Functional Immunomics of the Squash Bug, Anasa tristis (De Geer) (Heteroptera: Coreidae)
Abstract
:1. Introduction
2. Experiment
2.1. Insects, Infections and RNA Isolation
2.2. Illumina Sequence Generation and Assembly Procedures
2.3. Identification of Differentially Regulated Genes
3. Results and Discussion
3.1. Anasa Tristis RNA-Seq
| Parameter | Output |
|---|---|
| Total Sequences | 211, 532, 043 |
| Input Sequences | 115, 101, 048 (54.4%) |
| Contigs a | 130,000 |
| Contigs > 300 bp | 96,480 |
| Annotated b | |
| Contigs >300 bp | 37,327 |
| (Ave = 1,588 bp; Range 301 to 16,368) | |
| Contigs > 5 kb | 858 |
| Contigs > 4 kb < 5 kb | 1,068 |
| Contigs > 3 kb < 4 kb | 2,450 |
| Contigs > 2 kb < 3 kb | 5,601 |
| Contigs > 1 kb < 2 kb | 12,216 |
| Contigs > 301 bp < 1 kb | 15,135 |

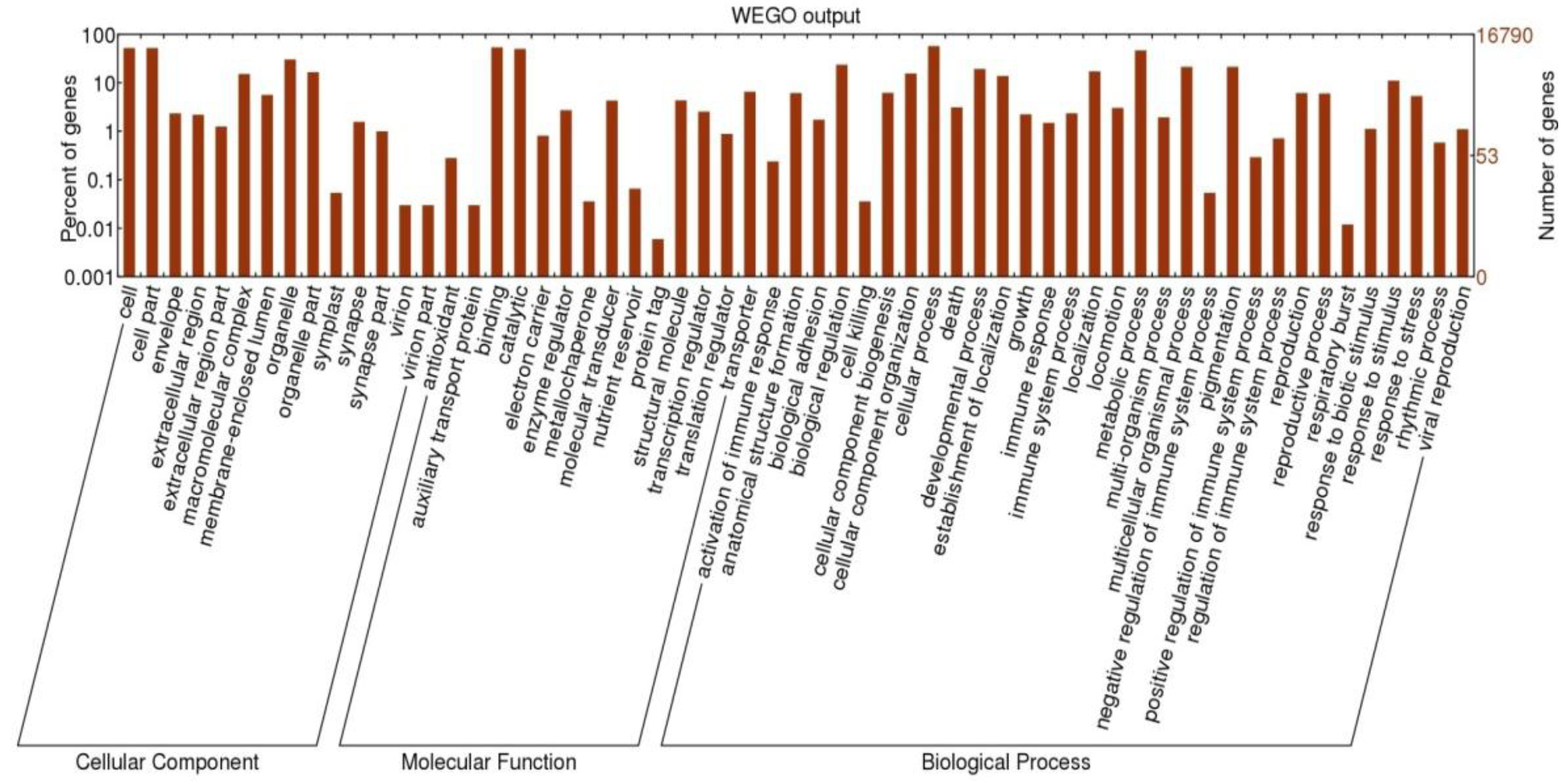
3.2. Immunity Related Transcripts Identified in the A. Tristis Transcriptome
3.3. Pattern Recognition and Signal Transduction
| Transcript ID | NR Annotation | MeanControl a | Mean Bacterial a | B/C b | Mean Fungal a | F/C b | NRid | Score | Eval | Best Match |
|---|---|---|---|---|---|---|---|---|---|---|
| 2224c0seq1 | 15-hydroxyprostaglandin dehydrogenase | 325.96 | 771.19 | 2.36 | 508 | 1.56 | ref|XP_001943808.2 | 204 | 1E-51 | Acyrthosiphon pisum |
| 3518c0seq1 | β-1,3-glucan recognition protein 4a | 306.34 | 381.82 | 1.25 | 363 | 1.19 | gb|ADZ45541.1 | 247 | 2E-64 | Hepialus pui |
| 20931c0seq2 | Croquemort | 6.74 | 16.1 | 2.39 | 13.17 | 1.96 | gb|EFN76142.1 | 323 | 2E-87 | Harpegnathos saltator |
| 18080c0seq1 | C-type Lectin | 16.67 | 5.33 | 0.32 | 15 | 0.9 | gb|ABD60991.1 | 192 | 6E-49 | Glossina morsitans |
| 7613c1seq8 | Dorsal | 29.84 | 42.47 | 1.42 | 33.62 | 1.13 | gb|ABU96698.1 | 555 | 1E-157 | Rhodnius prolixus |
| 17785c0seq3 | Dscam | 0.96 | 14.69 | 15.25 | 9.83 | 10.2 | ref|XP_003395940.1 | 1423 | 0 | Bombus terrestris |
| 1470c3seq3 | FADD | 266 | 313 | 1.18 | 134 | 0.51 | ref|NP_001189465.1 | 61.2 | 4E-08 | Bombyx mori |
| 6326c0seq5 | Galectin | 28.92 | 82 | 2.84 | 39.5 | 1.37 | ref|XP_001656933.1 | 157 | 3E-37 | Aedes aegypti |
| 12793c0seq5 | JNK-interacting protein 3 | 2.07 | 1.74 | 0.84 | 2 | 0.96 | ref|XP_396524.4 | 428 | 1E-118 | Apis mellifera |
| 10217c0seq1 | Leucine-rich repeat-containing protein 4B | 225 | 172 | 0.77 | 100 | 0.45 | ref|XP_972275.1 | 526 | 1E-148 | Tribolium castaneum |
| 14846c0seq15 | MAP kinase-activating death domain | 19.3 | 13.33 | 0.69 | 12.28 | 0.64 | gb|EFN64544.1 | 585 | 1E-166 | Camponotus floridanus |
| 2228c0seq2 | NF-κ-B inhibitor alpha | 132 | 130 | 0.99 | 118 | 0.9 | ref|XP_002427786.1 | 237 | 3E-61 | Pediculus humanus |
| 255c0seq1 | PGRP S2-like protein | 2462 | 2134 | 0.87 | 2648 | 1.08 | ref|NP_001164436.1 | 156 | 2E-37 | Nasonia vitripennis |
| 109335c0seq1 | Phospholipase A2 inhibitor 31 kDa | 2 | 0 | 0 | 0.33 | 0.16 | ref|XP003222879.1 | 87 | 1E-15 | Anolis carolinensis |
| 7436c0seq2 | Phospholipase A2, iPLA-2 | 282.73 | 254 | 0.89 | 174 | 0.61 | ref|XP_970721.2 | 631 | 1E-179 | Tribolium castaneum |
| 9865c1seq1 | Phospholipase A-2-activating protein | 144.90 | 157 | 1.08 | 107 | 0.74 | ref|XP_002423298.1 | 445 | 1E-123 | Pediculus humanus |
| 5036c1seq1 | Phospholipase A2 | 129.37 | 117 | 0.90 | 141 | 1.09 | ref|NP_001191907.1 | 201 | 1E-50 | Acyrthosiphon pisum |
| 8868c0seq1 | Prostaglandin E synthase 2 | 162 | 209.3 | 1.28 | 125 | 0.77 | ref|XP_002432321.1 | 202 | 1E-101 | Pediculus humanus |
| 28963c0seq2 | Prostaglandin E2 receptor EP3 subtype | 29 | 0 | 0 | 7.3 | 0.25 | ref|XP_001943957.2 | 237 | 4E-61 | Acyrthosiphon pisum |
| 6943c0seq1 | Prostaglandin reductase 1 | 100 | 216 | 2.15 | 116 | 1.15 | ref|XP_001603755.1 | 373 | 1E-102 | Nasonia vitripennis |
| 111273c0seq1 | Relish | 0.67 | 0 | 0 | 0 | 0 | gb|ACT66912.1 | 50.8 | 3E-06 | Apis andreniformis |
| 20920c0seq4 | Scavenger receptor class B | 0.09 | 12.95 | 149 | 10.77 | 124 | ref|XP_001947205.2 | 173 | 2E-42 | Acyrthosiphon pisum |
| 16467c0seq1 | Easter | 73.33 | 18.67 | 0.25 | 54 | 0.74 | gb|EFN62640.1 | 176 | 2E-43 | Camponotus floridanus |
| 15721c0seq1 | Snake | 45 | 52.67 | 1.17 | 11 | 0.24 | ref|XP_003247331.1 | 245 | 1E-63 | Acyrthosiphon pisum |
| 36985c0seq2 | Spaetzle 4 | 1 | 4.56 | 4.56 | 2.12 | 2.12 | ref|NP_001153592.1 | 132 | 5E-30 | Acyrthosiphon pisum |
| 19735c0seq1 | toll | 84 | 84 | 1 | 69 | 0.83 | ref|XP_002424097.1 | 1514 | 0 | Pediculus humanus |
3.4. Melanization, Coagulation and Antimicrobial Activities
| Transcript | NR Annotation | Mean Contro l a | Mean Bacterial a | B/C b | Mean Fungal a | F/C b | NRid | Score | Eval | Best Match |
|---|---|---|---|---|---|---|---|---|---|---|
| 693c0seq4 | Alaserpin-like | 196.48 | 246.79 | 1.26 | 159.80 | 0.81 | ref|XP003399187.1 | 240 | 3E-62 | Bombus terrestris |
| 3354c0seq1 | Amyloid beta A4 precursor | 144.59 | 304.18 | 2.10 | 161.73 | 1.12 | ref|XP001943320.1 | 406 | 1E-111 | Acyrthosiphon pisum |
| 1603c0seq10 | CLIP-associating protein | 307.15 | 372.83 | 1.21 | 305.06 | 0.99 | ref|XP003246351.1 | 404 | 1E-111 | Acyrthosiphon pisum |
| 83136c0seq1 | Coagulation factor IX | 1.67 | 5.33 | 3.20 | 2.33 | 1.40 | ref|XP001943624.2 | 256 | 2E-67 | Acyrthosiphon pisum |
| 23755c0seq2 | Coagulation factor X | 1.49 | 1.87 | 1.26 | 4.02 | 2.70 | gb|EFN83879.1 | 150 | 2E-35 | Harpegnathos saltator |
| 4923c0seq1 | Complement C1r-B subcomponent | 366.47 | 492.67 | 1.34 | 100.31 | 0.27 | ref|XP001120594.2 | 261 | 2E-68 | Apis mellifera |
| 393c1seq1 | Defensin “Anasin” | 1014.84 | 533.22 | 0.53 | 1665.55 | 1.64 | sp|P80407.1 | 85.5 | 2E-16 | Triatoma brasiliensis |
| 15509c0seq1 | Dual oxidase | 82.33 | 102.67 | 1.25 | 45.67 | 0.55 | ref|XP_001951113.2 | 2294 | 0 | Acyrthosiphon pisum |
| 8c0seq1 | ferritin | 48316 | 56283 | 1.16 | 57436 | 1.19 | gb|ABR27877.1 | 291 | 3E-77 | Triatoma infestans |
| 82370c0seq1 | Ferritin, heavy subunit | 2.67 | 0.00 | 0.00 | 1.67 | 0.63 | gb|ACO15170.1 | 130 | 2E-30 | Caligus clemensi |
| 20023c0seq1 | Ferritin, middle subunit | 10.67 | 56.72 | 5.32 | 39.93 | 3.74 | gb|ACO10415.1 | 201 | 7E-51 | Caligus rogercresseyi |
| 344c0seq1 | Gelsolin precursor | 7089.52 | 4998.67 | 0.71 | 1494.70 | 0.21 | ref|XP001657431.1 | 743 | 0 | Aedes aegypti |
| 33115c0seq1 | Hemiptericin “Anacin” | 1.33 | 22.07 | 16.55 | 3.00 | 2.25 | gb|ABW16857.1 | 79.7 | 8E-17 | Pyrrhocoris apterus |
| 2804c0seq1 | Hemolectin | 61.16 | 38.67 | 0.63 | 16 | 0.26 | ref|XP_002430354.1 | 52 | 7E-06 | Pediculus humanus |
| 533c2seq8 | Lysozyme | 167.25 | 144.80 | 0.87 | 118.97 | 0.71 | gb|ABX11554.1 | 159 | 7E-38 | Rhodnius prolixus |
| 2227c0seq2 | Multiple coagulation factor deficiency protein | 304.36 | 245.79 | 0.81 | 258.63 | 0.85 | gb|EGI57786.1 | 157 | 2E-37 | Acromyrmex echinatior |
| 17478c0seq1 | NADPH oxidase | 184.00 | 130.33 | 0.71 | 146.67 | 0.80 | tpd|FAA00348.1 | 58.2 | 5E-07 | Apis mellifera |
| 5121c0seq1 | Nitric oxide synthase | 342.29 | 1154.75 | 3.37 | 160.71 | 0.47 | sp|Q26240.1 | 1784 | 0 | Apis mellifera |
| 8767c1seq1 | Plasma kallikrein | 11.66 | 26.19 | 2.25 | 17.23 | 1.48 | gb|EFN62475.1 | 54.3 | 7E-07 | Camponotus floridanus |
| 6324c0seq4 | Platelet-activating factor | 62.28 | 75.57 | 1.21 | 111.93 | 1.80 | ref|XP002427586.1 | 311 | 9E-84 | Pediculus humanus |
| 4507c0seq2 | Proclotting enzyme | 219.76 | 357.26 | 1.63 | 177.83 | 0.81 | gb|EFN62552.1 | 296 | 5E-79 | Camponotus floridanus |
| 10065c0seq1 | Prophenoloxidase subunit 2 | 107.67 | 103.33 | 0.96 | 53.33 | 0.50 | ref|XP967179.2 | 688 | 0 | Tribolium castaneum |
| 15584c0seq1 | Prophenoloxidase subunit A3 | 16.00 | 8.00 | 0.50 | 8.00 | 0.50 | ref|NP001011627.1 | 298 | 3E-80 | Apis mellifera |
| 14386c0seq4 | Serpin | 5.50 | 0.10 | 0.02 | 0.00 | 0.00 | gb|EFA12666.1 | 112 | 8E-24 | Tribolium castaneum |
| 254c0seq1 | Transferrin | 7350.06 | 14070.74 | 1.91 | 10554.93 | 1.44 | gb|AAD02419.1 | 939 | 0 | Riptortus clavatus |
3.5. RNAi Pathways
3.6. Viral Sequences
| Transcript | NR Annotation | Mean Control a | Mean Bacterial a | B/C b | Mean Fungal a | F/C b | NRid | Score | Eval | Best Match |
|---|---|---|---|---|---|---|---|---|---|---|
| 10966c0seq13 | argonaute-1 | 33 | 34.96 | 1.06 | 43 | 1.3 | gb|ACO40482.1 | 133 | 5E-29 | Nasonia vitripennis |
| 1860c0seq1 | argonaute-2 | 1233.65 | 1107.58 | 0.9 | 634.29 | 0.51 | ref|XP001607164.1 | 721 | 0 | Nasonia vitripennis |
| 108824c0seq1 | aubergine | 0.67 | 0 | 0 | 0 | 0 | ref|NP001159378.1 | 221 | 2E-57 | Apis mellifera |
| 44766c0seq1 | dicer-1 | 9 | 14 | 1.56 | 11.67 | 1.3 | emb|CAX68236.1 | 754 | 0 | Blattella germanica |
| 12963c0seq2 | dicer-2 | 11.08 | 1.69 | 0.15 | 12.46 | 1.12 | ref|NP001107840.1 | 793 | 0 | Tribolium castaneum |
| 12963c0seq3 | Endoribonuclease Dcr-1 | 94.37 | 110.64 | 1.17 | 75.62 | 0.8 | gb|EFN62420.1 | 432 | 1E-120 | Camponotus floridanus |
| 17917c0seq1 | microprocessor subunit DGCR8 | 91.33 | 130.33 | 1.43 | 69 | 0.76 | ref|XP003397039.1 | 575 | 1E-162 | Bombus terrestris |
| 7746c0seq1 | PIWI | 235.33 | 304.67 | 1.29 | 125 | 0.53 | ref|XP001652945.1 | 722 | 0 | Aedes aegypti |
| 12734c0seq1 | RISC-loading complex subunit | 92.92 | 51.74 | 0.56 | 58.93 | 0.63 | ref|XP001601132.2 | 380 | 1E-104 | Nasonia vitripennis |
| 2817c0seq1 | Translin-like | 327.0 | 373.67 | 1.14 | 213.0 | 0.65 | dbj|BAG65665.1 | 611 | 1E-174 | Nasonia vitripennis |
4. Conclusions
Acknowledgments
Conflicts of Interest
Disclaimer
References
- United States Department of Agriculture, National Agricultural Statistics Service (USDA-NASS). Agriculture Statistics 2012; United States Government Printing Office: Washington, DC, USA, 2012.
- Pair, S.D.; Bruton, B.D.; Mitchell, F.; Fletcher, J.; Wayadande, A.; Melcher, U. Overwintering squash bugs harbor and transmit the causal agent of Cucurbit Yellow Vine Disease. J. Econ. Entomol. 2009, 97, 74–78. [Google Scholar]
- Nechols, J.R.; Tracy, J.L.; Vogt, E.A. Comparative ecological studies of indiginous egg parastioids (Hymenoptera: Scelionidae; Encyrtidae) of the squash bug Anasa tristis (Hemiptera: Coreidae). J. Kans. Entomol. Soc. 1989, 62, 177–188. [Google Scholar]
- Olson, D.L.; Nechols, J.R. Effects of squash leaf trichome exudates and honey on adult feeding, survival, and fecundity of the squash bug (Heteroptera: Coreidae) egg parasitoid Gryon pennsylvanicum (Hymenoptera: Scelionidae). Envion. Entomol. 1995, 24, 454–458. [Google Scholar]
- Vogt, E.A.; Nechols, J.R. Responses of the squash bug (Hemiptera: Coreidae) and its egg parasitoid, Gryon pennsylvanicum (Hymenoptera: Scelionidae) to three Curcurbita cultivars. Environ. Entomol. 1993, 22, 238–245. [Google Scholar]
- Decker, K.B.; Yeargan, K.V. Seasonal phenology and natural enemies of the squash bug (Hemiptera: Coreidae) in Kentucky. Environ. Entomol. 2009, 37, 670–678. [Google Scholar] [CrossRef]
- Nechols, J.R. Photoperiodic responses of the squash bug (Heteroptera: Coreidae): Diapause induction and maintenance. Envion. Entomol. 1988, 17, 427–431. [Google Scholar]
- Nechols, J.R. Voltinism, seasonal reproduction, and diapause in the squash bug (Heteroptera: Coreidae) in Kansas. Envion. Entomol. 1987, 16, 269–273. [Google Scholar]
- Tunaz, H.; Stanley, D.W. An immunological axis of biocontrol: Infections in field-trapped insects. Naturwissenschaften 2009, 96, 1115–1119. [Google Scholar] [CrossRef]
- Smilanich, A.M.; Dyer, L.A.; Gentry, G.L. The insect immune response and other putative defenses as effective predictors of parasitism. Ecology 2009, 90, 1434–1440. [Google Scholar] [CrossRef]
- Strand, M.R. The interactions between polydnavirus-carrying parasitoids and their Lepidopteran hosts. In Molecular Biology and Genetics of the Lepidoptera; Goldsmith, M.R., Marec, F., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 321–336. [Google Scholar]
- Shelby, K.S.; Popham, H.J.R. Dietary selenium supplementation elevates HzSNPV resistance of Heliothis virescens larvae. J. Invertebrate Pathol. 2007, 95, 77–83. [Google Scholar] [CrossRef]
- Popham, H.J.R.; Shelby, K.S. Effect of inorganic and organic forms of selenium supplementation on development of larval Heliothis virescens. Entomol. Exp. Appl. 2007, 125, 171–178. [Google Scholar] [CrossRef]
- Popham, H.J.R.; Shelby, K.S. Uptake of dietary micronutrients from artificial diets by larval Heliothis virescens. J. Insect Physiol. 2006, 52, 771–777. [Google Scholar] [CrossRef]
- Popham, H.J.R.; Shelby, K.S.; Popham, T.W. Effect of dietary selenium supplementation on resistance to baculovirus infection. Biol. Control. 2005, 32, 419–426. [Google Scholar] [CrossRef]
- Popham, H.J.R.; Shelby, K.S. Ascorbic acid influences the development and immunocompetence of larval Heliothis virescens. Entomol. Exp. Appl. 2009, 133, 57–64. [Google Scholar] [CrossRef]
- Coudron, T.A.; Shelby, K.S.; Ellersieck, M.R.; Winston, B.R.; Popham, H.J.R. Developmental response of the beneficial predator Podisus maculiventris to change in dietary ascorbic acid concentration. Entomol. Exp. Appl. 2011, 139, 235–241. [Google Scholar] [CrossRef]
- Coudron, T.A.; Shelby, K.S.; Ellersieck, M.R.; Odoom, E.D.; Lim, E.E.; Popham, H.J.R. Developmental response of Euplectrus comstockii (Hymenoptera: Eulophidae) to ascorbic acid in the diet of the larval host, Heliothis virescens (Lepidoptera: Noctuidae). BioControl 2009, 54, 175–182. [Google Scholar] [CrossRef]
- Eleftherianos, I.; Felfoldi, G.; ffrench-Constant, R.H.; Reynolds, S.E. Induced nitric oxide synthesis in the gut of Manduca sexta protects against oral infection by the bacterial pathogen Photorhabdus luminescens. Insect Mol. Biol. 2009, 18, 507–516. [Google Scholar] [CrossRef]
- Zhou, X.; Wheeler, M.M.; Oi, F.M.; Scharf, M.E. RNA interference in the termite Reticulitermes flavipes through ingestion of double-stranded RNA. Insect Biochem. Mol. Biol. 2008, 38, 805–815. [Google Scholar] [CrossRef]
- Zhuang, S.; Kelo, L.; Nardi, J.B.; Kanost, M.R. Multiple alpha subunits of integrin are involved in cell-mediated responses of the Manduca immune system. Dev. Comp. Immunol. 2008, 32, 365–379. [Google Scholar] [CrossRef]
- Zhuang, S.; Kelo, L.; Nardi, J.B.; Kanost, M.R. An integrin-tetraspanin interaction required for cellular innate immune respones of an insect, Manduca sexta. J. Biol. Chem. 2007, 282, 22563–22572. [Google Scholar] [CrossRef]
- Terenius, O.; Popham, H.J.R.; Shelby, K.S. Bacterial, but not baculoviral infections stimulate Hemolin expression in noctuid moths. Dev. Comp. Immunol. 2009, 33, 1176–1185. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.-C.; Miao, X.-X. Feasibility, limitation and possible solutions of RNAi-based technology for insect pest control. Insect Sci. 2013, 20, 15–30. [Google Scholar] [CrossRef]
- Aronstein, K.; Oppert, B.; Lorenzen, M.D. RNAi in agriculturaly-important arthropods. In RNA Processing; Grabowski, P., Ed.; InTech Publisher: Rijeka, Croatia, 2011; pp. 157–180. [Google Scholar]
- Burand, J.P.; Hunter, W.B. RNAi: Future in insect management. J. Invertebrate Pathol. 2013, 112, S68–S74. [Google Scholar] [CrossRef]
- Hunter, W.B.; van Engelsdorp, D.; Hayes, J.; Westervelt, D.; Glick, E.; Williams, M.; Sela, I.; Maori, E.; Pettis, J.; Cox-Foster, D.; et al. Large-scale field application of RNAi technology reducing Israeli Acute Paralysis Virus disease in Honey Bees (Apis mellifera, Hymenoptera: Apidae). PLoS Pathog. 2010, 6, e1001160. [Google Scholar] [CrossRef]
- Paldi, N.; Glick, E.; Oliva, M.; Zilberberg, Y.; Aubin, L.; Pettis, J.; Evans, J.D. Effective gene silencing in a microsporidian parasite associated with honeybee (Apis mellifera) colony declines. Appl. Environ. Microbiol. 2010, 76, 5960–5964. [Google Scholar] [CrossRef]
- Zha, W.; Peng, X.; Chen, R.; Du, B.; Zhu, L.; He, G. Knockdown of midgut genes by dsRNA-transgenic plant-mediated RNA interference in the hemipteran insect Nilaparvata lugens. PLoS One 2011, 6, e20504. [Google Scholar]
- Pitino, M.; Coleman, A.D.; Maffei, M.E.; Ridout, C.J.; Hogenhout, S.A. Silencing of aphid genes by dsRNA feeding from plants. PLoS One 2011, 6, e25709. [Google Scholar]
- Price, D.R.G.; Gatehouse, J.A. RNAi-mediated crop protection against insects. Trends Biotechnol. 2008, 26, 393–400. [Google Scholar] [CrossRef]
- Baum, J.A.; Bogaert, T.; Clinton, W.; Heck, G.R.; Feldmann, P.; Ilagan, O.; Johnson, S.; Plaetinck, G.; Munyikwa, T.; Pleau, M.; et al. Control of coleopteran insect pests through RNA interference. Nat. Biotechnol. 2007, 25, 1322–1326. [Google Scholar] [CrossRef]
- Xue, X.-Y.; Mao, Y.-B.; Tao, X.-Y.; Huang, Y.-P.; Chen, X.-Y. New approaches to agricultural insect pest control based on RNA interference. In Advances in Insect Physiology; Jockusch, E.L., Ed.; Academic Press: New York, NY, USA, 2012; pp. 73–118. [Google Scholar]
- Zhao, Y.; Yang, G.; Wang-Pruski, G.; You, M. Phyllotreta striolata (Coleoptera: Chrysomelidae): Arginine kinase cloning and RNAi-based pest control. Eur. J. Entomol. 2008, 105, 815–822. [Google Scholar]
- Gong, L.; Yang, X.; Zhang, B.; Zhong, G.; Hu, M.-Y. Silencing of Rieske iron-sulfur protein using chemically synthesised siRNA as a potential biopesticide against Plutella xylostella. Pest Manag. Sci. 2011, 67, 514–520. [Google Scholar] [CrossRef]
- Bulmer, M.S.; Bachelet, I.; Raman, R.; Rosengaus, R.B.; Sasisekharan, R. Targeting an antimicrobial effector function in insect immunity as a pest control strategy. Proc. Natl. Acad. Sci. USA 2009, 106, 12652–12657. [Google Scholar]
- Shelby, K.S.; Popham, H.J.R. RNA-seq study of microbially induced hemocyte transcripts from larval Heliothis virescens (Lepidoptera: Noctuidae). Insects 2012, 3, 743–762. [Google Scholar] [CrossRef]
- Breitenbach, J.E.; Shelby, K.S.; Popham, H.J.R. Baculovirus induced transcripts in hemocytes from the noctuid moth, Heliothis virescens. Viruses 2011, 3, 2047–2064. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 8, 469–477. [Google Scholar]
- Gotz, S.; Garcia-Gomez, J.M.; Terol, J.; Williams, T.D.; Naagaraj, S.H.; Nueda, M.J.; Robles, M.; Talon, M.; Dopazo, J.; Conesa, A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008, 36, 3420–3435. [Google Scholar] [CrossRef]
- Conesa, A.; Gotz, S. Blast2GO: A comprehensive suite for functional analysis in plant genomics. Int. J. Plant Genomics 2008, 2008. [Google Scholar] [CrossRef]
- Johnston, S.; Texas A & M University, College Station, TX, USA. personal communication, 2011.
- Wayadande, A.; Bruton, B.; Fletcher, J.; Pair, S.; Mitchell, F. Retention of cucurbit yellow vine disease bacterium Serratia marcescens through transstadial molt of vector Anasa tristis (Hemiptera: Coreidae). Ann. Entomol. Soc. Am. 2009, 98, 770–774. [Google Scholar]
- Boucias, D.G.; Garcia-Maruniak, A.; Cherry, R.; Lu, H.; Maruniak, J.E.; Lietze, V.-U. Detection and characterization of bacterial symbionts in the Heteropteran, Blissus insularis. FEMS Microbiol. Ecol. 2012, 82, 629–641. [Google Scholar] [CrossRef]
- Hail, D.; Lauziere, I.; Dowd, S.E.; Bextine, B. Culture independent survey of the microbiota of the Glassy-winted sharpshooter (Homalodisca vitripennis) using 454 pyrosequencing. Environ. Entomol. 2011, 40, 23–29. [Google Scholar] [CrossRef]
- Koga, R.; Meng, X.-Y.; Tsuchida, T.; Fukatsu, T. Cellular mechanism for selective vertical transmission of an obligate insect symbiont at the bacterocyte-embryo interface. Proc. Natl. Acad. Sci. USA 2012, 109, 7697–7598. [Google Scholar]
- Boucias, D.; Texas A & M University, College Station, TX, USA. personal communication, 2012.
- Brucker, R.M.; Funkhouser, L.J.; Setia, S.; Pauly, R.; Bordenstein, S.R. Insect innate immunity database (IIID): An annotation tool for identifying immune genes in insect genomes. PLoS One 2012, 7, e45125. [Google Scholar]
- Blander, J.M.; Sander, L.E. Beyond pattern recognition: Five immune checkpoints for scaling the microbial threat. Nat. Revs. Immunol. 2012, 12, 215–225. [Google Scholar] [CrossRef]
- Jiang, H.; Vilcinskas, A.; Kanost, M.R. Immunity in Lepidopteran insects. In Invertebrate Immunity; Springer: New York, NY, USA, 2011; pp. 163–180. [Google Scholar]
- Stanley, D.; Haas, E.; Miller, J. Eicosanoids: Exploiting insect immunity to improve biological control programs. Insects 2012, 3, 492–510. [Google Scholar] [CrossRef]
- Stanley, D.W. Eicosanoids: Progress towards manipulating insect immunity. J. Appl. Entomol. 2011, 135, 534–545. [Google Scholar] [CrossRef]
- Garcia, E.S.; Castro, D.P.; Figueiredo, M.B.; Genta, F.A.; Azambuja, P. Trypanosoma rangeli: A new perspective for studying the modulation of immune reactions of Rhodnius prolixus. Parasites Vectors 2009, 2. [Google Scholar] [CrossRef]
- Kanost, M.R.; Gorman, M.J. Phenoloxidases in insect immunity. In Insect Immunology, 1st ed.; Beckage, N., Ed.; Academic Press: New York, NY, USA, 2008; pp. 69–96. [Google Scholar]
- Nappi, A.J.; Christensen, B.M. Melanogenesis and associated cytotoxic reactions: Applications to insect innate immunity. Insect Biochem. Mol. Biol. 2005, 35, 443–449. [Google Scholar] [CrossRef]
- Shelby, K.S.; Popham, H.J.R. Plasma phenoloxidase of the larval tobacco budworm, Heliothis virescens, is virucidal. J. Insect Sci. 2006, 6. [Google Scholar] [CrossRef]
- Schmidt, O.; Soderhall, K.; Theopold, U.; Faye, I. Role of adhesion in arthropod immune recognition. Ann. Rev. Entomol. 2010, 54, 485–504. [Google Scholar]
- Broderick, N.A.; Welchman, D.P.; Lemaitre, B. Recognition and response to microbial infection in Drosophila. In Insect Infection and Immunity: Evolution, Ecology, and Mechanisms; Rolff, J., Reynolds, S.E., Eds.; Oxford University Press: New York, NY, USA, 2009; pp. 13–33. [Google Scholar]
- Renwick, J.; Reeves, E.P.; Wientjes, F.B.; Kavanagh, K. Translocation of proteins homologous to human neutrophil p47phox and p67phox to the cell membrane in activated hemocytes of Galleria mellonella. Dev. Comp. Immunol. 2007, 31, 347–359. [Google Scholar] [CrossRef]
- Bruton, B.D.; Mitchell, F.; Fletcher, J.; Pair, S.D.; Wayadande, A.; Melcher, U.; Brady, J.; Bextine, B.; Popham, T.W. Serratia marcescens, a phloem-colonizing squash bug-transmitted bacterium: Causal agent of cucurbit yellow vine disease. Plant Dis. 2003, 87, 937–944. [Google Scholar] [CrossRef]
- Rascoe, J.B.M.; Melcher, U.; Mithcell, F.L.; Bruton, B.D.; Pair, S.D.; Fletcher, J. Identification, phylogenetic analysis, and biological characterization of Serratia marcescens strains causing curcurbit yellow vine disease. Phytopathology 2003, 93, 1233–1239. [Google Scholar] [CrossRef]
- Ali, A.; Abdalla, O.; Bruton, B.; Fish, W.; Sikora, E.; Zhang, S.; Taylor, M. Occurence of viruses infecting watermelon, other cucurbits and weeds in the parts of Southern United States. Plant Health Progress 2012. [Google Scholar] [CrossRef]
- Perera, O.P.; Snodgrass, G.L.; Allen, K.C.; Jackson, R.E.; Becnel, J.J.; O’Leary, P.F.; Luttrell, R.G. The complete genome sequence of a single-stranded RNA virus from the tarnished plant bug, Lygus lineolaris (Palisot de Beauvois). J. Invertebrate Pathol. 2012, 109, 11–19. [Google Scholar] [CrossRef]
- Liu, S.; Vijayendran, D.; Bonning, B.C. Next generation sequencing technologies for insect virus discovery. Viruses 2011, 3, 1849–1869. [Google Scholar] [CrossRef]
- Wu, Q.; Luo, Y.; Lu, R.; Lau, N.; Lai, E.C.; Li, W.-X.; Ding, S.-W. Virus discovery by deep sequencing and assembly of virus-derived small silencing RNAs. Proc. Natl. Acad. Sci. USA 2010, 107, 1606–1611. [Google Scholar]
- Vodovar, N.; Goic, B.; Blanc, H.; Saleh, M.-C. In silico reconstruction of viral genomes from small RNAs improves virus-derived small interfering RNA profiling. J. Virol. 2011, 85, 11016–11021. [Google Scholar] [CrossRef]
- Ma, M.; Huang, Y.; Gong, Z.; Zhuang, L.; Li, C.; Yang, H.; Tong, Y.; Liu, W.; Cao, W. Discovery of DNA viruses in wild-caught mosquitoes using small RNA high throughput sequencing. PLoS One 2011, 6, e24758. [Google Scholar]
- Rosario, K.; Breitbart, M. Exploring the viral world through metagenomics. Curr. Opin. Virol. 2011, 1, 289–297. [Google Scholar] [CrossRef]
- Ng, T.F.F.; Duffy, S.; Polston, J.E.; Bixby, E.; Vallad, G.E.; Breitbart, M. Exploring the diversity of plant DNA viruses and their satellites using vector-enabled metagenomics on Whiteflies. PLoS One 2011, 6, e19050. [Google Scholar]
- Cox-Foster, D.L.; Conlan, S.; Holmes, E.C.; Palacios, G.; Evans, J.D.; Moran, N.A.; Quan, P.L.; Briese, T.; Hornig, M.; Geiser, D.M.; et al. A metagenomic survey of microbes in honey bee colony collapse disorder. Science 2007, 318, 283–287. [Google Scholar] [CrossRef]
- Ng, T.F.F.; Willner, D.L.; Lim, Y.W.; Schmieder, R.; Chau, B.; Nilsson, C.; Anthony, S.; Ruan, Y.; Rohwer, F.; Breitbart, M. Broad surveys of DNA viral diversity obtained through viral metagenomics of mosquitoes. PLoS One 2011, 6, e20579. [Google Scholar] [CrossRef]
- Al Rwahnih, M.; Daubert, S.; Urbez-Torres, J.R.; Cordero, F.; Rowhani, A. Deep sequencing evidence from single grapevine plants reveals a virome dominated by mycoviruses. Arch. Virol. 2011, 156, 397–403. [Google Scholar] [CrossRef]
- Coaetzee, B.; Freeborough, M.-J.; Maree, H.J.; Celton, J.-M.; Reese, D.J.G.; Burger, J.T. Deep sequencing analysis of viruses infecting grapevines: Virome of a vineyard. Virology 2010, 400, 157–163. [Google Scholar] [CrossRef]
- Li, L.; Victoria, J.G.; Wang, C.; Jones, M.; Fellers, G.M.; Kunz, T.H.; Delwart, E. Bat guano virome: Predominance of dietary viruses from insects and plants plus novel mammalian viruses. J. Virol. 2010, 84, 6955–6965. [Google Scholar]
- Li, Z.; Zhang, Z.; Yan, P.; Huang, S.; Fei, Z.; Lin, K. RNA-Seq improves annotation of protein-coding genes in the cucumber genome. BMC Genomics 2011, 12. [Google Scholar] [CrossRef]
- Sattar, S.; Song, Y.; Anstead, J.A.; Sunkar, R.; Thompson, G.A. Cucumis melo microRNA expression profile during herbivory in a resistant and susceptible interaction. Mol. Plant Microbe Interact. 2012, 25, 839–848. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shelby, K.S. Functional Immunomics of the Squash Bug, Anasa tristis (De Geer) (Heteroptera: Coreidae). Insects 2013, 4, 712-730. https://doi.org/10.3390/insects4040712
Shelby KS. Functional Immunomics of the Squash Bug, Anasa tristis (De Geer) (Heteroptera: Coreidae). Insects. 2013; 4(4):712-730. https://doi.org/10.3390/insects4040712
Chicago/Turabian StyleShelby, Kent S. 2013. "Functional Immunomics of the Squash Bug, Anasa tristis (De Geer) (Heteroptera: Coreidae)" Insects 4, no. 4: 712-730. https://doi.org/10.3390/insects4040712
APA StyleShelby, K. S. (2013). Functional Immunomics of the Squash Bug, Anasa tristis (De Geer) (Heteroptera: Coreidae). Insects, 4(4), 712-730. https://doi.org/10.3390/insects4040712




