Action on the Surface: Entomopathogenic Fungi versus the Insect Cuticle
Abstract
:1. Introduction
2. Infection by Entomopathogenic Fungi
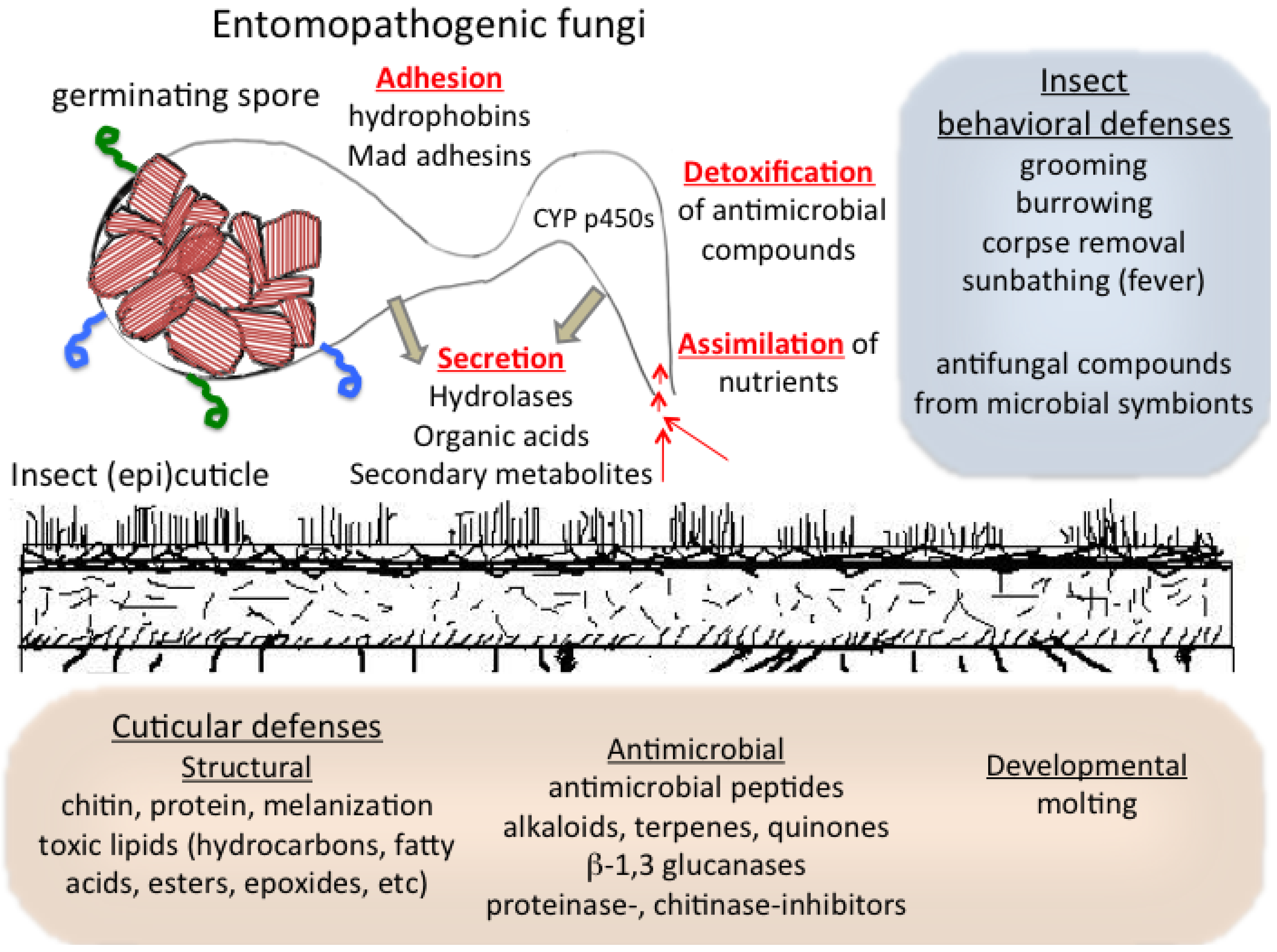
3. Interactions with and Assimilation of the Host Cuticle by Entomopathogenic Fungi
4. Cuticular Mechanisms of Host Resistance to Microbes: Structural and Chemical
5. Cuticular Developmental, Symbiotic, and Behavioral Defenses against Microbes
6. Conclusions
Acknowledgments
Conflict of Interest
References
- Porter, R. Bassi, a bicentennial (1773–1973). Bacteriol. Rev. 1973, 37, 284–288. [Google Scholar] [Green Version]
- Xiao, G.; Ying, S.H.; Zheng, P.; Wang, Z.L.; Zhang, S.; Xie, X.Q.; Shang, Y.; St Leger, R.J.; Zhao, G.P.; Wang, C.; et al. Genomic perspectives on the evolution of fungal entomopathogenicity in Beauveria bassiana. Sci. Rep. 2012, 2, 483. [Google Scholar] [Green Version]
- Zheng, P.; Xia, Y.L.; Xiao, G.H.; Xiong, C.H.; Hu, X.; Zhang, S.W.; Zheng, H.J.; Huang, Y.; Zhou, Y.; Wang, S.Y.; et al. Genome sequence of the insect pathogenic fungus Cordyceps militaris, a valued traditional chinese medicine. Genome Biol. 2011, 12. [Google Scholar] [CrossRef]
- Zheng, P.; Xia, Y.L.; Zhang, S.W.; Wang, C.S. Genetics of Cordyceps and related fungi. Appl. Microbiol. Biotechnol. 2013, 97, 2797–2804. [Google Scholar] [CrossRef]
- Vilcinskas, A. Coevolution between pathogen-derived proteinases and proteinase inhibitors of host insects. Virulence 2010, 1, 206–214. [Google Scholar] [CrossRef]
- Fang, W.G.; Feng, J.; Fan, Y.H.; Zhang, Y.J.; Bidochka, M.J.; Leger, R.J.S.; Pei, Y. Expressing a fusion protein with protease and chitinase activities increases the virulence of the insect pathogen Beauveria bassiana. J. Invertebr. Pathol. 2009, 102, 155–159. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Feng, M.G.; Fan, Y.H.; Luo, Z.B.; Yang, X.Y.; Wu, D.; Pei, Y. A cuticle-degrading protease (CDEP-1) of Beauveria bassiana enhances virulence. Biocontr. Sci. Technol. 2008, 18, 551–563. [Google Scholar]
- Fan, Y.H.; Fang, W.G.; Guo, S.J.; Pei, X.Q.; Zhang, Y.J.; Xiao, Y.H.; Li, D.M.; Jin, K.; Bidochka, M.J.; Pei, Y. Increased insect virulence in Beauveria bassiana strains overexpressing an engineered chitinase. Appl. Environ. Microbiol. 2007, 73, 295–302. [Google Scholar] [CrossRef]
- Kirkland, B.H.; Eisa, A.; Keyhani, N.O. Oxalic acid as a fungal acaracidal virulence factor. J. Med. Entomol. 2005, 42, 346–351. [Google Scholar] [CrossRef]
- Bidochka, M.J.; Khachatourians, G.G. The implication of metabolic acids produced by Beauveria bassiana in pathogenesis of the migratory grasshopper, Melanoplus sanguinipes. J. Invertebr. Pathol. 1991, 58, 106–117. [Google Scholar] [CrossRef]
- Nelson, D.R.; Blomquist, G.J. Insect waxes. In Waxes: Chemistry, Molecular Biology, and Functions; Hamilton, R.J., Ed.; The Oily Press, LTD: Dundee, Scotland, UK, 1995; pp. 1–90. [Google Scholar]
- Renobales, M.d.; Nelson, D.R.; Blomquist, G.J. Cuticular lipids. In Physiology of the Insect Epidermis; Binnington, K., Retnakaran, A., Eds.; CSIRO: Melbourne, Australia, 1991. [Google Scholar]
- Boucias, D.; Pendland, J. Attachment of mycopathogens to cuticle. In The Fungal Spore and Disease Initiation in Plants and Animals; Cole, G.T., Hoch, H.C., Eds.; Plenum Press: New York, NY, USA, 1991; pp. 101–127. [Google Scholar]
- Boucias, D.G.; Pendland, J.C.; Latge, J.P. Nonspecific factors involved in attachment of entomopathogenic Deuteromycetes to host insect cuticle. Appl. Environ. Microbiol. 1988, 54, 1795–1805. [Google Scholar]
- Holder, D.J.; Keyhani, N.O. Adhesion of the entomopathogenic fungus Beauveria (Cordyceps) bassiana to substrata. Appl. Environ. Microbiol. 2005, 71, 5260–5266. [Google Scholar] [CrossRef]
- Holder, D.J.; Kirkland, B.H.; Lewis, M.W.; Keyhani, N.O. Surface characteristics of the entomopathogenic fungus Beauveria (Cordyceps) bassiana. Microbiology 2007, 153, 3448–3457. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Xia, Y.X.; Kim, B.; Keyhani, N.O. Two hydrophobins are involved in fungal spore coat rodlet layer assembly and each play distinct roles in surface interactions, development and pathogenesis in the entomopathogenic fungus, Beauveria bassiana. Mol. Microbiol. 2011, 80, 811–826. [Google Scholar] [CrossRef]
- Cho, E.M.; Kirkland, B.H.; Holder, D.J.; Keyhani, N.O. Phage display cDNA cloning and expression analysis of hydrophobins from the entomopathogenic fungus Beauveria (Cordyceps) bassiana. Microbiology 2007, 153, 3438–3447. [Google Scholar] [CrossRef]
- Wang, C.S.; St Leger, R.J. The MAD1 adhesin of Metarhizium anisopliae links adhesion with blastospore production and virulence to insects, and the MAD2 adhesin enables attachment to plants. Eukaryot. Cell 2007, 6, 808–816. [Google Scholar] [CrossRef]
- Wanchoo, A.; Lewis, M.W.; Keyhani, N.O. Lectin mapping reveals stage-specific display of surface carbohydrates in in vitro and haemolymph-derived cells of the entomopathogenic fungus Beauveria bassiana. Microbiology 2009, 155, 3121–3133. [Google Scholar] [CrossRef]
- Howard, R.W.; Blomquist, G.J. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Ann. Rev. Entomol. 2005, 50, 371–393. [Google Scholar] [CrossRef]
- Andersen, S.O. Insect cuticular sclerotization: A review. Insect Biochem. Mol. Biol. 2010, 40, 166–178. [Google Scholar] [CrossRef]
- Lockey, K.H. Lipids of the insect cuticle: Origin, composition, and function. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1988, 89B, 595–645. [Google Scholar]
- Zhang, S.; Widemann, E.; Bernard, G.; Lesot, A.; Pinot, F.; Pedrini, N.; Keyhani, N.O. CYP52X1, representing new cytochrome P450 subfamily, displays fatty acid hydroxylase activity and contributes to virulence and growth on insect cuticular substrates in entomopathogenic fungus Beauveria bassiana. J. Biol. Chem. 2012, 287, 13477–13486. [Google Scholar]
- Pedrini, N.; Zhang, S.; Juarez, M.P.; Keyhani, N.O. Molecular characterization and expression analysis of a suite of cytochrome P450 enzymes implicated in insect hydrocarbon degradation in the entomopathogenic fungus Beauveria bassiana. Microbiology 2010, 156, 2549–2557. [Google Scholar] [CrossRef]
- Stleger, R.J.; Cooper, R.M.; Charnley, A.K. Utilization of alkanes by entomopathogenic fungi. J. Invertebr. Pathol. 1988, 52, 356–359. [Google Scholar] [CrossRef]
- Napolitano, R.; Juarez, M.P. Entomopathogenous fungi degrade epicuticular hydrocarbons of Triatoma infestans. Arch. Biochem. Biophys. 1997, 344, 208–214. [Google Scholar] [CrossRef]
- Crespo, R.; Juarez, M.P.; Cafferata, L.F.R. Biochemical interaction between entomopathogenous fungi and their insect-host-like hydrocarbons. Mycologia 2000, 92, 528–536. [Google Scholar] [CrossRef]
- Crespo, R.; Pedrini, N.; Juarez, M.P.; Dal Bello, G.M. Volatile organic compounds released by the entomopathogenic fungus Beauveria bassiana. Microbiol. Res. 2008, 163, 148–151. [Google Scholar] [CrossRef]
- Pedrini, N.; Juárez, M.; Crespo, R.; de Alaniz, M. Clues on the role of Beauveria bassiana catalases in alkane degradation events. Mycologia 2006, 98, 528–534. [Google Scholar] [CrossRef]
- Pedrini, N.; Crespo, R.; Juarez, M.P. Biochemistry of insect epicuticle degradation by entomopathogenic fungi. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 2007, 146, 124–137. [Google Scholar] [CrossRef]
- Pedrini, N.; Ortiz-Urquiza, A.; Huarte-Bonnet, C.; Zhang, S.; Keyhani, N.O. Targeting of insect epicuticular lipids by the entomopathogenic fungus Beauveria bassiana: Hydrocarbon oxidation within the context of a host-pathogen interaction. Front. Microbiol. 2013, 4, 24. [Google Scholar]
- Lida, T.; Sumita, T.; Ohta, A.; Takagi, M. The cytochrome P450ALK multigene family of an n-alkane-assimilating yeast, Yarrowia lipolytica: Cloning and characterization of genes coding for new CYP52 family members. Yeast 2000, 16, 1077–1087. [Google Scholar] [CrossRef]
- Wang, Z.L.; Zhang, L.B.; Ying, S.H.; Feng, M.G. Catalases play differentiated roles in the adaptation of a fungal entomopathogen to environmental stresses. Environ. Microbiol. 2013, 15, 409–418. [Google Scholar] [CrossRef]
- Da Silva, W.O.B.; Santi, L.; Correa, A.P.F.; Silva, L.A.D.; Bresciani, F.R.; Schrank, A.; Vainstein, M.H. The entomopathogen Metarhizium anisopliae can modulate the secretion of lipolytic enzymes in response to different substrates including components of arthropod cuticle. Fungal Biol. 2010, 114, 911–916. [Google Scholar] [CrossRef]
- Crespo, R.; Juarez, M.P.; Dal Bello, G.M.; Padin, S.; Fernandez, G.C.; Pedrini, N. Increased mortality of Acanthoscelides obtectus by alkane-grown Beauveria bassiana. Biocontrol 2002, 47, 685–696. [Google Scholar] [CrossRef]
- Jarrold, S.L.; Moore, D.; Potter, U.; Charnley, A.K. The contribution of surface waxes to pre-penetration growth of an entomopathogenic fungus on host cuticle. Mycol. Res. 2007, 111, 240–249. [Google Scholar] [CrossRef]
- Lecuona, R.; Riba, G.; Cassier, P.; Clement, J.L. Alterations of insect epicuticular hydrocarbons during infection with Beauveria bassiana or B. brongniartii. J. Invertebr. Pathol. 1991, 58, 10–18. [Google Scholar] [CrossRef]
- Boucias, D.G.; Pendland, J.C. Nutritional requirements for conidial germination of several host range pathotypes of the entomopathogenic fungus Nomuraea rileyi. J. Invertebr. Pathol. 1984, 43, 288–292. [Google Scholar] [CrossRef]
- Akbar, W.; Lord, J.C.; Nechols, J.R.; Howard, R.W. Diatomaceous earth increases the efficacy of Beauveria bassiana against Tribolium castaneum larvae and increases conidia attachment. J. Econ. Entomol. 2004, 97, 273–280. [Google Scholar] [CrossRef]
- Stephou, V.K.; Tjamos, S.E.; Paplomatas, E.J.; Athanassiou, C.G. Transformation and attachment of Beauveria bassiana conidia on the cuticle of Tribolium confusum and Sitophilus oryzae in conjunction with diatomaceous earth. J. Pest Sci. 2012, 85, 387–394. [Google Scholar]
- Lord, J.C.; Howard, R.W. A proposed role for the cuticular fatty amides of Liposcelis bostrychophila (Psocoptera: Liposcelidae) in preventing adhesion of entomopathogenic fungi with dry-conidia. Mycopathologia 2004, 158, 211–217. [Google Scholar] [CrossRef]
- Sosa-Gomez, D.R.; Boucias, D.G.; Nation, J.L. Attachment of Metarhizium anisopliae to the southern green stink bug Nezara viridula cuticle and fungistatic effect of cuticular lipids and aldehydes. J. Invertebr. Pathol. 1997, 69, 31–39. [Google Scholar] [CrossRef]
- Smith, R.J.; Grula, E.A. Toxic components on the larval surface of the Corn-Earworm (Heliothis zea) and their effects on germination and growth of Beauveria bassiana. J. Invertebr. Pathol. 1982, 39, 15–22. [Google Scholar] [CrossRef]
- Gross, J.; Schumacher, K.; Schmidtberg, H.; Vilcinskas, A. Protected by fumigants: Beetle perfumes in antimicrobial defense. J. Chem. Ecol. 2008, 34, 179–188. [Google Scholar] [CrossRef]
- Gross, J.; Muller, C.; Vilcinskas, A.; Hilker, M. Antimicrobial activity of exocrine glandular secretions, hemolymph, and larval regurgitate of the mustard leaf beetle Phaedon cochlearia. J. Invertebr. Pathol. 1998, 72, 296–303. [Google Scholar] [CrossRef]
- Saito, T.; Aoki, J. Toxicity of free fatty acids on the larval surfaces of 2 Lepidopterous insects towards Beauveria bassiana (Bals) Vuill and Paecilomyces fumosoroseus (Wize) Brown Et Smith (Deuteromycetes, Moniliales). Appl. Entomol. Zool. 1983, 18, 225–233. [Google Scholar]
- Urbanek, A.; Szadziewski, R.; Stepnowski, P.; Boros-Majewska, J.; Gabriel, I.; Dawgul, M.; Kamysz, W.; Sosnowska, D.; Golebiowski, M. Composition and antimicrobial activity of fatty acids detected in the hygroscopic secretion collected from the secretory setae of larvae of the biting midge Forcipomyia nigra (Diptera: Ceratopogonidae). J. Insect Physiol. 2012, 58, 1265–1276. [Google Scholar] [CrossRef]
- Lecuona, R.; Clement, J.L.; Riba, G.; Joulie, C.; Juarez, P. Spore germination and hyphal growth of Beauveria spp. on insect lipids. J. Econ. Entomol. 1997, 90, 119–123. [Google Scholar]
- Kerwin, J.L. Fatty acid regulation of the germination of Erynia variabilis conidia on adults and puparia of the lesser housefly, Fannia canicularis. Can. J. Microbiol. 1984, 30, 158–161. [Google Scholar] [CrossRef]
- Latge, J.P.; Sampedro, L.; Brey, P.; Diaquin, M. Aggressiveness of Conidiobolus obscurus against the pea aphid - influence of cuticular extracts on ballistospore germination of aggressive and nonaggressive strains. J. Gen. Microbiol. 1987, 133, 1987–1997. [Google Scholar]
- Degenkolb, T.; During, R.A.; Vilcinskas, A. Secondary metabolites released by the burying beetle Nicrophorus vespilloides: Chemical analyses and possible ecological functions. J. Chem. Ecol. 2011, 37, 724–735. [Google Scholar] [CrossRef]
- Kirkland, B.H.; Cho, E.M.; Keyhani, N.O. Differential susceptibility of Amblyomma maculatum and Amblyomma americanum (Acari: Ixodidea) to the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae. Biol. Contr. 2004, 31, 414–421. [Google Scholar] [CrossRef]
- Ment, D.; Churchill, A.C.L.; Gindin, G.; Belausov, E.; Glazer, I.; Rehner, S.A.; Rot, A.; Donzelli, B.G.G.; Samish, M. Resistant ticks inhibit Metarhizium infection prior to haemocoel invasion by reducing fungal viability on the cuticle surface. Environ. Microbiol. 2012, 14, 1570–1583. [Google Scholar] [CrossRef]
- Golebiowski, M.; Bogus, M.I.; Paszkiewicz, M.; Stepnowski, P. Cuticular lipids of insects as potential biofungicides: methods of lipid composition analysis. Anal. Bioanal. Chem. 2011, 399, 3177–3191. [Google Scholar] [CrossRef]
- Storey, G.K.; Vandermeer, R.K.; Boucias, D.G.; Mccoy, C.W. Effect of fire ant (Solenopsis invicta) venom alkaloids on the invitro germination and development of selected entomogenous fungi. J. Invertebr. Pathol. 1991, 58, 88–95. [Google Scholar] [CrossRef]
- Storey, G.K.; Aneshansley, D.J.; Eisner, T. Parentally provided alkaloid does not protect eggs of Utetheisa ornatrix (Lepidoptera, Arctiidae) against entomopathogenic fungi. J. Chem. Ecol. 1991, 17, 687–693. [Google Scholar] [CrossRef]
- Hamilton, C.; Lay, F.; Bulmer, M.S. Subterranean termite prophylactic secretions and external antifungal defenses. J. Insect Physiol. 2011, 57, 1259–1266. [Google Scholar] [CrossRef]
- Bulmer, M.S.; Bachelet, I.; Raman, R.; Rosengaus, R.B.; Sasisekharan, R. Targeting an antimicrobial effector function in insect immunity as a pest control strategy. Proc. Natl. Acad. Sci. USA 2009, 106, 12652–12657. [Google Scholar] [CrossRef]
- Hamilton, C.; Bulmer, M.S. Molecular antifungal defenses in subterranean termites: RNA interference reveals in vivo roles of termicins and GNBPs against a naturally encountered pathogen. Dev. Comp. Immunol. 2012, 36, 372–377. [Google Scholar] [CrossRef]
- Villaverde, M.L.; Girotti, J.R.; Mijailovsky, S.J.; Pedrini, N.; Juarez, M.P. Volatile secretions and epicuticular hydrocarbons of the beetle Ulomoides dermestoides. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009, 154, 381–386. [Google Scholar] [CrossRef]
- Stleger, R.J.; Cooper, R.M.; Charnley, A.K. The Effect of melanization of Manduca sexta cuticle on growth and infection by Metarhizium anisopliae. J. Invertebr. Pathol. 1988, 52, 459–470. [Google Scholar] [CrossRef]
- Wilson, K.; Cotter, S.C.; Reeson, A.F.; Pell, J.K. Melanism and disease resistance in insects. Ecol. Lett. 2001, 4, 637–649. [Google Scholar] [CrossRef]
- Vandenberg, J.D.; Ramos, M.; Altre, J.A. Dose-response and age- and temperature-related susceptibility of the diamondback moth (Lepidoptera: Plutellidae) to two isolates of Beauveria bassiana (Hyphomycetes: Moniliaceae). Environ. Entomol. 1998, 27, 1017–1021. [Google Scholar]
- Kim, J.J.; Roberts, D.W. The relationship between conidial dose, moulting and insect developmental stage on the susceptibility of cotton aphid, Aphis gossypii, to conidia of Lecanicillium attenuatum, an entomopathogenic fungus. Biocontr. Sci. Tech. 2012, 22, 319–331. [Google Scholar] [CrossRef]
- Samuels, R.I.; Reynolds, S.E. Proteinase inhibitors from the molting fluid of the pharate adult tobacco hornworm, Manduca sexta. Arch. Insect Biochem. Physiol. 2000, 43, 33–43. [Google Scholar] [CrossRef]
- Milner, R.J.; Prior, C. Susceptibility of the Australian plague locust, Chortoicetes terminifera, and the wingless grasshopper, Phaulacridium vittatum, to the fungi Metarhizium spp. Biol. Contr. 1994, 4, 132–137. [Google Scholar] [CrossRef]
- Kiuchi, M.; Yasui, H.; Hayasaka, S.; Kamimura, M. Entomogenous fungus Nomuraea rileyi inhibits host insect molting by C22-oxidizing inactivation of hemolymph ecdysteroids. Arch. Insect Biochem. Physiol. 2003, 52, 35–44. [Google Scholar] [CrossRef]
- Zindel, R.; Gottlieb, Y.; Aebi, A. Arthropod symbioses: A neglected parameter in pest- and disease-control programmes. J. Appl. Ecol. 2011, 48, 864–872. [Google Scholar] [CrossRef]
- Panteleev, D.Y.; Goryacheva, I.I.; Andrianov, B.V.; Reznik, N.L.; Lazebny, O.E.; Kulikov, A.M. The endosymbiotic bacterium Wolbachia enhances the nonspecific resistance to insect pathogens and alters behavior of Drosophila melanogaster. Russ. J. Genet. 2007, 43, 1066–1069. [Google Scholar] [CrossRef]
- Kaltenpoth, M.; Gottler, W.; Herzner, G.; Strohm, E. Symbiotic bacteria protect wasp larvae from fungal infestation. Curr. Biol. 2005, 15, 475–479. [Google Scholar] [CrossRef]
- Boucias, D.G.; Garcia-Maruniak, A.; Cherry, R.; Lu, H.J.; Maruniak, J.E.; Lietze, V.U. Detection and characterization of bacterial symbionts in the Heteropteran, Blissus insularis. FEMS Microbiol. Ecol. 2012, 82, 629–641. [Google Scholar] [CrossRef]
- Folgarait, P.; Gorosito, N.; Poulsen, M.; Currie, C.R. Preliminary in vitro insights into the use of natural fungal pathogens of leaf-cutting ants as biocontrol agents. Curr. Microbiol. 2011, 63, 250–258. [Google Scholar] [CrossRef]
- Little, A.E.F.; Murakami, T.; Mueller, U.G.; Currie, C.R. Defending against parasites: fungus-growing ants combine specialized behaviours and microbial symbionts to protect their fungus gardens. Biol. Lett. 2006, 2, 12–16. [Google Scholar] [CrossRef]
- Tragust, S.; Mitteregger, B.; Barone, V.; Konrad, M.; Ugelvig, L.V.; Cremer, S. Ants disinfect fungus-exposed brood by oral uptake and spread of their poison. Curr. Biol. 2013, 23, 76–82. [Google Scholar] [CrossRef]
- Shimizu, S.; Yamaji, M. Effect of density of the termite, Reticulitermes speratus Kolbe (Isoptera : Rhinotermitidae), on the susceptibilities to Metarhizium anisopliae. Appl. Entomol. Zool. 2003, 38, 125–130. [Google Scholar] [CrossRef]
- Yanagawa, A.; Yokohari, F.; Shimizu, S. Defense mechanism of the termite, Coptotermes formosanus Shiraki, to entomopathogenic fungi. J. Invertebr. Pathol. 2008, 97, 165–170. [Google Scholar] [CrossRef]
- Yanagawa, A.; Fujiwara-Tsujii, N.; Akino, T.; Yoshimura, T.; Yanagawa, T.; Shimizu, S. Odor aversion and pathogen-removal efficiency in grooming behavior of the termite Coptotermes formosanus. PLoS One 2012, 7, e47412. [Google Scholar]
- Yanagawa, A.; Yokohari, F.; Shimizu, S. The role of antennae in removing entomopathogenic fungi from cuticle of the termite, Coptotermes formosanus. J. Insect Sci. 2009, 9. [Google Scholar] [CrossRef]
- Chouvenc, T.; Su, N.Y. Apparent synergy among defense mechanisms in subterranean termites (Rhinotermitidae) against epizootic events: Limits and potential for biological control. J. Econ. Entomol. 2010, 103, 1327–1337. [Google Scholar] [CrossRef]
- Heinrich, B. Insect thermoregulation. Endeavour 1995, 19, 28–33. [Google Scholar] [CrossRef]
- Gardner, S.N.; Thomas, M.B. Costs and benefits of fighting infection in locusts. Evol. Ecol. Res. 2002, 4, 109–131. [Google Scholar]
- Carruthers, R.I.; Larkin, T.S.; Firstencel, H.; Feng, Z.D. Influence of thermal ecology on the mycosis of a rangeland grasshopper. Ecology 1992, 73, 190–204. [Google Scholar] [CrossRef]
- Chown, S.L.; Addo-Bediako, A.; Gaston, K.J. Physiological variation in insects: Large-scale patterns and their implications. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2002, 131, 587–602. [Google Scholar] [CrossRef]
- Wang, Y.D.; Yang, P.C.; Cui, F.; Kang, L. Altered immunity in crowded locust reduced fungal (Metarhizium anisopliae) pathogenesis. Plos Pathogens 2013, 9, e1003102. [Google Scholar] [CrossRef]
- Schwarz, J.J.; Punja, Z.; Goettel, M.; Gries, G. Do Western boxelder bugs sunbathe for sanitation? Inferences from in vitro experiments. Entomol. Exp. Appl. 2012, 145, 38–49. [Google Scholar] [CrossRef]
- Fernandes, E.K.K.; Rangel, D.E.N.; Moraes, A.M.L.; Bittencourt, V.R.E.P.; Roberts, D.W. Variability in tolerance to UV-B radiation among Beauveria spp. isolates. J. Invertebr. Pathol. 2007, 96, 237–243. [Google Scholar] [CrossRef]
- Rangel, D.E.N.; Braga, G.U.L.; Flint, S.D.; Anderson, A.J.; Roberts, D.W. Variations in UV-B tolerance and germination speed of Metarhizium anisopliae conidia produced on insects and artificial substrates. J. Invertebr. Pathol. 2004, 87, 77–83. [Google Scholar] [CrossRef]
- Sun, Q.; Zhou, X.G. Corpse management in social insects. Int. J. Biol. Sci. 2013, 9, 313–321. [Google Scholar] [CrossRef]
- Quintela, E.D.; McCoy, C.W. Conidial attachment of Metarhizium anisopliae and Beauveria bassiana to the larval cuticle of Diaprepes abbreviatus (Coleoptera: Curculionidae) treated with imidacloprid. J. Invertebr. Pathol. 1998, 72, 220–230. [Google Scholar] [CrossRef]
- Wilson, K. Evolutionary ecology of insect host-parasite interactions: An ecological immunology perspective. In Insect Evolutionary Ecology; Fellowes, M., Holloway, G., Rolff, J., Eds.; CABI Publishing: Wallingford, Oxon, UK, 2005; pp. 289–341. [Google Scholar]
- Quintela, E.D.; McCoy, C.W. Synergistic effect of imidacloprid and two entomopathogenic fungi on the behavior and survival of larvae of Diaprepes abbreviatus (Coleoptera: Curculionidae) in soil. J. Econ. Entomol. 1998, 91, 110–122. [Google Scholar]
- Paula, A.R.; Carolino, A.T.; Paula, C.O.; Samuels, R.I. The combination of the entomopathogenic fungus Metarhizium anisopliae with the insecticide Imidacloprid increases virulence against the dengue vector Aedes aegypti (Diptera: Culicidae). Parasit. Vectors 2011, 4. [Google Scholar] [CrossRef]
- Galvanho, J.P.; Carrera, M.P.; Moreira, D.D.O.; Erthal, M.; Silva, C.P.; Samuels, R.I. Imidacloprid inhibits behavioral defences of the leaf-cutting ant Acromyrmex subterraneus subterraneus (Hymenoptera: Formicidae). J. Insect. Behav. 2013, 26, 1–13. [Google Scholar] [CrossRef]
- Fan, Y.; Pereira, R.M.; Kilic, E.; Casella, G.; Keyhani, N.O. Pyrokinin beta-neuropeptide affects necrophoretic behavior in fire ants (S. invicta), and expression of beta-NP in a mycoinsecticide increases its virulence. PLoS One 2012, 7, e26924. [Google Scholar]
- de Crecy, E.; Jaronski, S.; Lyons, B.; Lyons, T.J.; Keyhani, N.O. Directed evolution of a filamentous fungus for thermotolerance. BMC Biotechnol. 2009, 9. [Google Scholar] [CrossRef]
- Brito, E.S.; de Paula, A.R.; Vieira, L.P.; Dolinski, C.; Samuels, R.I. Combining vegetable oil and sub-lethal concentrations of Imidacloprid with Beauveria bassiana and Metarhizium anisopliae against adult guava weevil Conotrachelus psidii (Coleoptera: Curculionidae). Biocontr. Sci. Tech. 2008, 18, 665–673. [Google Scholar] [CrossRef]
- Cohen, E.; Joseph, T. Photostabilization of Beauveria bassiana conidia using anionic dyes. Appl. Clay Sci. 2009, 42, 569–574. [Google Scholar] [CrossRef]
- Gosselin, M.E.; Belair, G.; Simard, L.; Brodeur, J. Toxicity of spinosad and Beauveria bassiana to the black cutworm, and the additivity of subletal doses. Biocontr. Sci. Tech. 2009, 19, 201–217. [Google Scholar] [CrossRef]
- Inglis, G.D.; Johnson, D.L.; Cheng, K.J.; Goettel, M.S. Use of pathogen combinations to overcome the constraints of temperature on entomopathogenic hyphomycetes against grasshoppers. Biol. Contr. 1997, 8, 143–152. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ortiz-Urquiza, A.; Keyhani, N.O. Action on the Surface: Entomopathogenic Fungi versus the Insect Cuticle. Insects 2013, 4, 357-374. https://doi.org/10.3390/insects4030357
Ortiz-Urquiza A, Keyhani NO. Action on the Surface: Entomopathogenic Fungi versus the Insect Cuticle. Insects. 2013; 4(3):357-374. https://doi.org/10.3390/insects4030357
Chicago/Turabian StyleOrtiz-Urquiza, Almudena, and Nemat O. Keyhani. 2013. "Action on the Surface: Entomopathogenic Fungi versus the Insect Cuticle" Insects 4, no. 3: 357-374. https://doi.org/10.3390/insects4030357
APA StyleOrtiz-Urquiza, A., & Keyhani, N. O. (2013). Action on the Surface: Entomopathogenic Fungi versus the Insect Cuticle. Insects, 4(3), 357-374. https://doi.org/10.3390/insects4030357




