Habitat Re-Creation (Ecological Restoration) as a Strategy for Conserving Insect Communities in Highly Fragmented Landscapes
Abstract
:1. Introduction
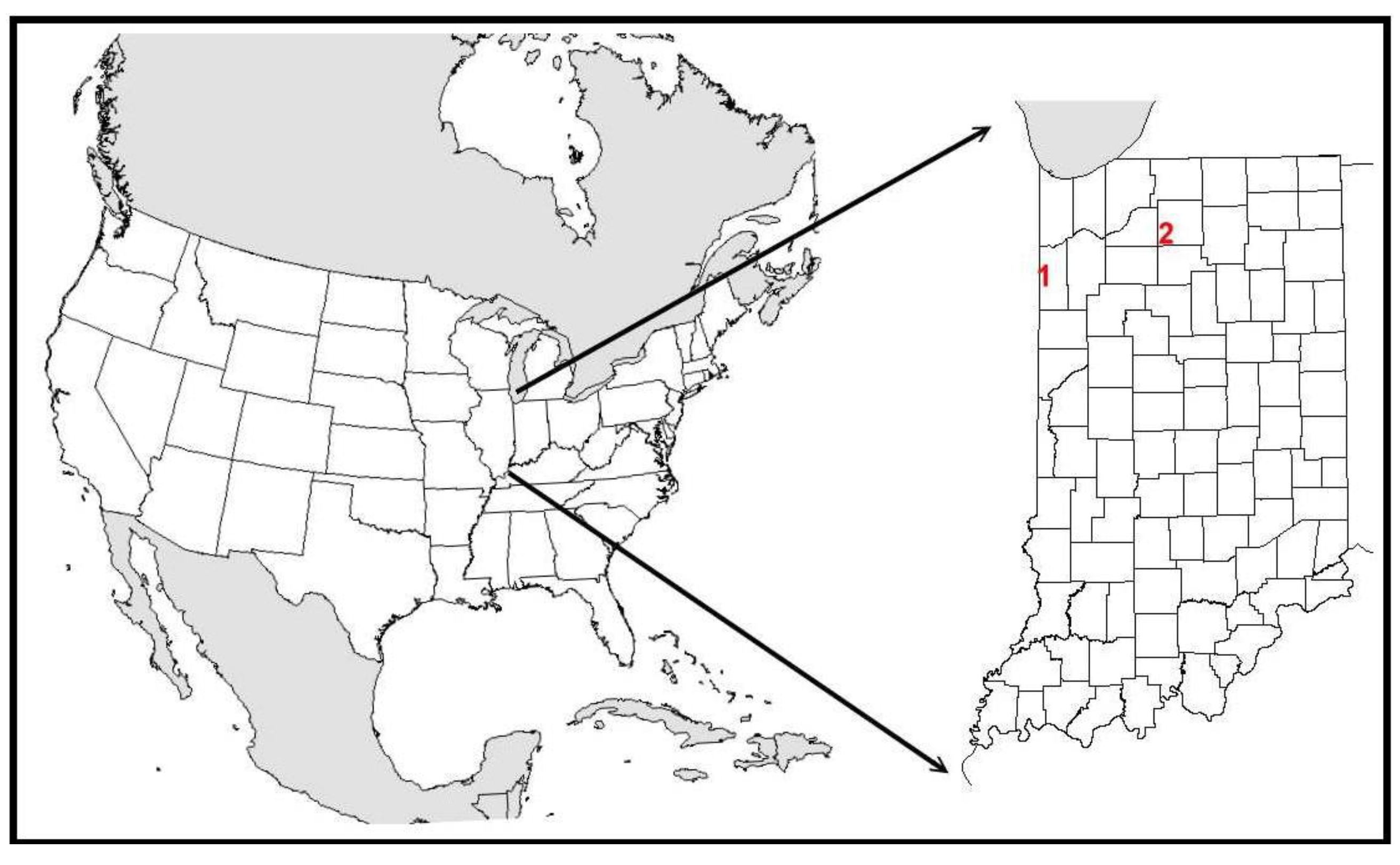
2. Generalized Conservation Threats to Remnant-Dependent Insect Communities in Highly Fragmented Landscapes
2.1. Small Population Size
2.2. Population Isolation/Disrupted Population Dynamics
2.3. Inappropriately Scaled Disturbance Regimes/Ecological Processes
2.4. Future Predicted Climate Regimes
3. Application of Generalized Restoration Strategies at Real-World Conservation Sites
3.1. Kankakee Sands
3.1.1. Site Description
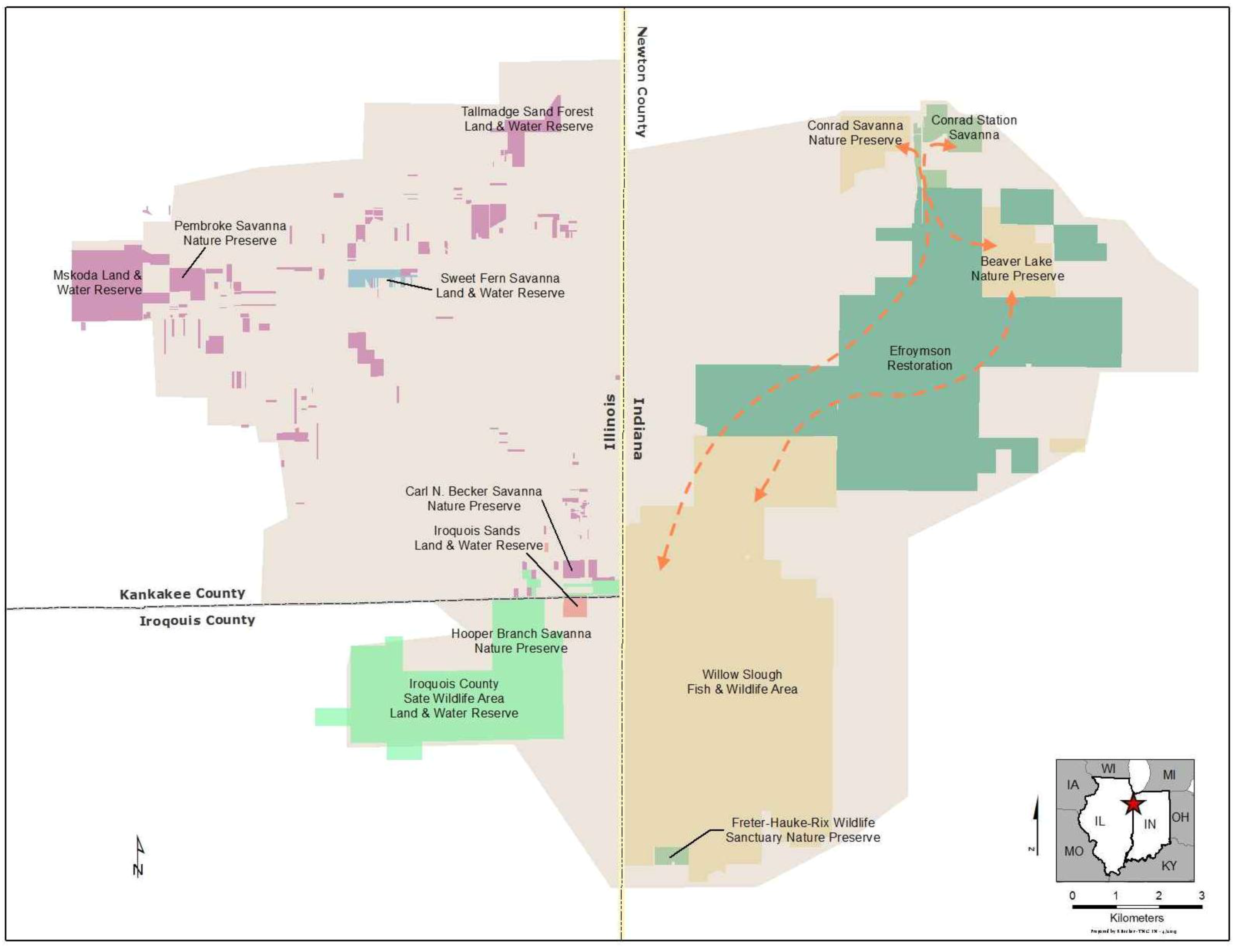
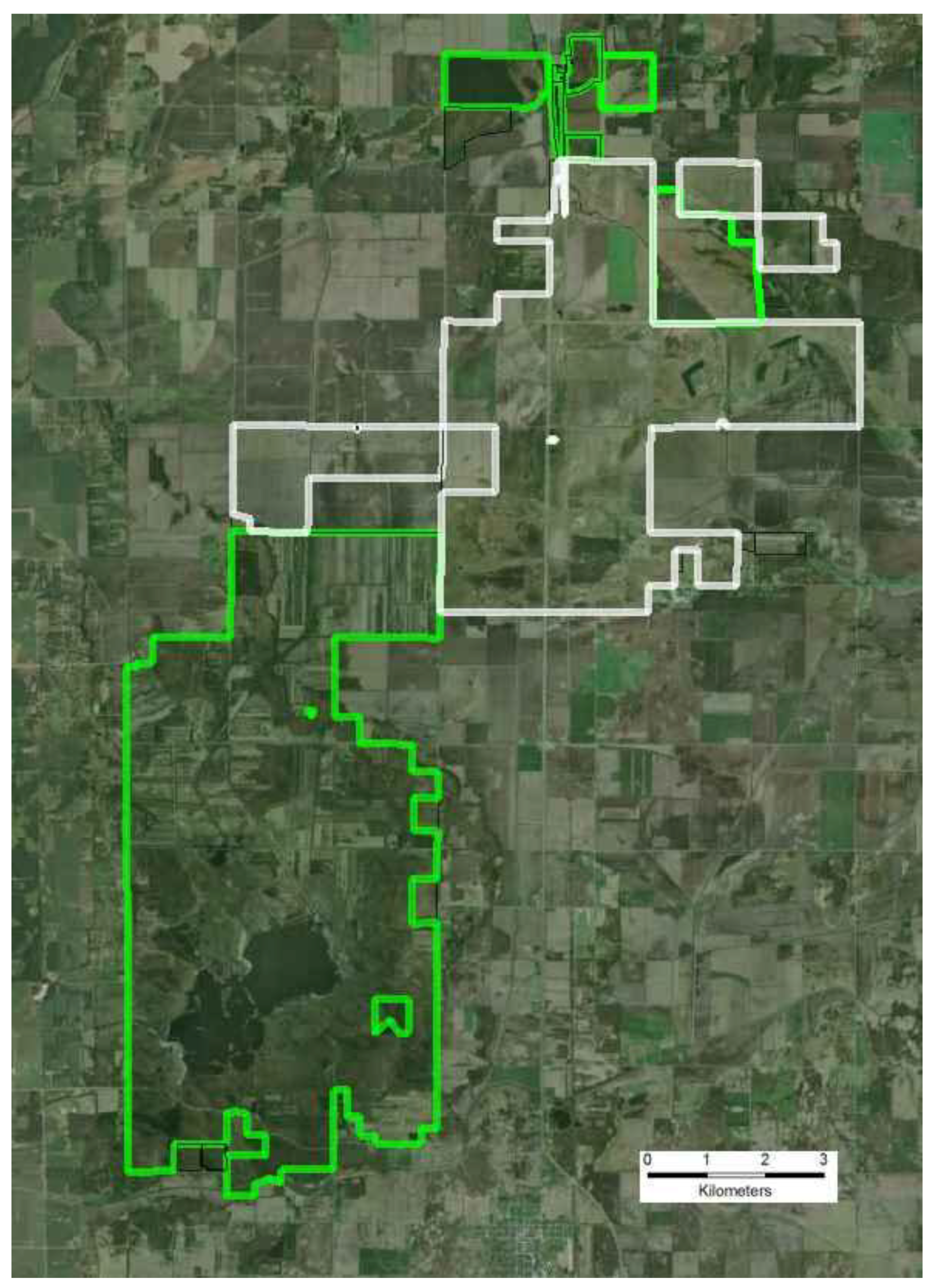
3.1.2. Small Population Size
- Additional habitats adjacent to ecosystem remnants would be restored to increase available habitat for r-d insect communities. Note that the scale of this “restoration for habitat expansion for insects” was overridden by the demands for restoring connectivity at the site and by the habitat needs of density dependent grassland birds [26] and by the need to address connectivity across the site.
- Hydrology would be restored to capture the entire range of habitats used by r-d species. R-d insects at the site use habitats that range from xeric sand dunes to seasonally flooded wet prairie. Adjacent to the remnants, wetlands and mesic habitats had been drained to accommodate agricultural production. To the greatest extent possible we restored hydrology to bring back emergent wetland hydrology, allowing the restoration of a diverse range of habitats types across the entire hydrological gradient.
- The entire native plant community would be planted into the restoration. Because many r-d insect species are presumed to be monophagous, and we do not know hostplant requirements for all species, we decided to restore all native vascular plant species known from the three ecosystem remnants into the restoration. Seeds and plugs for 621 species have been planted in appropriate hydrologic zones to kick-start ecological healing for the entire botanical community in an attempt to establish hostplants for all potential r-d insects.
- Local genotype plant materials are exclusively used for the restoration. While this criterion is in part based on our desire to conserve local plant ecotypes, an underlying concern involves co-evolutionary relationships with native insect communities at regional scales. While we don’t know for certain if local insect populations are adapted to local plant populations, the conservative approach is to assure that we do not disrupt any local adaptations that may have evolved locally.
3.1.3. Population Isolation/Disrupted Population Dynamics
3.1.4. Inappropriately Scaled Disturbance Regimes
3.1.5. Future Predicted Climate Regimes

3.2. Houghton Lake Wetlands
3.2.1. Site Description


3.2.2. Small Population Size
- Hydrology would be restored to maximize groundwater flow into the lake basin and adjacent drained wetlands to restore wetland hydrology to adjacent drained muck soils. Fens are fed by alkaline groundwater discharges, but agricultural practices intercepted groundwater and diverted it away from the lake and surrounding fields (Figure 5). As best as possible, groundwater flow was restored to enhance subsurface recharge of surrounding muck soils at the site in an attempt to recreate appropriate conditions for fen and sedge meadow restoration. In agricultural fields surrounding the wetland, we filled ditches and removed tile drainage to the maximum extent possible. This included creating upland basins which captured overland flow in order to increase infiltration of surface water flow into near-surface ground water flow. In addition, the Houghton Lake outfall was raised to help re-wet muck soils surrounding the wetlands (Figure 7).
- For fen habitat, the entire native plant community would be seeded into the restoration. Because the fen is assumed to support r-d species that are monophagous, we seeded as many of the known vascular plant species as possible into the restoration. This included over 110 species planted as seed over bare soil. Because sedges often do not establish well from seed, we planted approximately 10,000 plugs of late-successional rhizominous Carex species to aid in the initial establishment of sedge meadows habitat. The agricultural uplands surrounding wetlands were not restored as r-d insect habitat. Because no remnant upland grasslands persisted at the site, there was no concern for r-d insects in this habitat type. Uplands were restored to meet habitat criteria for regionally rare vertebrates known to occur at the site and to facilitate groundwater recharge. Uplands were planted at a relatively low cost to moderate diversity grasslands.
- Local genotype plant materials are exclusively used for the restoration of fen habitats. Similar to Kankakee Sands, we desire to conserve local plant ecotypes and potential coevolutionary relationships with native insect communities. Seeds for many species were collected on site from sedge meadow and fen for use in the adjacent restorations.

3.2.3. Future Predicted Climate Regimes
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Panzer, R.; Shuey, J.; Stillwaugh, D. Characterizing insects within fragmented landscapes. Nat. Areas J. 1997, 17, 53–55. [Google Scholar]
- Lessons from Amazonia: The Ecology and Conservation of a Fragmented Forest; Bierregaard, R.; Gascon, C.; Lovejoy, T.; Mesquita, R. (Eds.) Yale University Press: New Haven, CT, USA, 2001.
- Lindenmayer, D.; Fischer, J. Habitat Fragmentation and Landscape Change: An Ecological and Conservation Synthesis; Island Press: Washington, DC, USA, 2006. [Google Scholar]
- Hanski, I. Habitat connectivity, habitat continuity, and metapopulations in dynamic landscapes. Oikos 1999, 87, 209–219. [Google Scholar] [CrossRef]
- Hamilton, K. Bugs reveal an extensive, long-lost northern tallgrass prairie. BioScience 2005, 55, 49–59. [Google Scholar] [CrossRef]
- Metzler, E.; Shuey, J.; Ferge, L.; Henderson, R.; Goldstein, P. Contributions to the Understanding of Tallgrass Prairie-Dependent Butterflies and Moths (Lepidoptera) and their Biogeography in the United States; Bulletin of the Ohio Biological Survey; Ohio Biological Survey: Columbus, OH, USA, 2005; Volume 15, p. Number 1. [Google Scholar]
- Panzer, R. Management of prairie remnants for insect conservation. Nat. Areas J. 1988, 8, 83–90. [Google Scholar]
- Panzer, R.; Stillwaugh, D.; Gnaedinger, R.; Derkovitz, G. Prevalence of remnant-dependence among the prairie- and savanna-inhabiting insects of the Chicago region. Nat. Areas J. 1995, 15, 101–116. [Google Scholar]
- Panzer, R.; Gnaedinger, R.; Derkovitz, G. The prevalence and status of conservative prairie and sand savanna insects in the Chicago wilderness region. Nat. Areas J. 2010, 30, 73–81. [Google Scholar] [CrossRef]
- Panzer, R.; Schwartz, M.W. Effectiveness of a vegetation-based approach to insect conservation. Conserv. Biol. 1998, 12, 693–702. [Google Scholar] [CrossRef]
- Association for Biodiversity Information. Plant Communities of the Midwest: Classification in an Ecological Context; Faber-Langendoen, D., Ed.; Association for Biodiversity Information: Arlington, VA, USA, 2001. [Google Scholar]
- Groves, C.; Jensen, D.; Valutis, L.; Redford, K.; Shaffer, M.; Scott, J.; Baumgartner, J.; Higgins, J.; Beck, M.; Anderson, M. Planning for biodiversity conservation: Putting conservation science into practice. BioScience 2002, 52, 499–511. [Google Scholar] [CrossRef]
- Das, A.; Krishnaswamya, J.; Bawaa, K.; Kirana, M.; Srinivasc, V.; Kumarc, N.; Karanth, K. Prioritization of conservation areas in the Western Ghats, India. Biol. Conserv. 2006, 133, 16–13. [Google Scholar] [CrossRef]
- Howard, P.; Viskanic, P.; Davenport, T.; Kigenyi, F.; Baltzer, M.; Dickenson, C.; Lwanga, J.; Matthews, R.A.; Balmford, A. Complementarity and the use of indicator groups for reserve selection in Uganda. Nature 1998, 392, 472–475. [Google Scholar]
- Shuey, J. The Status of butterfly conservation in Indiana, Assessing the effectiveness of a complimentary system of habitat reserves relative to species at risk and divergent populations. Am. Midland Nat. 2005, 153, 117–127. [Google Scholar]
- Hanski, I. Metapopulation dynamics. Nature 1998, 396, 41–49. [Google Scholar] [CrossRef]
- Sampson, F.; Knopf, F. Prairie conservation in North America. BioScience 1994, 44, 418–421. [Google Scholar] [CrossRef]
- Biedermann, R.; Achtziger, R.; Nickel, H.; Stewart, A. Conservation of grassland leafhoppers: A brief review. J. Insect Conserv. 2005, 9, 229–243. [Google Scholar] [CrossRef]
- Summerville, K.; Crist, T. Contrasting effects of habitat quantity and quality on moth communities in fragmented landscapes. Ecography 2004, 27, 3–12. [Google Scholar] [CrossRef]
- Jonas, J.; Whiles, M.; Charlton, R. Aboveground invertebrate response to land management differences in a central Kansas Grassland. Environ. Entomol. 2002, 31, 1142–1152. [Google Scholar] [CrossRef]
- Orlofske, J.; Ohnesorg, W.; Debinski, D. A comparison of the arthropod communities in remnant, restored, and reconstructed Iowa tallgrass prairies. Nat. Areas J. 2011, 31, 148–155. [Google Scholar] [CrossRef]
- Brand, R.; Dunn, C. Diversity and abundance of springtails (insecta: collembola) in native and restored tallgrass prairies. Am. Midland Nat. 1998, 139, 235–242. [Google Scholar] [CrossRef]
- Rowe, H.; Holland, J. High plant richness in prairie reconstructions support diverse leafhopper communities. Restor. Ecol. 2013, 21, 174–180. [Google Scholar] [CrossRef]
- Shepherd, S.; Debinski, D. Evaluation of isolated and integrated prairie reconstructions as habitat for prairie butterflies. Biol. Conserv. 2005, 126, 51–61. [Google Scholar] [CrossRef]
- Woodcock, B.; Vogiatzakis, I.; Westbury, D.; Lawson, C.; Edwards, A.; Brook, A.; Harris, S.; Lock, K.; Maczey, N.; Masters, G.; et al. The role of management and landscape context in the restoration of grassland phytophagous beetles. J. Appl. Ecol. 2010, 47, 366–376. [Google Scholar] [CrossRef]
- Herkert, J. The effects of habitat fragmentation on Midwestern grassland bird communities. Ecol. Appl. 1994, 4, 461–471. [Google Scholar] [CrossRef]
- Semlitsch, R. Principles for management of aquatic breeding amphibians. J. Wildlife Manag. 2000, 64, 615–631. [Google Scholar] [CrossRef]
- Shepherd, S.; Debinski, D. Reintroduction of Regal Fritillary (Speyeria idalia) to a restored prairie. Ecol. Restor. 2005, 23, 244–250. [Google Scholar] [CrossRef]
- Schultz, C. Dispersal behavior and its implications for reserve design in a rare Oregon butterfly. Conserv. Biol. 1998, 12, 284–292. [Google Scholar] [CrossRef]
- Schultz, C. Restoring resources for an endangered butterfly. J. Appl. Ecol. 2001, 38, 1007–1019. [Google Scholar] [CrossRef]
- Samways, M. Insect Conservation Biology; Chapman and Hall: New York, NY, USA, 1997. [Google Scholar]
- New, T. Butterfly Conservation; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Naeem, S. Biodiversity and Ecosystem Functioning in Restored Ecosystems: Extracting Principles for a Synthetic Perspective. In Foundations of Restoration Ecology; Falk, D., Palmer, M., Zedler, J., Eds.; Island Press: Washington, DC, USA, 2006; pp. 210–237. [Google Scholar]
- Assembly Rules and Restoration Ecology—Bridging the Gap between Theory and Practice; Temperton, V.; Hobbs, R.; Nuttle, T.; Halle, S. (Eds.) Island Press: Washington, DC, USA, 2004.
- Schwartz, M.; Brigham, C.; Hoeksema, J.; Lyons, K.; Mills, M.; van Mantgem, P. Linking biodiversity to ecosystem function: implications for conservation ecology. Oecologia 2000, 122, 297–305. [Google Scholar] [CrossRef]
- Conservation Measures Partnership. 2013 The Open Standards for the Practice of Conservation, Version 3.0. Available online: http://www.conservationmeasures.org/wp-content/uploads/2013/05/CMP-OS-V3–0-Final.pdf (accessed on 2 November 2013).
- Gilpin, M.; Soule, M. Minimum Viable Populations: Processes of Species Extinction. In Conservation Biology: The Science of Scarcity and Diversity; Soule, M., Ed.; Sinauer Associates: Sunderland, MA, USA, 1986; pp. 19–34. [Google Scholar]
- Szymanski, J.; Shuey, J.; Oberhauser, K. Population structure of the endangered Mitchell’S satyr, Neonympha mitchellii mitchellii (French): Implications for conservation. Am. Midland Nat. 2004, 152, 304–322. [Google Scholar] [CrossRef]
- Wells, C.; Williams, R.; Walker, G.; Haddad, N. Effects of corridors on genetics of a butterfly in a landscape experiment. South. Nat. 2009, 8, 709–722. [Google Scholar] [CrossRef]
- Hamilton, K. Evaluation of Leafhoppers and Their Relatives (Insecta: Homoptera: Auchenorrhyncha) as Indicators of Prairie Preserve Quality. In Proceedings of the Fourteenth North American Prairie Conference, Manhattan, KS, USA, 12–16 July 1994.
- Panzer, R.; Schwartz, M. Effects of management burning on prairie insect species richness within a system of small, highly fragmented reserves. Biol. Conserv. 2000, 96, 363–369. [Google Scholar] [CrossRef]
- Woo, I.; Zedler, J. Can nutrients alone shift a sedge meadow towards dominance by the invasive Typha × Glauca. Wetlands 2002, 22, 509–521. [Google Scholar] [CrossRef]
- Climate Wizard Custom. 2013. Available online: http://www.climatewizardcustom.org/ (accessed on 2 November 2013).
- O’Leary, C.; Shuey, J. Ecosystem Restoration at the Landscape-Scale: Design and Implementation at the Efroymson Restoration. In Proceedings of the 18th North American Prairie Conference, Mason City, IA, USA, 16–20 July 2000.
- Shuey, J.; Metzler, E.; Tungesvick, K. Moth communities correspond with plant communities in Midwestern (Indiana, USA) sand prairies and oak barrens and their degradation endpoints. Am. Midland Nat. 2012, 167, 273–284. [Google Scholar]
- Forman, R. Land Mosaics. The Ecology of Landscapes and Regions; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Braun, E.L. Glacial and post-glacial plant migrations indicated by relic colonies of southern Ohio. Ecology 1928, 9, 284–302. [Google Scholar] [CrossRef]
- Shane, L. Late-glacial vegetational and climatic history of the Allegheny Plateau and the Till Plains of Ohio and Indiana, USA. Boreas 1987, 16, 1–20. [Google Scholar] [CrossRef]
- Gustafson, E.; Sturtevant, B. Modeling forest mortality caused by drought stress: Implications for climate change. Ecosystems 2013, 16, 60–74. [Google Scholar] [CrossRef]
- Dale, V.; Joyce, L.; McNulty, S.; Neilson, R.; Ayres, M.; Flannigan, M.; Hanson, P.; Irland, L.; Lugo, A.; Peterson, C.; et al. Climate change and forest disturbances. BioScience 2001, 51, 723–734. [Google Scholar] [CrossRef]
- Panzer, R. Northeastern Illinois University: Chicago, IL, USA, Unpublished work. 2009.
- Shuey, J. Habitat associations of wetland butterflies near the glacial Maxima in Ohio, Indiana, and Michigan. J. Res. Lepidoptera 1985, 24, 176–186. [Google Scholar]
- Landis, D.; Fiedler, A.; Hamm, C.; Cuthrell, D.; Schools, E.; Pearsall, D.; Herbert, M.; Doran, P. Insect conservation in Michigan prairie fen: Addressing the challenge of global change. J. Insect Conserv. 2012, 16, 131–142. [Google Scholar] [CrossRef]
- Andreas, B. The relationship between Ohio peatland distribution and buried river valleys. Ohio J. Sci. 1985, 85, 116–125. [Google Scholar]
- Swinehart, A.; Parker, G. Palaeoecology and development of peatlands in Indiana. Am. Midland Nat. 2000, 143, 267–297. [Google Scholar] [CrossRef]
- Shirey, P.; Lamberti, G. Assisted colonization under the U.S. Endangered Species Act. Conserv. Lett. 2010, 3, 45–52. [Google Scholar] [CrossRef]
- Losey, J.; Vaughan, M. The economic value of ecological services provided by insects. BioScience 2006, 56, 311–323. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shuey, J.A. Habitat Re-Creation (Ecological Restoration) as a Strategy for Conserving Insect Communities in Highly Fragmented Landscapes. Insects 2013, 4, 761-780. https://doi.org/10.3390/insects4040761
Shuey JA. Habitat Re-Creation (Ecological Restoration) as a Strategy for Conserving Insect Communities in Highly Fragmented Landscapes. Insects. 2013; 4(4):761-780. https://doi.org/10.3390/insects4040761
Chicago/Turabian StyleShuey, John A. 2013. "Habitat Re-Creation (Ecological Restoration) as a Strategy for Conserving Insect Communities in Highly Fragmented Landscapes" Insects 4, no. 4: 761-780. https://doi.org/10.3390/insects4040761
APA StyleShuey, J. A. (2013). Habitat Re-Creation (Ecological Restoration) as a Strategy for Conserving Insect Communities in Highly Fragmented Landscapes. Insects, 4(4), 761-780. https://doi.org/10.3390/insects4040761



