics. Ecol. Lett. 2010, 13, 989–997. [Google Scholar]
- Bolnick, D.L.; Amarasekare, P.; Araújo, M.S.; Burger, R.; Levine, J.M.; Noval, M.; Rudolf, V.H.W.; Schreiber, S.J.; Urban, M.C.; Vasseur, D.A. Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol. 2011, 26, 183–192. [Google Scholar] [CrossRef]
- Pearse, I.S.; Altermatt, F. Predicting novel trophic interactions in a non-native world. Ecol. Lett. 2013, 16, 1088–1094. [Google Scholar] [CrossRef]
- Mattson, W.J.; Vanhanen, H.; Veteli, T.; Sivonen, S.; Niemelä, P. Few immigrant phytophagous insects on woody plants in Europe: Legacy of the European crucible? Biol. Invas. 2007, 9, 957–974. [Google Scholar] [CrossRef]
- Boettner, G.H.; Elkinton, J.S.; Boettner, C.J. Effects of a biological control introduction on three nontarget native species of saturniid moths. Cons. Biol. 2000, 14, 524–531. [Google Scholar]
- Elkinton, J.S.; Liebhold, A.M. Population dynamics of the gypsy moth in North America. Annu. Rev. Entomol. 1990, 35, 571–596. [Google Scholar] [CrossRef]
- Johnson, K.S.; Scriber, J.M.; Nitao, J.N.; Smitley, D.R. Toxicity of Bacillus thuringiensis var. kurstaki to three non-target Lepidoptera in field studies. Environ. Entomol. 1995, 24, 288–297. [Google Scholar]
- Sample, B.E.; Butler, L.; Zivkovich, C.; Whitmore, R.C.; Reardon, R. Effects of Bacillus thuringiensis Berliner var. kurstaki and defoliation by gypsy moth (Lymantria dispar (L.), Lepidoptera: Lymantriidae) on native arthropods in West Virginia. Can. Entomol. 1996, 128, 573–592. [Google Scholar] [CrossRef]
- Rastall, K.; Kondo, V.; Strazanac, J.S.; Butler, L. Lethal effects of biological insecticide applications on non-target lepidopterans in two Appalachian forests. Environ. Entomol. 2003, 32, 1364–1369. [Google Scholar] [CrossRef]
- Redman, A.; Scriber, J.M. Competition between gypsy moth, Lymantria dispar, and the northern tiger swallowtail, Papilio canadensis: Interactions mediated by host plant chemistry, pathogens, and parasitoids. Oecologia 2000, 125, 218–228. [Google Scholar] [CrossRef]
- Jones, C.G.; Osfeld, R.S.; Richards, M.P.; Schauber, E.M.; Wolff, J.O. Chain reactions linking acorns to gypsy moth outbreaks and Lyme disease risk. Science 1998, 279, 1023–1026. [Google Scholar] [CrossRef]
- Soga, M.; Yamaura, Y.; Koike, S. From ecological pessimism to conservation change: Reviving living dead in changing landscapes. Anim. Conserv. 2013, 16, 16–18. [Google Scholar] [CrossRef]
- Pimentel, D.; Lach, L.; Zuniga, R.; Morrison, D. Environmental and economic costs associated with non-indigenous species in the United States. Bioscience 2000, 50, 53–65. [Google Scholar] [CrossRef]
- Lindroth, R.L.; Scriber, J.M.; Hsia, M.T.S. Chemical ecology of the tiger swallowtail: Mediation of host use by phenolic glycosides. Ecology 1988, 69, 814–822. [Google Scholar] [CrossRef]
- Herms, D.; Mattson, W.J. The dilemma of plants: To grow or defend. Quart. Rev. Biol. 1992, 67, 283–335. [Google Scholar]
- Scriber, J.M.; Lindroth, R.L.; Nitao, J.K. Differential toxicity of a phenolic glycoside from quaking aspen to Papilio glaucus butterfly species, subspecies, hybrids and backcrosses. Oecologia 1989, 81, 186–191. [Google Scholar]
- Scriber, J.M.; Weir, K.; Parry, D.; Deering, J. Using hybrid and backcross larvae of Papilio canadensis and Papilio glaucus to detect induced phytochemical resistance in hybrid Poplar trees experimentally dfoliated by gypsy moths. Ent. Exp. Appl. 1999, 91, 233–236. [Google Scholar]
- Craig, T.P.; Itami, J.K.; Ohgushi, T.; Ando, Y.; Utsumi, S. Bridges and barriers to host shifts resulting from host plant genotypic variation. J. Plant Interact. 2011, 6, 141–145. [Google Scholar] [CrossRef]
- Cavender-Bares, J.; Kozak, K.H.; Fine, P.V.A.; Kemberyl, S.W. The merging of community ecology and phylogenetic biology. Ecol. Lett. 2009, 12, 693–715. [Google Scholar] [CrossRef]
- Agosta, S.J.; Janz, N.; Brooks, D.R. How specialists can be generalists: Resolving the “parasite paradox” and implications for emerging infectious disease. Zoologia 2011, 27, 151–162. [Google Scholar] [CrossRef]
- Utsumi, S. Evolutionary community ecology of plant-associated arthropods in terrestrial ecosystems. Ecol. Res. 2013, 28, 359–371. [Google Scholar] [CrossRef]
- Zvereva, E.L.; Kozlov, M.V. Responses of terrestrial arthropods to air pollution: A meta-analysis. Environ. Sci. Pollut. Res. 2010, 17, 297–311. [Google Scholar] [CrossRef]
- Gross, J.; Fatouros, N.E.; Neuvonen, S.; Hilker, M. The importance of specialist natural enemies for Chrysolmela lapponica in pioneering a new host plant. Ecol. Entomol. 2004, 29, 584–593. [Google Scholar] [CrossRef]
- Sunday, J.M.; Bates, A.E.; Dulvy, N.K. Thermal tolerance and global redistribution of animals. Nat. Clim. Chang 2012, 2, 686–690. [Google Scholar]
- Fox, L.R.; Morrow, P.A. Specialization-species property or local phenomenon. Science 1981, 211, 887–893. [Google Scholar]
- Gourbieres, S.; Mallet, J. Are species real: The shape of the species boundary with expanded failures, reinforcement, and the missing snowball. Evolution 2010, 64, 1–24. [Google Scholar] [CrossRef]
- Berlocher, S.H.; Feder, J.K. Sympatric speciation in phytophagous insects: Moving beyond controversy? Annu. Rev. Entom. 2002, 47, 773–815. [Google Scholar] [CrossRef]
- Emelianov, I.; Marec, F.; Mallet, J. Genomic evidence for divergence with gene flow in host races of the larcxh budmoth. Proc. R. Soc. Lond. B Biol. Sci. 2004, 271, 97–105. [Google Scholar] [CrossRef]
- Xue, H.-J.; Li, W.-Z.; Nie, R.-E.; Yang, X.-K. Recent speciation in three closely related sympatric specialists: Inferences using multi-locus sequence, post-mating isolation and endosymbiont data. PLoS One 2011, 6, e27834. [Google Scholar]
- Stireman, J.O.; Devlin, H.; Abbot, P. Rampant host- and defensive phenotype-associated differentiation in a goldenrod gall midge. J. Evol. Biol. 2012, 25, 1991–2004. [Google Scholar] [CrossRef]
- Mikheyev, A.S.; McBride, C.S.; Mueller, U.G.; Parmesan, C.; Smee, M.R.; Stefenscu, C.; Wee, B.; Singer, M.C. Host-associated genomic differentiation in congeneric butterflies: Now you see it, now you do not. Mol. Ecol. 2013, 22, 4753–4766. [Google Scholar] [CrossRef]
- McBride, C.S.; van Velzen, R.; Larsen, T.B. Allopatric origin of cryptic butterfly species that were discovered feeding on distinct host plants in sympatry. Mol. Ecol. 2009, 18, 3639–3651. [Google Scholar] [CrossRef]
- Mercader, R.J.; Aardema, M.L.; Scriber, J.M. Hybridization leads to host-use divergence in a polyphagous butterfly sibling species pair. Oecologia 2009, 158, 651–662. [Google Scholar] [CrossRef]
- Schwarz, D.; Matta, B.M.; Shakir-Botteri, N.L.; McPheron, B.A. Host shift to an invasive plant triggers rapid animal hybrid speciation. Nature 2006, 436, 546–549. [Google Scholar]
- Nolte, A.W.; Tautz, D. Understanding the onset of hybrid speciation. Trends Ecol. Evol. 2010, 26, 54–58. [Google Scholar]
- Mullen, S.P.; Dopman, E.B.; Harrison, R.G. Hybrid zone origins, species boundaries, and the evolution of wing-pattern diversity in a polytypic species complex of North American Admiral butterflies (Nymphalidae: Limenitis). Evolution 2008, 62, 1400–1417. [Google Scholar] [CrossRef]
- Macholán, M.; Baird, S.J.E.; Dufková, P.; Munclinger, P.; Bimová, B.V.; Piálek, J. Assessing multilocus introgression patterns: A case study on the mouse X chromosome in central Europe. Evolution 2011, 65, 1428–1446. [Google Scholar] [CrossRef]
- Nachman, M.W.; Payseur, B.A. Recombination rate variation and speciation: Theoretical predictions and empirical results from rabbits and mice. Phil. Trans. R. Soc. B Biol. Sci. 2012, 367, 409–421. [Google Scholar] [CrossRef]
- Carneiro, M.; Baird, S.J.E.; Afonso, S.; Ramirez, E.; Tarroso, P.; Teotonio, H.; Villafuerte, R.; Nachman, M.W.; Ferrand, N. Step clines within a highly permeable genome across a hybrid zone between two subspecies of the European rabbit. Mol. Ecol. 2013, 22, 2511–2525. [Google Scholar] [CrossRef]
- Sperling, F.A.H. Butterfly Molecular Systematics: From Species Definitions to Higher Level Phylogenies. In Butterflies: Ecology and Evolution Taking Flight; Boggs, C.L., Watt, W.B., Ehrlich, P.R., Eds.; University Chicago Press: Chicago, IL, USA, 2003; pp. 431–458. [Google Scholar]
- Hendry, A.P.; Bolnick, D.I.; Berner, D.; Peichel, C.L. Along the speciation continuum in sticklebaclks. J. Fish Biol. 2009, 75, 2000–2036. [Google Scholar] [CrossRef]
- Mallet, J. A species definition for the modern synthesis. Trends Ecol. Evol. 2005, 10, 294–299. [Google Scholar] [CrossRef]
- Mallet, J.; Beltrán, M.; Neukirchen, W.; Linares, M. Natural hybridization in heliconiine butterflies: The species boundary as a continuum. BMC Evol. Biol. 2007, 7, 28. [Google Scholar] [CrossRef]
- Dopman, E.B.; Perez, L.; Bogdanowicz, S.M.; Harrison, R.G. Consequences of reproductive barriers for genealogical discordance in the European corn borer. Proc. Natl. Acad. Sci. USA 2005, 102, 14706–14711. [Google Scholar]
- Dopman, E.B.; Robbins, P.S.; Seaman, A. Components of reproductive isolation between North American pheromone strains of the European corn borer. Evolution 2010, 64, 881–902. [Google Scholar] [CrossRef]
- Nosil, P.; Harmon, L.J.; Seehausen, O. Ecological explanations for (incomplete) speciation. Trends Ecol. Evol. 2009, 24, 145–156. [Google Scholar] [CrossRef]
- Smadja, C.M.; Butlin, R.K. A framework for comparing processes of speciation in the presence of gene flow. Mol. Ecol. 2011, 20, 5123–5140. [Google Scholar] [CrossRef]
- Nosil, P.; Feder, J.L. Genomic divergence during speciation: Causes and consequences. Phil. Trans. R. Soc. B 2012, 367, 332–342. [Google Scholar] [CrossRef]
- Hagen, R.H.; Scriber, J.M. Sex chromosomes and speciation in the Papilio glaucus group. In Swallowtail Butterflies: Their Ecology and Evolutionary Biology; Scriber, J.M., Tsubaki, Y., Lederhouse, R.C., Eds.; Scientific Publishers, Inc.: Gainesville, FL, USA, 1995; pp. 211–228. [Google Scholar]
- Lee, Y.; Collier, T.C.; Sanford, M.R.; Marsden, C.D.; Fofana, A.; Comel, A.J.; Lanzaro, G.C. Chromosome inversions, genomic differentiation and speciation in the African malaria mosquito, Anopheles gambia. PLoS One 2013, 8, e5787. [Google Scholar]
- Sperling, F.A.H. Sex-linked genes and species differences in Lepidoptera. Can. Entomol. 1994, 126, 807–818. [Google Scholar] [CrossRef]
- Pashley-Prowell, D. Sex Linkage and Speciation in Lepidoptera. Endless Forms: Species and Speciation; Howard, D.J., Berlocher, S.H., Eds.; Oxford University Press: New York, NY, USA, 1998; pp. 309–319. [Google Scholar]
- Elgvin, T.O.; Hermansen, J.S.; Fijarczyk, A.; Bonnet, T.; Borge, T.; Saether, S.A.; Voje, K.L.; Saetre, G.-P. Hybrid speciation in sparrows II: A role for sex chromosomes? Mol. Ecol. 2011, 20, 3823–3837. [Google Scholar] [CrossRef]
- Rauch, E.M.; Bar-Yam, Y. Theory predicts the uneven distribution of genetic diversity within species. Nature 2004, 431, 449–450. [Google Scholar] [CrossRef]
- Eckert, C.G.; Samis, K.E.; Lougheed, S.C. Genetic variation across species’ geographic ranges: The central-marginal hypothesis and beyond. Mol. Ecol. 2008, 17, 1170–1188. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gaudeul, M.; Debusasche, M. Conservation value of sites of hybridization in peripheral populations of rare plant species. Conserv. Biol. 2009, 24, 236–245. [Google Scholar] [CrossRef]
- Lopez-Pujol, J.; Garcia-Jacas, N.; Susanna, A.; Vilatersana, R. Should we conserve pure species or hybrid species? Delimiting hybridization and introgression in the Iberian endemic Centaurea podospermifolia. Biol. Conserv. 2012, 152, 271–279. [Google Scholar] [CrossRef]
- Cozzolino, S.; Naqrdella, A.M.; Impagliazzo, S.; Widmer, A.; Lexer, C. Hybridization and conservation of Mediterranean orchids: Should we protect the orchid hybrids or the orchid hybrid zones? Biol. Conserv. 2005, 129, 14–23. [Google Scholar]
- Hill, J.K.; Hughes, C.L.; Dytham, C.; Searle, J.B. Genetic diversity in butterflies: Interactive effects of habitat fragmentation and climate-driven range expansion. Biol. Lett. 2009, 2, 152–154. [Google Scholar]
- Pelini, S.L.; Dzurisin, J.D.K.; Prior, K.M.; Williams, C.M.; Marisco, T.D.; Sinclair, B.J.; Hellmann, J.J. Translocation experiments with butterflies reveal limits to enhancement of poleward populations under climate change. Proc. Natl. Acad. Sci. USA 2009, 106, 11160–11165. [Google Scholar] [CrossRef]
- Kotiaho, J.S.; Kaitala, V.; Komonen, A.; Päivien, J. Predicting the risk of extinction from shared ecological characteristics. Proc. Natl. Acad. Sci. USA 2005, 102, 1963–1967. [Google Scholar] [CrossRef]
- Mattila, N.; Kaitala, V.; Komonen, A.; Päivinen, J.; Kotiaho, J.S. Ecological correlates of distribution change and range shift in butterflies. Insect Conserv. Divers. 2011, 4, 239–246. [Google Scholar] [CrossRef]
- Moritz, C. Strategies to protect biological diversity and the evolutionary processes that sustain it. Syst. Biol. 2002, 51, 238–254. [Google Scholar] [CrossRef]
- Mace, G.M.; Purvis, A. Evolutionary biology and practical conservation: Bridging a widening gap. Mol. Ecol. 2008, 17, 9–19. [Google Scholar] [CrossRef]
- Sagarin, R.D.; Gaines, S.D.; Gaylord, B. Moving beyond assumptions to understand abundance distributions across ranges of species. Trends Ecol. Evol. 2006, 21, 524–530. [Google Scholar] [CrossRef]
- McGill, B.; Collins, C. A unified theory for macroecology based on spatial patterns of abundance. Evol. Ecol. Res. 2003, 5, 469–492. [Google Scholar]
- Van Heerewaarden, B.; Kellermann, V.; Schiffer, M.; Blacker, M.; Sgro, C.M.; Hoffmann, A.A. Testing evolutionary hypotheses about species borders: Patterns of genetic variation towards the southern borders of two rainforest Drosophila and a related habitat generalist. Proc. R. Soc. B 2009, 276, 1517–1526. [Google Scholar] [CrossRef]
- Weeks, A.R.; Sgro, C.; Young, A.G.; Frankham, R.; Mitchell, N.J.; Miller, K.A.; Byrne, M.; Coates, D.J.; Eldridge, M.D.B.; Sunnucks, P.; et al. Assessing the benefits and risks of translocations in changing environments: A genetic perspective. Evol. Appl. 2011, 4, 709–725. [Google Scholar] [CrossRef]
- Avise, J.C. The history and purview of phylogeography: A personal reflection. Mol. Ecol. 1998, 7, 371–379. [Google Scholar] [CrossRef]
- Hewitt, G.M. Speciation, hybrid zones and phylogeography- or seeing genes in space and time. Mol. Ecol. 2001, 10, 537–549. [Google Scholar] [CrossRef]
- Cooper, V.S.; Bennett, A.F.; Lenski, R.E. Evolution of thermal dependence of growth rate of Escherichia coli populations during 20,000 generations of a constant environment. Evolution 2001, 55, 889–896. [Google Scholar] [CrossRef]
- Maughan, H.; Masel, J.; Birky, C.W.; Nicholson, W.L. The roles of mutation accumulation and selection in loss of sporulation in experimental populations of Bacillus subtilis. Genetics 2007, 177, 937–948. [Google Scholar] [CrossRef]
- Hoffmann, A.A. A genetic perspective on insect climate specialists. Aust. J. Entomol. 2010, 49, 93–103. [Google Scholar] [CrossRef]
- Bonebrake, T.C. Conservation implications of adaptation to tropical climates from a historical perspective. J. Biogeogr. 2013, 40, 409–414. [Google Scholar] [CrossRef]
- Sunday, J.M.; Bates, A.E.; Dulvy, N.K. Global analysis of thermal tolerance and latitude in ectotherms. Proc. R. Soc. Lond. B 2011, 278, 1823–1830. [Google Scholar] [CrossRef]
- Scriber, J.M.; Keefover, K.; Nelson, S. Hot summer temperatures may stop movement of Papilio canadensis butterflies and genetic introgression south of the hybrid zone in the North American Great Lakes region. Ecography 2002, 25, 184–192. [Google Scholar] [CrossRef]
- Mercader, R.J.; Scriber, J.M. Diversification of host use in two polyphagous butterflies: Differences in oviposition specificity or host rank hierarchy. Ent. Exp. Appl. 2007, 125, 89–101. [Google Scholar] [CrossRef]
- Mercader, R.J.; Scriber, J.M. Divergence of ovipositional behavior in the Papilio glaucus group. Insect Sci. 2008, 15, 361–367. [Google Scholar] [CrossRef]
- Mercader, R.J.; Scriber, J.M. Asymmetrical thermal constraints on the parapatric species boundaries of two widespread generalist butterflies. Ecol. Entom. 2008, 33, 537–545. [Google Scholar] [CrossRef]
- Scriber, J.M.; Sonke, B. Effects of diurnal temperature range on adult size and emergence time from diapausing pupae in Papilio glaucus and P. canadensis (Papilionidae). Insect Sci. 2011, 18, 435–442. [Google Scholar] [CrossRef]
- Williams, C.M.; Marshall, K.E.; MacMillan, H.A.; Dzurisin, J.D.K.; Hellmann, J.J.; Sinclair, B.J. Thermal variability increases the impact of Autumnal warming and drives metabolic depression in an overwintering butterfly. PLoS One 2012, 7, e34470. [Google Scholar]
- Williams, C.M.; Hellmann, J.J.; Sinclair, B.J. Lepidopteran species differ in susceptibility to winter warming. Clim. Res. 2012, 53, 119–130. [Google Scholar] [CrossRef]
- Hurrell, J.W. Decadal trends in North Atlantic oscillation region temperatures and precipitation. Science 1995, 269, 676–679. [Google Scholar]
- Higgins, R.W.; Leetma, A.; Kousky, V.E. Relationships between climate variability and winter temperature extremes in the United States. J. Clim. 2002, 15, 1555–1572. [Google Scholar] [CrossRef]
- Gu, L.; Hanson, P.J.; Pool, W.M.; Kaiser, D.P.; Yang, B.; Nemani, R.; Pallardy, S.G.; Meyers, T. The 2007 Eastern USA Spring freeze: Increased cold damage in a warming world. BioScience 2008, 58, 253–262. [Google Scholar] [CrossRef]
- LePage, M. Wild winters. New Sci. 2011, 212, 42–44. [Google Scholar]
- Kukal, O.; Ayres, M.P.; Scriber, J.M. Cold tolerance of pupae in relation to the distribution of tiger swallowtails. Can. J. Zool. 1991, 69, 3028–3037. [Google Scholar] [CrossRef]
- Tesar, D.; Scriber, J.M. Growth season constraints in climatic cold pockets: Tolerance of subfreezing temperatures and compensatory growth by tiger swallowtail butterfly larvae (Lepidoptera: Papilionidae). Holarctic Lepidopt. 2005, 7, 39–44. [Google Scholar]
- Roland, J.; Matter, S.F. Variability in winter climate and winter extremes reduces population growth of an alpine butterfly. Ecology 2012, 94, 190–199. [Google Scholar] [CrossRef]
- Pöyry, J.; Luoto, M.; Heikinnen, R.K.; Kuussaari, M.; Saarinen, K. Species traits explain recent range shifts of Finnish butterflies. Glob. Chang Biol. 2009, 15, 732–743. [Google Scholar] [CrossRef]
- Wilson, R.J.; Gutierrez, D.; Gutierrez, J.; Monserrat, V.J. An elevational shift in butterfly species richness and composition accompanying recent climate change. Glob. Chang Biol. 2007, 13, 1873–1887. [Google Scholar] [CrossRef]
- Turlure, C.; Choutt, J.; Baguette, M.; van Dyck, H. Microclimatic buffering and resource-based habitat in a glacial relic butterfly: Significance for conservation under climate change. Glob. Chang Biol. 2010, 16, 1883–1893. [Google Scholar]
- Matter, S.F.; Doyle, A.; Illerbrun, K.; Wheeler, J.; Rolands, J. An assessment of direct and indirect effects of climate change for populations of the Rocky Mountain Apollo butterfly (Parnassius smintheus Doubleday). Insect Sci. 2011, 18, 385–392. [Google Scholar] [CrossRef]
- Scriber, J.M.; Stump, A.; Deering, M. Hybrid zone ecology and tiger swallowtail trait clines in North America. In Ecology and Evolution Taking Flight: Butterflies as Model Study Systems; Boggs, C., Watt, W., Ehrlich, P., Eds.; University of Chicago Press: Chicago, IL, USA, 2003; pp. 367–391. [Google Scholar]
- Scriber, J.M.; Maher, E.; Aardema, M.L. Differential effects of short term winter thermal stress on diapausing tiger swallowtail butterflies (Papilio spp.). Insect Sci. 2012, 19, 277–285. [Google Scholar] [CrossRef]
- Scriber, J.M.; Lederhouse, R.C. In the thermal environment as a resource dictating geographic patterns of feeding specialization of insect herbivores. In Effects of Resource Distribution on Animal–Plant Interactions; Hunter, M.R., Ohgushi, T., Price, P.W., Eds.; Academic Press: New York, NY, USA, 1992; pp. 429–466. [Google Scholar]
- Remington, C.L. Suture zones of hybrid interaction between recently joined biotas. In Evolutionary Biology; Dobzhanski, T., Hecht, M.K., Steere, W.C., Eds.; Plenum Press: New York, NY, USA, 1968; pp. 321–348. [Google Scholar]
- Luebke, H.J.; Scriber, J.M.; Yandell, B.S. Use of multivariate discriminant analysis of male wing morphometrics to delineate a hybrid zone for Papilio glaucus glaucus and P. g. canadensis in Wisconsin. Am. Midl. Nat. 1988, 119, 366–379. [Google Scholar] [CrossRef]
- Swensen, N.G.; Howard, D.J. Do suture zones exist? Evolution 2004, 58, 2391–2397. [Google Scholar]
- Hagen, R.H.; Lederhouse, R.C.; Bossart, J.L.; Scriber, J.M. Papilio canadensis and P. glaucus (Papilionidae) are distinct species. J. Lepid. Soc. 1991, 45, 245–258. [Google Scholar]
- Scriber, J.M.; Lederhouse, R.C. Temperature as a factor in the development and feeding ecology of tiger swallowtail caterpillars, Papilio glaucus. Oikos 1983, 40, 95–102. [Google Scholar] [CrossRef]
- Ayres, M.P.; Scriber, J.M. Local adaptations to regional climates in Papilio canadensis (Lepidoptera: Papilionidae). Ecol. Monogr. 1994, 64, 465–482. [Google Scholar] [CrossRef]
- Scriber, J.M. Climatic legacies and sex chromosomes: Latitudinal patterns of voltinism, diapause, size and host-plant selection in 2 species of swallowtail butterflies at their hybrid zone. In Insect Life-Cycle Polymorphism: Theory, Evolution and Ecological Consequences for Seasonality and Diapause Control; Danks, H.V., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; pp. 133–171. [Google Scholar]
- Scriber, J.M. A new cold pocket hypothesis to explain local host preference shifts in Papilio canadensis. Entomol. Exp. Appl. 1996, 80, 315–319. [Google Scholar] [CrossRef]
- Rockey, S.J.; Hainze, J.H.; Scriber, J.M. Evidence of a sex linked diapause response in Papilio glaucus subspecies and their hybrids. Physiol. Entomol. 1987, 12, 181–184. [Google Scholar] [CrossRef]
- Rockey, S.J.; Hainze, J.H.; Scriber, J.M. A latitudinal and obligatory diapause response in three subspecies of the eastern tiger swallowtail Papilio glaucus (Lepidoptera: Papilionidae). Am. Midl. Nat. 1987, 118, 162–168. [Google Scholar] [CrossRef]
- Eichenlaub, V.L.; Harman, J.R.; Nurnberger, F.V.; Stolle, H.J. The Climatic Atlas of Michigan; Notre Dame Press: Notre Dame, IN, USA, 1990; p. 165. [Google Scholar]
- Scriber, J.M.; Ording, G.J.; Mercader, R.J. Hybrid introgression and parapatric speciation in a hybrid zone. In Specialization, Speciation, and Radiation: The Evolutionary Biology of Herbivorous Insects; Tilmon, K.J., Ed.; University California Press: Berkeley, CA, USA, 2008; pp. 69–87. [Google Scholar]
- Higaki, M.; Toyama, M. Evidence for reversible change in the intensity of prolonged diapause in the Chestnut weevil, Curculio sikkimensis. J. Insect Physiol. 2012, 58, 56–60. [Google Scholar] [CrossRef]
- Feder, J.L.; Roethele, J.B.; Wlazlo, B.; Berlocher, S.H. Selective maintenance of allozyme dfferences among sympatric host races of the apple maggot fly. Proc. Natl. Acad. Sci. USA 1997, 94, 11417–11421. [Google Scholar] [CrossRef]
- Feder, J.L.; Stolz, U.; Lewis, K.M.; Perry, W.; Roethele, J.B.; Rogers, A. The effects of winter length on the genetics of apple and hawthorn races of Rhagoletis pomonella (Diptera: Tephritidae). Evolution 1997, 51, 1862–1876. [Google Scholar] [CrossRef]
- Masaki, S. Ecophysiological consequences of variability of diapause intensity. Eur. J. Entomol. 2002, 99, 143–154. [Google Scholar]
- Lehnert, M.S.; Scriber, J.M.; Gerard, P.D.; Emmel, T.C. The “converse of Bergmann’s Rule” in tiger swallowtail butterflies; Boundaries of species and subspecies wing traits are independent of thermal and host-plant induction. Am. Entom. 2012, 58, 156–165. [Google Scholar]
- Donovan, J.; Scriber, J.M. Detection and verification of a primary natural hybridization event between two tiger swallowtail butterfly species in northern Michigan. J. Lepid. Soc. 2003, 57, 25–35. [Google Scholar]
- Moczek, A.P. Phenotypic plasticity and diversity in insects. Phil. Trans. R. Soc. B Biol. Sci. 2010, 365, 593–603. [Google Scholar] [CrossRef]
- Schwander, T.; Leimar, O. Genes as leaders and followers in evolution. Trends Ecol. Evol. 2011, 26, 143–151. [Google Scholar] [CrossRef]
- Bonduriansky, R.; Crean, A.J.; Day, T. The implications of nongenetic inheritance for evolution in changing environments. Evol. Applic. 2012, 5, 192–201. [Google Scholar] [CrossRef]
- Pfennig, D.W.; Wund, M.A.; Snell-Rood, E.C.; Cruickshank, T.; Schlicting, C.D.; Moczek, A.P. Phenotypic plasticity’s impacts on diversification and speciation. Trends Ecol. Evol. 2010, 25, 459–467. [Google Scholar] [CrossRef]
- Koevoets, T.; vande Zande, L.; Beukeboom, L.W. Temperature stress increases hybrid incompatibilities in the parasitic wasp genus Nasonia. J. Evol. Biol. 2011, 25, 304–316. [Google Scholar]
- Arnold, M.L.; Martin, N.H. Adaptation by introgression. J. Biol. 2009, 8, 82. [Google Scholar] [CrossRef]
- Arnold, M.L.; Hodges, S.A. Are natural hybrids fit or unfit relative to their parents? Trends Ecol. Evol. 1995, 10, 67–71. [Google Scholar] [CrossRef]
- Baskett, M.L.; Gomulkiewicz, R. Introgressive hybridization as a mechanism for species rescue. Theoret. Ecol. 2011, 4, 223–239. [Google Scholar] [CrossRef]
- Franks, S.J.; Hoffmann, A.A. Genetics of climate change adaptation. Annu. Rev. Genet. 2012, 46, 185–208. [Google Scholar] [CrossRef]
- Balanya, J.; Oller, J.M.; Huey, R.B.; Gilchrist, G.W.; Serra, L. Global genetic change tracks global climate warming in Drosophila subobscura. Science 2006, 313, 1773–1775. [Google Scholar] [CrossRef]
- Gluesenkamp, D.; Chasse, M.; Frey, M.; Parker, V.T.; Vasey, M.; Young, B. Back from the brink: A second chance at discovery and conservation of the Franciscan Manzanita. Fremontia 2011, 38, 3–17. [Google Scholar]
- USFWS (United States Fish and Wildlife Service). 5-Year Review of Endangered Status of Raven’s Manzanita, June 2012. Available online: http://milliontrees.me/tag/ravens-manzanita/ (accessed on 19 December 2013).
- Barton, N.H. The role of hybridization in evolution. Mol. Ecol. 2001, 10, 551–568. [Google Scholar] [CrossRef]
- Coyne, J.A.; Orr, H.A. Speciation; Sinauer Associates: Sunderland, MA, USA, 2004. [Google Scholar]
- Orr, H.A.; Unckless, R.L. Population extinction and the genetics of adaptation. Am. Nat. 2008, 172, 160–169. [Google Scholar] [CrossRef]
- Huang, Y.; Lloyd, A.L.; Legros, M.; Gould, F. Gene-drive into insect populations with age and spatial structure: A theoretical assessment. Evol. Appl. 2011, 4, 415–428. [Google Scholar] [CrossRef]
- Hagen, R.H.; Scriber, J.M. Sex linked diapause, color, and allozyme loci in Papilio glaucus: Linkage analysis and significance in a hybrid zone. Heredity 1989, 80, 179–185. [Google Scholar]
- Hagen, R.H.; Scriber, J.M. Systematics of the Papilio glaucus and P. troilus groups (Lepidoptera: Papilionidae): Inferences from allozymes. Ann. Entom. Soc. Am. 1991, 84, 380–395. [Google Scholar]
- Scriber, J.M. Tiger tales: Natural history of native North American swallowtails. Am. Entomol. 1996, 42, 19–32. [Google Scholar]
- Scriber, J.M. Absence of behavioral induction in oviposition preference of Papilio glaucus (Lepidoptera: Papilionidae). Great Lakes Entomol. 1993, 26, 81–95. [Google Scholar]
- Scriber, J.M.; Lederhouse, R.C.; Dowell, R.V. Hybridization studies with North American swallowtails. In Swallowtail Butterflies: Their Ecology and Evolutionary Biology; Scriber, J.M., Tsubaki, Y., Lederhouse, R.C., Eds.; Scientific Publishers: Gainesville, FL, USA, 1995; pp. 269–281. [Google Scholar]
- Deering, M.D.; Scriber, J.M. Field bioassays show heterospecific mating preference asymmetry between hybridizing North American Papilio butterfly species (Lepidoptera: Papilionidae). J. Ethol. 2002, 20, 25–33. [Google Scholar] [CrossRef]
- Aardema, M.L.; Scriber, J.M. No evidence that male choice contributes to the maintenance of a shared, sex-limited trait in mimetic and non-mimetic female tiger swallowtail butterflies, Papilio glaucus. Evol. Biol. 2013, 40, 108–116. [Google Scholar] [CrossRef]
- Lederhouse, R.C. Comparative mating behavior and sexual selection in North American swallowtail butterflies. In Swallowtail Butterflies: Their Ecology and Evolutionary Biology; Scriber, J.M., Tsubaki, Y., Lederhouse, R.C., Eds.; Scientific Publishers: Gainesville, FL, USA, 1995; pp. 117–131. [Google Scholar]
- Stump, A.; Scriber, J.M. Sperm precedence in experimental interspecific multiple matings of hybridizing North American tiger swallowtail butterflies (Lepidoptera: Papilionidae)? J. Lepidopt. Soc. 2006, 60, 65–78. [Google Scholar]
- Ae, S.A. Ecological and evolutionary aspects of hybridization in some Papilio butterflies. In Swallowtail Butterflies, Their Ecology and Evolutionary Biology; Scriber, J.M., Tsubaki, Y., Lederhouse, R.C., Eds.; Scientific Publication: Gainesville, FL, USA, 1995; pp. 229–235. [Google Scholar]
- Fox, C.W.; Reed, D.H. Inbreeding depression increases with environmental stress: An experimental study and meta-analysis. Evolution 2011, 65, 246–258. [Google Scholar] [CrossRef]
- Reed, D.H. Albatrosses, eagles and newts, oh my! Exceptions to the prevailing paradigm concerning genetic diversity and population viability. Anim. Conserv. 2010, 13, 448–457. [Google Scholar] [CrossRef]
- Charpentier, M.J.E.; Williams, C.V.; Drea, C.M. Inbreeding depression in ring-tailed lemurs (Lemur catta): Genetic diversity predicts parasitism, immunocompetence, and survivorship. Conserv. Genet. 2008, 9, 1605–1615. [Google Scholar] [CrossRef]
- Bijlsma, R.; Bundgaard, J.; Boerema, A.C. Does inbreeding affect the extinction risk of small populations? Predictions from Drosophila. J. Evol. Biol. 2000, 13, 502–514. [Google Scholar]
- Reed, D.H.; Briscoe, D.A.; Frankham, R. Inbreeding and extinction: The effect of environmental stress and lineage. Conserv. Genet. 2002, 3, 301–307. [Google Scholar] [CrossRef]
- Liao, W.; Reed, D.H. Inbreeding-environment interactions increase extinction risk. Anim. Conserv. 2009, 12, 54–61. [Google Scholar] [CrossRef]
- Frankham, R.J.D.; Ballou, M.D.B.; Eldridge, R.C.; Lacy, R.C.; Ralls, K.; Dudash, M.R.; Fenster, C.B. Predicting the probability of outbreeding depression. Conserv. Biol. 2011, 25, 465–475. [Google Scholar] [CrossRef]
- Gay, L.; Crochet, P.-A.; Bell, D.A.; Lenormand, T. Comparing clines on molecular and phenotypic traits in hybrid zones: A window on tension zone models. Evolution 2008, 62, 2789–2806. [Google Scholar] [CrossRef]
- Stevens, V.M.; Turlure, C.; Baguette, M. A meta-analysis of dispersal in butterflies. Biol. Rev. 2010, 85, 625–642. [Google Scholar]
- Huxel, G.R. Rapid displacement of native species by invasive species: Effects of hybridization. Biol. Conserv. 1999, 89, 143–152. [Google Scholar]
- Wolf, D.E.; Takebayashi, N.; Rieseberg, L.H. Predicting the risks of extinction through hybridization. Conserv. Biol. 2001, 15, 1039–1053. [Google Scholar] [CrossRef]
- Seehausen, O. Conservation: Losing biodiversity by reverse speciation. Curr. Biol. 2006, 16, R334–R337. [Google Scholar] [CrossRef]
- Taylor, E.B.; Boughman, J.W.; Groenenboom, M.; Sniatynski, M.; Schluter, D.; Gow, J.L. Speciation in reverse: Morphological and genetic evidence of the collapse of a three-spined stickleback (Gasterosteus aculeatus) species pair. Evolution 2006, 15, 343–355. [Google Scholar]
- Gilman, R.T.; Behm, J.E. Hybridization, species collapse, and species reemergence after disturbance to premating mechanisms of reproductive isolation. Evolution 2011, 65, 2592–2260. [Google Scholar] [CrossRef]
- Mallet, J.; Wynne, I.R.; Thomas, C.D. Hybridisation and climate change: Brown argus butterflies in Britain (Polyommatus subgenus Aricia). Insect Conserv. Divers. 2011, 4, 192–199. [Google Scholar] [CrossRef]
- Buggs, R.J.A. Empirical study of hybrid zone movement. Heredity 2007, 99, 301–312. [Google Scholar] [CrossRef]
- Currat, M.; Ruedi, M.; Petit, R.J.; Excoffier, L. The hidden side of invasions: Massive introgression by local genes. Evolution 2008, 62, 1908–1920. [Google Scholar]
- Hopper, S.D. Evolutionary networks: Natural hybridization and its conservation significance. In Nature Conservation 4: The Role of Networks; Saunders, D.A., Craig, J.L., Mattiske, E.M., Eds.; Surrey Beatty and Sons: Sydney, Australia, 1995; pp. 51–66. [Google Scholar]
- Soltis, P.S.; Soltis, D.E. The role of hybridization in plant speciation. Annu. Rev. Plant Biol. 2009, 60, 561–588. [Google Scholar] [CrossRef]
- Evans, L.M.; Allan, G.J.; Whitham, T.G. Populus hybrid hosts drive divergence in herbivorous mite, Aceria parapopuli: Implications for conservation of plant hybrid zone as essential habitat. Cons. Genet. 2012, 13, 1601–1609. [Google Scholar] [CrossRef]
- Whitham, T.G.; Morrow, P.A.; Potts, B.M. Conservation of hybrid plants. Science 1991, 254, 779–780. [Google Scholar]
- Whitham, T.G.; Martinsen, G.D.; Floate, K.D.; Dungey, H.S.; Potts, B.M.; Keim, P. Plant hybrid zones affect biodiversity: Tools for a genetically-based understanding of community structure. Ecology 1999, 80, 416–428. [Google Scholar] [CrossRef]
- Floate, K.D.; Whitham, T.G. The “hybrid bridge” hypothesis: Host shifting via plant hybrid swarms. Am. Nat. 1993, 141, 651–662. [Google Scholar]
- Floate, K.D.; Kearsley, M.J.C.; Whitham, T.G. Elevated herbivory in plant hybrid zones: Chrysomela confluens, Populus and phenological sinks. Ecology 1993, 74, 2056–2065. [Google Scholar] [CrossRef]
- Fritz, R.S. Resistance of hybrid plants to herbivores: Genes, environment, or both? Ecology 1999, 80, 382–391. [Google Scholar] [CrossRef]
- Fritz, R.S.; Moulia, C.; Newcombe, G. Resistance of hybrid plants and animals to herbivores, pathogens, and parasites. Annu. Rev. Ecol. Syst. 1999, 30, 565–591. [Google Scholar] [CrossRef]
- Dungey, H.S.; Potts, B.M.; Whitham, T.G.; Li, H.F. Plant genetics affects arthropod community richness and composition: Evidence from a synthetic eucalypt hybrid population. Evolution 2000, 54, 1938–1946. [Google Scholar]
- McIntyre, P.M.; Whitham, T.G. Plant genotype affects long-term herbivore population dynamics and extinction: Conservation implications. Ecology 2003, 84, 311–322. [Google Scholar] [CrossRef]
- Hochwender, C.G.; Fritz, R.S. Plant genetic differences influence herbivore community structure: Evidence from a hybrid willow system. Oecologia 2004, 138, 547–557. [Google Scholar] [CrossRef]
- Schweitzer, J.A.; Bailey, J.K.; Rehill, B.J.; Martinsen, G.D.; Hart, S.C.; Lindroth, R.L.; Keim, P.; Whitham, T.G. Genetically-based trait in a dominant tree affects ecosystem processes. Ecol. Lett. 2004, 7, 127–134. [Google Scholar] [CrossRef]
- Bangert, E.O.; Turek, R.J.; Martinsen, G.D.; Wimp, G.M.; Bailey, J.K.; Whitham, T.G. Benefits of conservation of plant genetic diversity on arthropod diversity. Conserv. Biol. 2005, 19, 379–390. [Google Scholar] [CrossRef]
- Rehill, B.; Clauss, A.; Wieczorek, L.; Whitham, T.G.; Lindroth, R.L. Foliar phenolic glycosides from Populus fremontii, Populus angustifolia, and their hybrids. Biochem. Syst. Ecol. 2005, 33, 125–131. [Google Scholar] [CrossRef]
- Wimp, G.M.; Martinsen, G.D.; Floate, K.D.; Bangert, R.K.; Whitham, T.G. Plant genetic determinants of arthropod community structure and diversity. Evolution 2005, 59, 61–69. [Google Scholar]
- LeRoy, C.J.; Whitham, T.G.; Keim, P.; Marks, J.C. Plant genes link forests and streams. Ecology 2006, 87, 255–261. [Google Scholar] [CrossRef]
- Lamit, L.J.; Wojtowicz, T.; Kovacs, Z.; Wooley, S.C.; Zinkgraf, M.; Whitham, T.G.; Lindroth, R.L.; Gehring, C.A. Hybridization among foundation tree species influences the structure of associated understory plant communities. Botany 2011, 89, 165–174. [Google Scholar] [CrossRef]
- Tovar-Sanchez, E.; Oyama, K. Effect of hybridization of the Quercus crassifolia x Quercus crassipes complex on community structure of endophagous insects. Oecologia 2006, 147, 702–713. [Google Scholar] [CrossRef]
- Whitham, T.G. Plant hybrid zones as sinks for pests. Science 1989, 244, 1490–1493. [Google Scholar] [CrossRef]
- Scriber, J.M.; Tingey, W.M.; Gracen, V.E.; Sullivan, S.L. Leaf-feeding resistance to the European corn borer in genotypes of tropical (low-DIMBOA) and U.S. Inbred (High-DIMBOA) maize. J. Econ. Entomol. 1975, 68, 823–826. [Google Scholar]
- Manuwoto, S.; Scriber, J.M. Consumption and utilization of 3 Maize genotypes by the southern armyworm, Spodoptera eridania (Noctuidae). J. Econ. Entom. 1982, 75, 163–167. [Google Scholar]
- Manuwoto, S.; Scriber, J.M. Neonate larval survival of European corn borers, Ostrinia nubilalis, on high and low DIMBOA genotypes of maize-effects of light intensity and degree of insect inbreeding. Agric. Ecosyst. Environ. 1985, 14, 221–236. [Google Scholar] [CrossRef]
- Strauss, S.Y. Levels of herbivory and parasitism in host hybrid zones. Trends Ecol. Evol. 1994, 9, 209–214. [Google Scholar] [CrossRef]
- Moulia, C. Parasitism of plant and animal hybrids: Are facts and fates the same? Ecology 1999, 80, 392–406. [Google Scholar] [CrossRef]
- Slansky, F.; Scriber, J.M. Food Consumption and Utilization. In Comprehensive Insect Physiology, Biochemistry, and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Permagon Press: Oxford, UK, 1985. [Google Scholar]
- Mattson, W.J.; Scriber, J.M. Nutritional ecology of insect folivores of woody plants: Water, nitrogen, fiber, and mineral considerations. In Nutritional Ecology of Insects, Mites, and Spiders; Slansky, F., Jr., Rodriques, J.G., Eds.; Wiley: New York, NY, USA, 1987; pp. 105–146. [Google Scholar]
- Lindtke, D.; Buerkle, C.A.; Barbara, T.; Heinze, B.; Castiglione, S.; Bartha, D.; Lexer, C. Recombinant hybrids retain heterozygosity at many loci: Insights into the genomics of reproductive isolation in Populus. Mol. Ecol. 2012, 21, 5042–5048. [Google Scholar] [CrossRef]
- Altizer, S.; Ostfeld, R.S.; Johnson, T.J.; Kutz, S.; Harvell, C.D. Climate change and infectious diseases: From evidence to a predictive framework. Science 2013, 341, 514–519. [Google Scholar] [CrossRef]
- Martin, L.B.; Hopkins, W.A.; Mydlarz, L.D.; Rohr, J.R. The effects of anthropogenic global changes on immune functions and disease resistance. Ann. N. Y. Acad. Sci. 2010, 1195, 129–148. [Google Scholar] [CrossRef]
- Altizer, S.; Bartel, R.; Han, B.A. Animal migration and infectious disease risk. Science 2011, 331, 296–302. [Google Scholar] [CrossRef]
- Bartel, R.A.; Oberhauser, K.S.; deRoode, J.C.; Altizer, S. Monarch butterfly migration and mparasite transmission in eastern North America. Ecology 2011, 92, 342–351. [Google Scholar] [CrossRef]
- Harvell, C.D.; Mitchell, C.E.; Ward, J.R.; Altizer, S.; Dobson, A.P.; Osfeld, R.S.; Samual, M.D. Climate warming and disease risks for terrestrial and marine biota. Science 2002, 296, 2158–2162. [Google Scholar] [CrossRef]
- David, P. Heterozygosity-fitness correlations: New perspectives on old problems. Heredity 1998, 80, 531–537. [Google Scholar] [CrossRef]
- Watt, W.B. Allozymes in evolutionary genetics: Self-imposed burden or extraordinary tool? Genetics 1994, 136, 11–16. [Google Scholar]
- Britten, H.B. Meta-analysis of the association between multilocus heterozygosity and fitness. Evolution 1996, 50, 2158–2164. [Google Scholar] [CrossRef]
- Thelan, G.C.; Allendorf, F.W. Heterozygosity-fitness correlations in rainbow trout: Effects of allozyme loci or associative overdominance? Evolution 2001, 55, 1180–1187. [Google Scholar]
- Federici, B.A. Pathogens of Insects. In Encyclopedia of Insects; Resh, V.H., Carde, R.T., Eds.; Academic Press: New York, NY, USA, 2003; pp. 856–865. [Google Scholar]
- Lafferty, L.A.; Gerber, L.R. Good medicine for conservation biology: The intersection of epidemiology and conservation theory. Cons. Biol. 2002, 16, 593–604. [Google Scholar] [CrossRef]
- Sage, R.D.; Heyneman, D.; Lim, K.-C.; Wilson, A.C. Wormy mice in a hybrid zone. Nature 1986, 324, 60–63. [Google Scholar] [CrossRef]
- Hafner, M.S.; Demastes, J.W.; Hafner, D.J.; Spradling, T.A.; Sudman, P.D.; Nadler, S.A. Age and movement of a hybrid zone: Implications for dispersal distance in pocket gophers and their chewing lice. Evolution 1998, 52, 278–282. [Google Scholar] [CrossRef]
- Sammataro, D.; Gerson, U.; Needam, G. Parasitic mites of honey bees: Life history, implications, and impact. Annu. Rev. Entomol. 2000, 45, 519–548. [Google Scholar] [CrossRef]
- Scriber, J.M.; Hagen, R.H.; Lederhouse, R.C. Genetics of mimicry in the tiger swallowtail butterflies, Papilio glaucus and P. canadensis (Lepidoptera: Papilionidae). Evolution 1996, 50, 222–236. [Google Scholar] [CrossRef]
- Donovan, J. Multiple Fitness Indicators for Laboratory Hybrid Larvae of Papilio glaucus and Papilio canadensis (Lepidoptera: Papilionidae). MSc. Thesis, Michigan State University, East Lansing, MI, USA, June 2001. [Google Scholar]
- Johannesen, J.; Keyghobadi, N.; Schuler, H.; Stauffer, C.; Vogt, H. Invasion genetics of American cherry fruit fly in Europe and signals of hybridization with the European cherry fruit fly. Entomol. Expt. Appl. 2013, 147, 61–72. [Google Scholar] [CrossRef]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef]
- Nice, C.C.; Gompert, Z.; Forister, M.L.; Fordyce, J.A. An unseen foe in arthropod conservation efforts: The case of Wolbachia infections in the Karner blue butterfly. Biol. Conserv. 2009, 142, 3137–3146. [Google Scholar] [CrossRef]
- Thomas, M.B.; Blanford, S. Thermal biology in insect-parasite interactions. Trends Ecol. Evol. 2003, 18, 344–350. [Google Scholar] [CrossRef]
- Lafferty, K.D. The ecology of climate change and infectious diseases. Ecology 2009, 90, 888–900. [Google Scholar] [CrossRef]
- Hoang, A. Immune response to parasitism reduces resistance of Drosophila melanogaster to desiccation and starvation. Evolution 2001, 55, 2353–2358. [Google Scholar]
- Lampert, E. Influences of plant traits on immune responses of specialist and generalist herbivores. Insects 2012, 3, 573–592. [Google Scholar] [CrossRef]
- Moore, J.; Freehling, M. Cockroach hosts in thermal gradients suppress parasite development. Oecologia 2002, 133, 261–266. [Google Scholar] [CrossRef]
- Brasier, C.M. Rapid evolution of introduced plant pathogens via interspecific hybridization. BioScience 2001, 51, 123–133. [Google Scholar] [CrossRef]
- Nunney, L.; Yuuan, X.; Bromley, R.; Hartung, J.; Montero-Astua, M.; Mauricio, M.L.; Ortiz, B.; Stouthamer, R. Population genomic analysis of a bacterial plant pathogen: Novel insight into the origin of Pierce’s dsisease of grapevine in the U.S. PLoS One 2010, 5. [Google Scholar] [CrossRef]
- Seddon, P.J.; Armstrong, D.P.; Maloney, R.F. Developing the science of reintroduction biology. Conserv. Biol. 2007, 21, 303–312. [Google Scholar] [CrossRef]
- Armstrong, D.P.; Seddon, P. Directions in reintroduction biology. Trends Ecol. Evol. 2008, 23, 20–25. [Google Scholar] [CrossRef]
- Fischer, J.; Lindenmayer, D.B. An assessment of the published results of animal relocations. Biol. Conserv. 2000, 96, 1–11. [Google Scholar] [CrossRef]
- Chatzimanolis, S.; Caterino, M.S. Phylogeography and conservation genetics of California coastal terrestrial communities: A comparative study using three beetles. Insect Conserv. Divers. 2008, 1, 222–232. [Google Scholar] [CrossRef]
- Lankau, R.; Jorgensen, P.S.; Harris, D.J.; Sih, A. Incorporating evolutionary principals into environmental management and policy. Evol. Appl. 2011, 4, 315–325. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Hughes, L.; McIntyre, S.; Lindenmayer, D.B.; Parmesan, C.; Possingham, H.P.; Thomas, C.D. Assisted colonization and rapid climate change. Science 2008, 321, 345–346. [Google Scholar] [CrossRef]
- Crozier, L. Field transplants reveal summer constraints on butterfly range expansions. Oecologia 2004, 141, 148–157. [Google Scholar] [CrossRef]
- Bale, J.S.; Hayward, S.A.L. Insect overwintering in a changing climate. J. Exp. Biol. 2010, 213, 980–994. [Google Scholar] [CrossRef]
- Hahn, D.A.; Denlinger, D.L. Energetics of insect diapause. Annu. Rev. Entomol. 2011, 56, 103–121. [Google Scholar] [CrossRef]
- Brower, L.P.; Taylor, O.R.; Williams, E.H.; Slayback, D.A.; Zubieta, R.R.; Isabel, M. Decline of monarch butterflies overwintering in Mexico: Is the migratory phenomenon at risk? Insect Conserv. Divers. 2011, 5, 95–100. [Google Scholar]
- Feder, J.L.; Forbes, A.A. Host fruit-odor discrimination and sympatric host-race formation. In Specialization, Speciation, and Radiation: The Evolutionary Biology of Herbivorous Insects; Tilmon, K.J., Ed.; University California Press: Berkeley, CA, USA, 2008. [Google Scholar]
- Carroll, S.P. Facing change: Forms and foundations of contemporary adaptation to biotic invasions. Mol. Ecol. 2008, 17, 361–372. [Google Scholar] [CrossRef]
- Carroll, S.P.; Loye, J.E.; Dingle, H.; Mathieson, M.; Famula, T.R.; Zalucki, M.P. And the beak shall inherit-evolution in response to invasion. Ecol. Lett. 2005, 8, 944–951. [Google Scholar] [CrossRef]
- Murphy, S.M. Enemy-free space maintains swallowtail butterfly host shift. Proc. Natl. Acad. Sci. USA 2004, 101, 18048–18052. [Google Scholar] [CrossRef]
- Slove, J.; Janz, N. The relationship between diet breadth and geographical range size in the butterfly subfamily Nymphalinae- as study of global scale. PLoS One 2011, 6, e16057. [Google Scholar] [CrossRef]
- Jahner, J.P.; Bonilla, M.M.; Badick, K.J.; Shapiro, A.M.; Forister, M.L. Use of exotic hosts by Lepidoptera: Widespread species colonize more novel hosts. Evolution 2011, 65, 2719–2724. [Google Scholar] [CrossRef]
- Scriber, J.M.; Hainze, J. Geographic variation in host utilization and the development of insect outbreaks. In Insect Outbreaks: Ecological and Evolutionary Processes; Barbosa, P., Schultz, J.C., Eds.; Academic Press: New York, NY, USA, 1987; pp. 433–468. [Google Scholar]
- Scriber, J.M.; Allen, G.R.; Walker, P.W. Ecological monophagy in Tasmanian Graphium macleayanum moggana and evolutionary reflections of ancient Angiosperm hosts. Insect Sci. 2006, 13, 325–338. [Google Scholar] [CrossRef]
- Ording, G.J. Isolated Hybrid Swarm: Introgressed Genes of Papilio glaucus in a P. canadensis Population far beyond Their Hybrid Zone. MSc. Thesis, Michigan State University, East Lansing, MI, USA, August 2001. [Google Scholar]
- Scriber, J.M.; Gage, S. Pollution and global climate change: Plant ecotones, butterfly hybrid zones, and biodiversity. In The Swallowtail Butterflies: Their Ecology and Evolutionary Biology; Scriber, J.M., Tsubaki, Y., Lederhouse, R.H., Eds.; Scientific Publishers, Inc.: Gainesville, FL, USA, 1995; pp. 319–344. [Google Scholar]
- Agrawal, A.A. Phenotypic plasticity in the interactions and evolution of species. Science 2001, 294, 321–326. [Google Scholar] [CrossRef]
- Condamine, F.L.; Sperling, F.A.X.; Kergoat, G.J. Global biogeographical pattern of swallowtail diversification demonstrates alternative colonization routes in the Northern and Southern hemispheres. J. Biogeogr. 2013, 40, 9–23. [Google Scholar] [CrossRef]
- Buerkle, C.A.; Morris, R.J.; Asmussen, M.A.; Rieseberg, L.H. The likelihood of homoploid hybrid speciation. Heredity 2000, 84, 441–451. [Google Scholar] [CrossRef]
- Lexer, C.; Stolting, K.N. Tracing the recombination and colonization history of hybrid species in space and time. Mol. Ecol. 2011, 20, 3701–3704. [Google Scholar] [CrossRef]
- Gompert, Z.; Lucas, L.K.; Nice, C.C.; Fordyce, J.A.; Buerkle, C.A.; Forister, M.L. Geographically multifarous phenotypic divergence during speciation. Ecol. Evol. 2013, 3, 595–613. [Google Scholar] [CrossRef]
- Nosil, P.; Gompert, Z.; Farkas, T.E.; Comeault, A.A.; Feder, J.L.; Buerkle, C.A.; Parchman, T.L. Genomic consequences of multiple speciation processes in a stick insect. Proc. R. Soc. B 2012. [Google Scholar] [CrossRef]
- Brower, L.P. Speciation in butterflies of the Papilio glaucus group. I. Morphological relationships and hybridization. Evolution 1959, 13, 40–63. [Google Scholar] [CrossRef]
- Moritz, C. Defining “evolutionarily significant units” for conservation. Trends Ecol. Evol. 1994, 9, 373–375. [Google Scholar] [CrossRef]
- Galtier, N.; Nabholz, B.; Glemin, S.; Hurst, G.D.D. Mitochondrial DNA as a marker of molecular diversity: A reappraisal. Mol. Ecol. 2009, 18, 4541–4550. [Google Scholar]
- Hendry, A.P.; Vamosi, S.M.; Latham, S.J.; Heilbuth, J.C.; Day, T. Questioning species realities. Conserv. Genet. 2000, 1, 67–76. [Google Scholar] [CrossRef]
- Hey, J. On the failure of modern species concepts. Trends Ecol. Evol. 2006, 21, 447–450. [Google Scholar] [CrossRef]
- Avise, J.C.; Walker, D. Abandon all species concepts? A response. Cons. Genet. 2000, 1, 77–80. [Google Scholar] [CrossRef]
- Harrison, R.G. Linking evolutionary pattern and process: The relevance of species concepts for the study of speciation. In Endless Forms: Species and Speciation; Howard, D.J., Berlocher, S.H., Eds.; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Howard, D.J.; Berlocher, S.H. Endless Forms: Species and Speciation; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Crozier, R.H. Preserving the information content of species: Genetic diversity, phylogeny, and conservation worth. Annu. Rev. Ecol. Syst. 1997, 28, 243–268. [Google Scholar] [CrossRef]
- Maroja, L.S.; Andres, J.A.; Harrison, R.G. Genealogical discordance and patterns of introgression and selection across a cricket hybrid zone. Evolution 2009, 63, 2999–3015. [Google Scholar] [CrossRef]
- Feder, J.L.; Flaxmann, S.M.; Egan, S.P.; Nosil, P. Hybridization and the build-up of genomic divergence during speciation. J. Evol. Biol. 2013, 26, 261–266. [Google Scholar] [CrossRef]
- Scriber, J.M. Interaction of introgression from Papilio glaucus canadensis and diapause in producing ‘spring form’ Eastern tiger swallowtail butterflies, P. glaucus. Great Lakes Entomol. 1990, 23, 127–138. [Google Scholar]
- Ording, G.J. An Analysis of Climate Induced Hybrid Speciation in Tiger Swallowtail Butterflies (Papilio). Ph.D. Dissertation, Michigan State University, East Lansing, MI, USA, June 2008. [Google Scholar]
- Wadsworth, C.B.; Woods, W.A.; Hahn, D.A.; Dopman, E.B. One phase of the dormancy development pathway is critical for the evolution of insect seasonality. J. Evol. Biol. 2013, 26, 2359–2368. [Google Scholar] [CrossRef]
- Condamine, F.L.; Rolland, J.; Morlon, H. Macroevolutionary perspectives to environmental change. Ecol. Lett. 2013, 16, 72–85. [Google Scholar] [CrossRef]
- Gompert, Z. Population genomics as a new tool for wildlife management. Mol. Ecol. 2012, 21, 1542–1544. [Google Scholar] [CrossRef]
- Nosil, P.; Feder, J.L. Genome evolution and speciation: Toward quantitative descriptions of pattern and process. Evolution 2013, 67, 2461–2467. [Google Scholar] [CrossRef]
- Scriber, J.M. Origins of the regional feeding abilities in the tiger swallowtail butterfly: Ecological monophagy and the Papilio glaucus australis subspecies in Florida. Oecologia 1986, 71, 94–103. [Google Scholar] [CrossRef]
- Sands, T.; Larson, M.; Gleeson, M.; Scriber, J.M. Personal communication. University of Queensland: QLD, Brisbane, Australia, 2006. [Google Scholar]
- Parry, D.; Herms, D.A.; Scriber, J.M. Michigan State University: East Lansing, MI, USA, 1996; Unpublished work.
- Thiem, S.; Bauer, L. Michigan State University: East Lansing, MI, USA, 1997; Unpublished work.
- Hamm, C. Michigan State University: East Lansing, MI, USA, 2009; Unpublished work.
- Scriber, J.M.; Maher, E.; Niblack, M.; McGuire, M.; Nguyen, J. Michigan State University: East Lansing, MI, USA, 2008–2012; Unpublished work.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Scriber, J.M. Climate-Driven Reshuffling of Species and Genes: Potential Conservation Roles for Species Translocations and Recombinant Hybrid Genotypes. Insects 2014, 5, 1-61. https://doi.org/10.3390/insects5010001
Scriber JM. Climate-Driven Reshuffling of Species and Genes: Potential Conservation Roles for Species Translocations and Recombinant Hybrid Genotypes. Insects. 2014; 5(1):1-61. https://doi.org/10.3390/insects5010001
Chicago/Turabian StyleScriber, Jon Mark. 2014. "Climate-Driven Reshuffling of Species and Genes: Potential Conservation Roles for Species Translocations and Recombinant Hybrid Genotypes" Insects 5, no. 1: 1-61. https://doi.org/10.3390/insects5010001
APA StyleScriber, J. M. (2014). Climate-Driven Reshuffling of Species and Genes: Potential Conservation Roles for Species Translocations and Recombinant Hybrid Genotypes. Insects, 5(1), 1-61. https://doi.org/10.3390/insects5010001