Immune Signaling and Antimicrobial Peptide Expression in Lepidoptera
Abstract
:1. Introduction
2. Insect Immunity
| Receptor/Effector | Class | Activity | Reference |
|---|---|---|---|
| BmPGRP-L1-L5 | PRR | Peptidoglycan recognition | [54,55] |
| βGRP1-3 | PRR | β–1,3 glucan recognition | [54,56,57] |
| Hemolin | PRP | Binds LPS and LTA; triggers cellular response | [58,59] |
| HP14 | PRR | Binds Lys-PGN; triggers proPO activation | [60] |
| Moricin | AMP | Antibacterial activity against Gram-positive and Gram-negative bacteria; targets cytoplasmic membrane; increases membrane permeability | [61] |
| Gloverin | AMP | Antimicrobial activity against fungi, and Gram-negative and Gram-positive bacteria; targets outer membrane; inhibition of outer membrane proteins | [62] |
| Lebocin | AMP | Antimicrobial activity against fungi, and Gram-negative and Gram-positive bacteria | [63,64] |
3. Signaling Pathways Involved in Antimicrobial Peptide Gene Expression
3.1. Induction of AMP Genes by the NF-kB Family of Transcription Factors
3.2. Recognition and Proteolytic Cascades
3.3. Toll Pathway
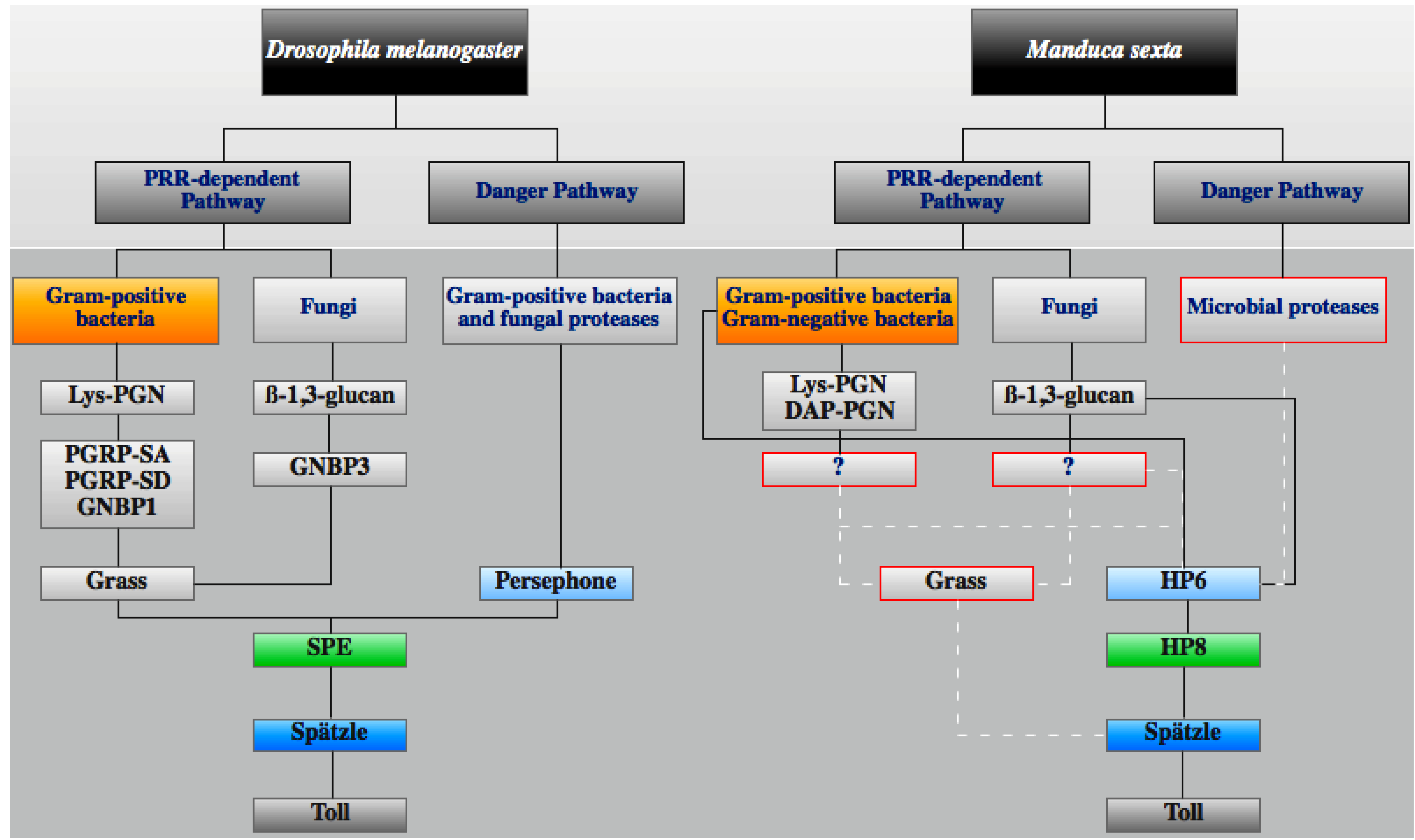
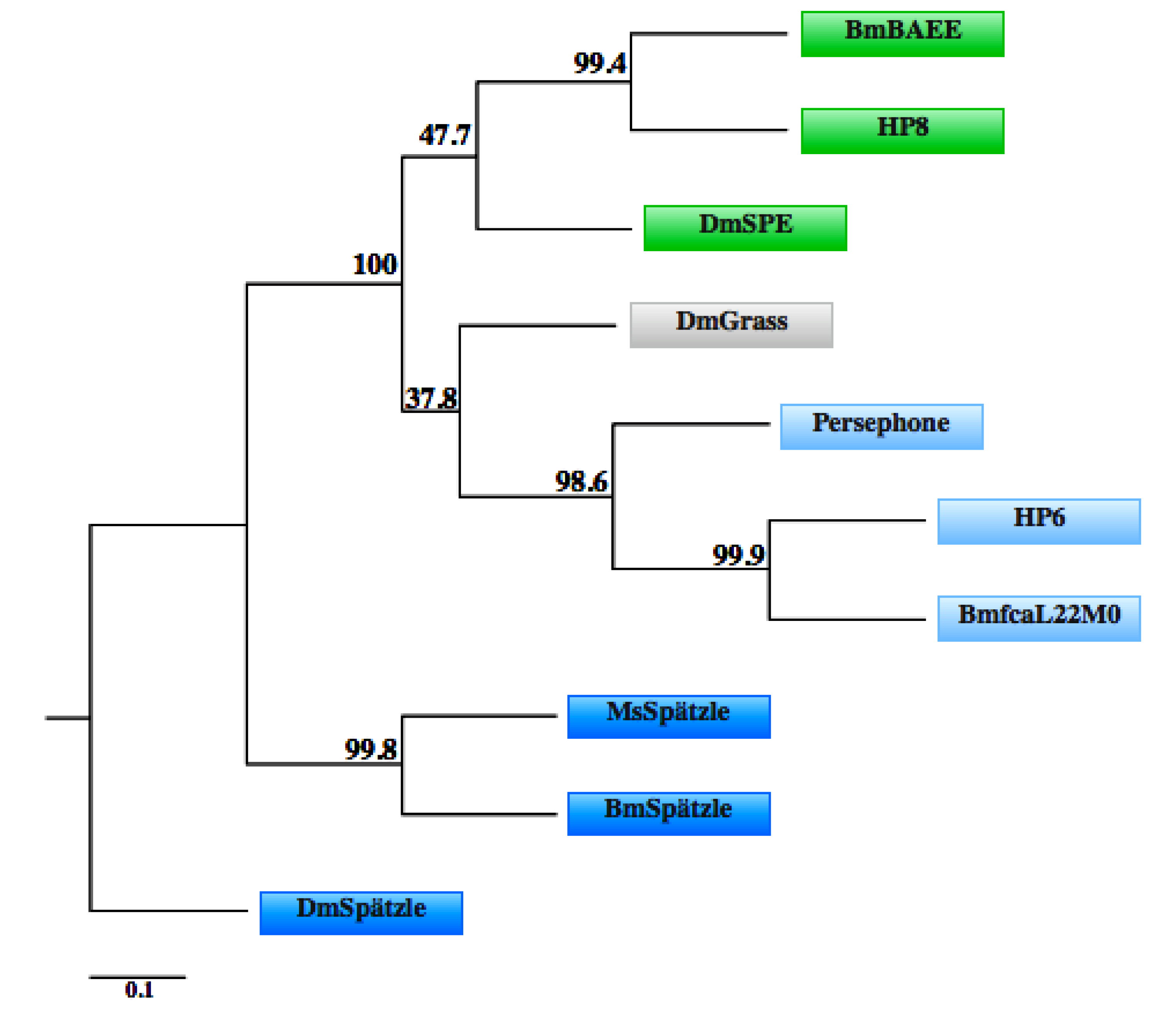
3.4. IMD Pathway
4. Conclusions
Acknowledgments
References
- Mohandass, S.; Arthur, F.H.; Zhu, K.Y.; Throne, J.E. Biology and management of Plodia interpunctella (Lepidoptera: Pyralidae) in stored products. J. Stored Prod. Res. 2007, 43, 302–311. [Google Scholar] [CrossRef]
- Nègre, V.; Hôtelier, T.; Volkoff, A.-N.; Gimenez, S.; Cousserans, F.; Mita, K.; Sabau, X.; Rocher, J.; López-Ferber, M.; D’Alençon, E.; et al. SPODOBASE: An EST database for the lepidopteran crop pest Spodoptera. BMC Bioinformatics 2006, 7, 322. [Google Scholar] [CrossRef]
- Vail, P.V.; Hoffman, D.F.; Tebbets, J.S. Effects of a fluorescent brightener on the activity of Anagrapha falcifera (Lepidoptera: Noctuidae) nuclear Polyhedrosis virus to four Noctuid pests. Biolog. Contr. 1996, 125, 121–125. [Google Scholar]
- Cordero, R.J.; Kuhar, T.P.; Speese, J.; Youngman, R.; Lewis, E.; Boomquist, J.; Kok, L.T.; Bratsch, A. Field efficacy of insecticides for control of lepidopteran pests on collards in Virginia. Plant Health Progr 2006. [Google Scholar] [CrossRef]
- James, R.R.; Xu, J. Mechanisms by which pesticides affect insect immunity. J. Invertebr. Pathol. 2012, 109, 175–182. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.-M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Zibaee, A.; Bandani, A.R. Effects of Artemisia annua L. (Asteracea) on the digestive enzymatic profiles and the cellular immune reactions of the Sunn pest, Eurygaster integriceps (Heteroptera: Scutellaridae), against Beauveria bassiana. Bull. Entomol. Res. 2010, 100, 185–196. [Google Scholar] [CrossRef]
- Azambuja, P.; Garcia, E.S.; Ratcliffe, N.A.; Warthen, D.J. Immune-depression in Rhodnius prolixus induced by the growth inhibitor, azadirachtin. J. Insect Physiol. 1991, 37, 771–777. [Google Scholar] [CrossRef]
- Franssens, V.; Smagghe, G.; Simonet, G.; Claeys, I.; Breugelmans, B.; De Loof, A.; Vanden Broeck, J. 20-Hydroxyecdysone and juvenile hormone regulate the laminarin-induced nodulation reaction in larvae of the flesh fly, Neobellieria bullata. Dev. Comp. Immunol. 2006, 30, 735–740. [Google Scholar] [CrossRef]
- Delpuech, J.-M.; Frey, F.; Carton, Y. Action of insecticides on the cellular immune reaction of Drosophila melanogaster against the parasitoid Leptopilina boulardi. Environ. Toxicol. Chem. 1996, 15, 2267–2271. [Google Scholar]
- Babu, S.R.; Subrahmanyam, B. Bio-potency of serine proteinase inhibitors from Acacia senegal seeds on digestive proteinases, larval growth and development of Helicoverpa armigera (Hübner). Pestic. Biochem. Physiol. 2010, 98, 349–358. [Google Scholar] [CrossRef]
- Nasr, H.M.; Badawy, M.E.I.; Rabea, E.I. Toxicity and biochemical study of two insect growth regulators, buprofezin and pyriproxyfen, on cotton leafworm Spodoptera littorali. Pestic. Biochem. Physiol. 2010, 98, 198–205. [Google Scholar] [CrossRef]
- Quintela, E.D.; McCoy, C. Pathogenicity enhancement of Metarhizium anisopliae and Beauveria bassiana to first instars of Diaprepes abbreviatus (Coleoptera: Curculionidae) with sublethal doses of imidacloprid. Environ. Entomol. 1997, 26, 1173–1182. [Google Scholar]
- Koppenhöfer, A.M.; Grewal, P.S.; Kaya, H.K. Synergism of imidacloprid and entomopathogenic nematodes against white grubs: The mechanism. Entomol. Exp. Appl. 2000, 94, 283–293. [Google Scholar]
- Lazzaro, B.P. Natural selection on the Drosophila antimicrobial immune system. Curr. Opin. Microbiol. 2008, 11, 284–289. [Google Scholar] [CrossRef]
- Kanost, M.R.; Jiang, H.; Yu, X.-Q. Innate immune responses of a lepidopteran insect, Manduca sexta. Immunol. Rev. 2004, 198, 97–105. [Google Scholar] [CrossRef]
- Lavine, M.D.; Strand, M.R. Insect hemocytes and their role in immunity. Insect Biochem. Mol. Biol. 2002, 32, 1295–1309. [Google Scholar] [CrossRef]
- Marmaras, V.J.; Lampropoulou, M. Regulators and signalling in insect haemocyte immunity. Cell. Signal. 2009, 21, 186–195. [Google Scholar] [CrossRef]
- Jiggins, F.M.; Kim, K.W. A screen for immunity genes evolving under positive selection in Drosophila. J. Evol. Biol. 2007, 20, 965–970. [Google Scholar] [CrossRef]
- Sackton, T.B.; Lazzaro, B.P.; Schlenke, T.A.; Evans, J.D.; Hultmark, D.; Clark, A.G. Dynamic evolution of the innate immune system in Drosophila. Nat. Genet. 2007, 39, 1461–1468. [Google Scholar] [CrossRef]
- Keebaugh, E.S.; Schlenke, T.A. Adaptive evolution of a novel Drosophila lectin induced by parasitic wasp attack. Mol. Biol. Evol. 2012, 29, 565–577. [Google Scholar] [CrossRef]
- Sackton, T.B.; Clark, A.G. Comparative profiling of the transcriptional response to infection in two species of Drosophila by short-read cDNA sequencing. BMC Genomics 2009, 10, 259–275. [Google Scholar] [CrossRef]
- Park, Y.; Herbert, E.E.; Cowles, C.E.; Cowles, K.N.; Menard, M.L.; Orchard, S.S.; Goodrich-Blair, H. Clonal variation in Xenorhabdus nematophila virulence and suppression of Manduca sexta immunity. Cell. Microbiol. 2007, 9, 645–656. [Google Scholar] [CrossRef]
- Ji, D.; Kim, Y. An entomopathogenic bacterium, Xenorhabdus nematophila, inhibits the expression of an antibacterial peptide, cecropin, of the beet armyworm, Spodoptera exigua. J. Insect Physiol. 2004, 50, 489–496. [Google Scholar] [CrossRef]
- Aymeric, J.-L.; Givaudan, A.; Duvic, B. Imd pathway is involved in the interaction of Drosophila melanogaster with the entomopathogenic bacteria, Xenorhabdus nematophila and Photorhabdus luminescens. Mol. Immunol. 2010, 47, 2342–2348. [Google Scholar] [CrossRef]
- Kearns, C.A. North American dipteran pollinators: Assessing their value and conservation status. Conserv. Ecol. 2001, 5, 1–10. [Google Scholar]
- Ssymank, A.; Kearns, C.A.; Pape, T.; Thompson, C. Pollinating flies (Diptera): A major contribution to plant diversity and agricultural production. Biodiversity 2007, 9, 86–89. [Google Scholar]
- Bulmer, M.S.; Bachelet, I.; Raman, R.; Rosengaus, R.B.; Sasisekharan, R. Targeting an antimicrobial effector function in insect immunity as a pest control strategy. Proc. Natl. Acad. Sci. USA 2009, 106, 12652–12657. [Google Scholar] [CrossRef]
- Clark, A.G.; Eisen, M.B.; Smith, D.R.; Bergman, C.M.; Oliver, B.; Markow, T.A.; Kaufman, T.C.; Kellis, M.; Gelbart, W.; Iyer, V.N.; et al. Evolution of genes and genomes on the Drosophila phylogeny. Nature 2007, 450, 203–218. [Google Scholar] [CrossRef]
- Eleftherianos, I.; Millichap, P.J.; Ffrench-Constant, R.H.; Reynolds, S.E. RNAi suppression of recognition protein mediated immune responses in the tobacco hornworm Manduca sexta causes increased susceptibility to the insect pathogen Photorhabdus. Dev. Comp. Immunol. 2006, 30, 1099–1107. [Google Scholar] [CrossRef]
- Huvenne, H.; Smagghe, G. Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: A review. J. Insect Physiol. 2010, 56, 227–235. [Google Scholar] [CrossRef]
- Zhang, S.; Gunaratna, R.T.; Zhang, X.; Najar, F.; Wang, Y.; Roe, B.; Jiang, H. Pyrosequencing-based expression profiling and identification of differentially regulated genes from Manduca sexta, a lepidopteran model insect. Insect Biochem. Mol. Biol. 2011, 41, 733–746. [Google Scholar]
- International, T.; Genome, S. The genome of a lepidopteran model insect, the silkworm Bombyx mori. Insect Biochem. Mol. Biol. 2008, 38, 1036–1045. [Google Scholar] [CrossRef]
- Tobacco Hornworm Genome Project. Available online: http://www.hgsc.bcm.tmc.edu/content/tobacco-hornworm-genome-project (accessed on 6 May 2013).
- Gunaratna, R.T.; Jiang, H. A comprehensive analysis of the Manduca sexta immunotranscriptome. Dev. Comp. Immunol. 2013, 39, 388–398. [Google Scholar] [CrossRef]
- Xu, Q.; Lu, A.; Xiao, G.; Yang, B.; Zhang, J.; Li, X.; Guan, J.; Shao, Q.; Beerntsen, B.T.; Zhang, P.; et al. Transcriptional profiling of midgut immunity response and degeneration in the wandering silkworm, Bombyx mori. PLoS One 2012, 7, 1–14. [Google Scholar]
- Pauchet, Y.; Wilkinson, P.; Vogel, H.; Nelson, D.R.; Reynolds, S.E.; Heckel, D.G.; Ffrench-Constant, R.H. Pyrosequencing the Manduca sexta larval midgut transcriptome: Messages for digestion, detoxification and defence. Insect Mol. Biol. 2010, 19, 61–75. [Google Scholar] [CrossRef]
- Zou, Z.; Najar, F.; Wang, Y.; Roe, B.; Jiang, H. Pyrosequence analysis of expressed sequence tags for Manduca sexta hemolymph proteins involved in immune responses. Insect Biochem. Mol. Biol. 2008, 38, 677–682. [Google Scholar] [CrossRef]
- Schmidt, O.; Theopold, U.; Strand, M. Innate immunity and its evasion and suppression by hymenopteran endoparasitoids. Bioessays 2001, 23, 344–351. [Google Scholar] [CrossRef]
- Nappi, A.J.; Kohler, L.; Mastore, M.; Funzionale, B. Signaling pathways implicated in the cellular innate immune responses of Drosophila. Invertebrate Surviv. J. 2004, 1, 5–33. [Google Scholar]
- Lamprou, I.; Tsakas, S.; Theodorou, G.L.; Karakantza, M.; Lampropoulou, M.; Marmaras, V.J. Uptake of LPS/E. coli/latex beads via distinct signalling pathways in medfly hemocytes: The role of MAP kinases activation and protein secretion. Biochim. Biophys. Acta 2005, 1744, 1–10. [Google Scholar] [CrossRef]
- Sideri, M.; Tsakas, S.; Markoutsa, E.; Lampropoulou, M.; Marmaras, V.J. Innate immunity in insects: Surface-associated dopa decarboxylase-dependent pathways regulate phagocytosis, nodulation and melanization in medfly haemocytes. Immunology 2008, 123, 528–537. [Google Scholar] [CrossRef]
- Bulet, P.; Hetru, C.; Dimarcq, J.L.; Hoffmann, D. Antimicrobial peptides in insects; structure and function. Dev. Comp. Immunol. 1999, 23, 329–344. [Google Scholar] [CrossRef]
- Bulet, P.; Stöcklin, R. Insect antimicrobial peptides: Structures, properties and gene regulation. Protein Pept. Lett. 2005, 12, 3–11. [Google Scholar] [CrossRef]
- González-Santoyo, I.; Córdoba-Aguilar, A. Phenoloxidase: A key component of the insect immune system. Entomol. Exp. Appl. 2012, 142, 1–16. [Google Scholar] [CrossRef]
- Zhao, P.; Li, J.; Wang, Y.; Jiang, H. Broad-spectrum antimicrobial activity of the reactive compounds generated in vitro by Manduca sexta phenoloxidase. Insect Biochem. Mol. Biol. 2007, 37, 952–959. [Google Scholar] [CrossRef]
- Hoffmann, J.A. Phylogenetic perspectives in innate immunity. Science 1999, 284, 1313–1318. [Google Scholar] [CrossRef]
- Gorman, M.J.; Kankanala, P.; Kanost, M.R. Bacterial challenge stimulates innate immune responses in extra-embryonic tissues of tobacco hornworm eggs. Insect Mol. Biol. 2004, 13, 19–24. [Google Scholar] [CrossRef]
- García-Olmedo, F.; Molina, A.; Alamillo, J.M.; Rodríguez-Palenzuéla, P. Plant defense peptides. Biopolymers 1998, 47, 479–491. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Ravi, C.; Jeyashree, A.; Renuka Devi, K. Antimicrobial peptides from insects: An overview. Res. Biotech. 2011, 2, 1–7. [Google Scholar]
- Haine, E.R.; Moret, Y.; Siva-Jothy, M.T.; Rolff, J. Antimicrobial defense and persistent infection in insects. Science 2008, 322, 1257–1259. [Google Scholar] [CrossRef]
- Meister, M.; Lemaitre, B.; Hoffmann, J.A. Antimicrobial peptide defense in Drosophila. BioEssays 1997, 19, 1019–1026. [Google Scholar] [CrossRef]
- Tanaka, H.; Ishibashi, J.; Fujita, K.; Nakajima, Y.; Sagisaka, A.; Tomimoto, K.; Suzuki, N.; Yoshiyama, M.; Kaneko, Y.; Iwasaki, T.; et al. A genome-wide analysis of genes and gene families involved in innate immunity of Bombyx mori. Insect Biochem. Mol. Biol. 2008, 38, 1087–1110. [Google Scholar] [CrossRef]
- Yu, X.-Q.; Zhu, Y.-F.; Ma, C.; Fabrick, J.A.; Kanost, M.R. Pattern recognition proteins in Manduca sexta plasma. Insect Biochem. Mol. Biol. 2002, 32, 1287–1293. [Google Scholar] [CrossRef]
- Ma, C.; Kanost, M.R. A β-1,3-glucan recognition protein from an insect, Manduca sexta, agglutinates microorganisms and activates the phenoloxidase cascade. J. Biol. Chem. 2000, 275, 7505–7514. [Google Scholar] [CrossRef]
- Fabrick, J.A.; Baker, J.E.; Kanost, M.R. cDNA cloning, purification, properties, and function of a β-1,3-glucan recognition protein from a pyralid moth, Plodia interpunctella. Insect Biochem. Mol. Biol. 2003, 33, 579–594. [Google Scholar] [CrossRef]
- Yu, X.; Kanost, M.R. Binding of hemolin to bacterial lipopolysaccharide and lipoteichoic acid: An immunoglobulin superfamily member from insects as a pattern-recognition receptor. Eur. J. Biochem. 2002, 1834, 1827–1834. [Google Scholar]
- Eleftherianos, I.; Gökçen, F.; Felföldi, G.; Millichap, P.J.; Trenczek, T.E.; Ffrench-Constant, R.H.; Reynolds, S.E. The immunoglobulin family protein Hemolin mediates cellular immune responses to bacteria in the insect Manduca sexta. Cell. Microbiol. 2007, 9, 1137–1147. [Google Scholar] [CrossRef]
- Ji, C.; Wang, Y.; Guo, X.; Hartson, S.; Jiang, H. A pattern recognition serine proteinase triggers the prophenoloxidase activation cascade in the tobacco hornworm, Manduca sexta. J. Biol. Chem. 2004, 279, 34101–34106. [Google Scholar] [CrossRef]
- Hara, S.; Yamakawa, M. Moricin, a novel type of antibacterial peptide isolated from the silkworm, Bombyx mori. J. Biol. Chem. 1995, 270, 29923–29927. [Google Scholar] [CrossRef]
- Xu, X.-X.; Zhong, X.; Yi, H.-Y.; Yu, X.-Q. Manduca sexta gloverin binds microbial components and is active against bacteria and fungi. Dev. Comp. Immunol. 2012, 38, 275–284. [Google Scholar] [CrossRef]
- Chowdhury, S.; Taniai, K.; Hara, S.; Kadono-Okuda, K.; Kato, Y.; Yamamoto, M.; Xu, J.; Su Kyung, C.; Debnath, N.C.; Choi, H.K.; et al. cDNA cloning and gene expression of lebocin, a novel member of antibacterial peptides from the silkworm, Bombyx mori. Biochem. Biophys. Res. Commun. 1995, 214, 271–278. [Google Scholar] [CrossRef]
- Rao, X.-J.; Xu, X.-X.; Yu, X.-Q. Functional analysis of two lebocin-related proteins from Manduca sexta. Insect Biochem. Mol. Biol. 2012, 42, 231–239. [Google Scholar] [CrossRef]
- Hoffmann, J.A. The immune response of Drosophila. Nature 2003, 426, 33–38. [Google Scholar] [CrossRef]
- Lemaitre, B.; Hoffmann, J.A. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef]
- Ip, Y.T.; Reach, M.; Engstrom, Y.; Kadalayil, L.; Cai, H.; González-Crespo, S.; Tatei, K.; Levine, M. Dif, a dorsal-related gene that mediates an immune response in Drosophila. Cell 1993, 75, 753–763. [Google Scholar] [CrossRef]
- Dushay, M.S.; Asling, B.; Hultmark, D. Origins of immunity: Relish, a compound Rel-like gene in the antibacterial defense of Drosophila. Proc. Natl. Acad. Sci. USA 1996, 93, 10343–10347. [Google Scholar] [CrossRef]
- Hedengren, M.; Asling, B.; Dushay, M.S.; Ando, I.; Ekengren, S.; Wihlborg, M.; Hultmark, D. Relish, a central factor in the control of humoral but not cellular immunity in Drosophila. Mol. Cell 1999, 4, 827–837. [Google Scholar] [CrossRef]
- Engstrom, Y.; Kadalayil, L.; Sun, S.-C.; Samakovlis, C.; Hultmark, D.; Faye, I. kB-like motifs regulate the induction of immune genes in Drosophila. J. Mol. Biol. 1993, 232, 327–333. [Google Scholar] [CrossRef]
- Stöven, S.; Ando, I.; Kadalayil, L.; Engström, Y.; Hultmark, D. Activation of the Drosophila NF-kappaB factor Relish by rapid endoproteolytic cleavage. EMBO Rep. 2000, 1, 347–352. [Google Scholar] [CrossRef]
- Kappler, C.; Meister, M.; Lagueux, M.; Gateff, E.; Hoffmann, J.A.; Reichhart, J. Insect immunity. Two 17 bp repeats nesting a kB-related sequence confer inducibility to the diptericin gene and bind a polypeptide in bacteria-challenge Drosophila. EMBO J. 1993, 12, 1561–1568. [Google Scholar]
- Ghosh, S.; May, M.J.; Kopp, E.B. NF-kappa B and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998, 16, 225–260. [Google Scholar] [CrossRef]
- Ryzhakov, G.; Teixeira, A.; Saliba, D.; Blazek, K.; Muta, T.; Ragoussis, J.; Udalova, I.A. Cross-species analysis reveals evolving and conserved features of the NF-κB proteins. J. Biol. Chem. 2013, 288, 11546–11554. [Google Scholar] [CrossRef]
- Rao, X.-J.; Xu, X.-X.; Yu, X.-Q. Manduca sexta moricin promoter elements can increase promoter activities of Drosophila melanogaster antimicrobial peptide genes. Insect Biochem. Mol. Biol. 2011, 41, 982–992. [Google Scholar] [CrossRef]
- Wang, Y.; Sumathipala, N.; Rayaprolu, S.; Jiang, H. Recognition of microbial molecular patterns and stimulation of prophenoloxidase activation by a β-1,3-glucanase-related protein in Manduca sexta larval plasma. Insect Biochem. Mol. Biol. 2011, 41, 322–331. [Google Scholar] [CrossRef]
- Yoshida, H.; Ochiai, M.; Ashida, M. ß-1,3-glucan receptor and peptidoglycan receptor are present as separate entities within insect prophenoloxidase activating system. Biochem. Biophys. Res. Commun. 1986, 141, 1177–1184. [Google Scholar] [CrossRef]
- Kang, D.; Liu, G.; Lundström, A.; Gelius, E.; Steiner, H. A peptidoglycan recognition protein in innate immunity conserved from insects to humans. Proc. Natl. Acad. Sci. USA 1998, 95, 10078–10082. [Google Scholar] [CrossRef]
- Ferrandon, D.; Imler, J.-L.; Hoffmann, J.A. Sensing infection in Drosophila: Toll and beyond. Semin. Immunol. 2004, 16, 43–53. [Google Scholar] [CrossRef]
- Choe, K.-M.; Werner, T.; Stöven, S.; Hultmark, D.; Anderson, K.V. Requirement for a peptidoglycan recognition protein (PGRP) in Relish activation and antibacterial immune responses in Drosophila. Science 2002, 296, 359–362. [Google Scholar] [CrossRef]
- Takehana, A.; Katsuyama, T.; Yano, T.; Oshima, Y.; Takada, H.; Aigaki, T.; Kurata, S. Overexpression of a pattern-recognition receptor, peptidoglycan-recognition protein-LE, activates imd/relish-mediated antibacterial defense and the prophenoloxidase cascade in Drosophila larva. Proc. Natl. Acad. Sci. USA 2002, 99, 13705–13710. [Google Scholar] [CrossRef]
- Gottar, M.; Gobert, V.; Michel, T.; Belvin, M.; Duyk, G.; Hoffmann, J.A.; Ferrandon, D.; Royet, J. The Drosophila immune response against Gram-negative bacteria is mediated by a peptidoglycan recognition protein. Nature 2002, 416, 640–644. [Google Scholar] [CrossRef]
- Rämet, M.; Manfruelli, P.; Pearson, A.; Mathey-Prevot, B.; Ezekowitz, R.A.B. Functional genomic analysis of phagocytosis and identification of a Drosophila receptor for E. coli. Nature 2002, 416, 644–648. [Google Scholar] [CrossRef]
- Gottar, M.; Gobert, V.; Matskevich, A.A.; Reichhart, J.M.; Wang, C.; Butt, T.M.; Belvin, M.; Hoffmann, J.A.; Ferrandon, D. Dual detection of fungal infections in Drosophila via recognition of glucans and sensing of virulence factors. Cell 2006, 127, 1425–1437. [Google Scholar] [CrossRef]
- Tanaka, H.; Yamakawa, M. Regulation of the innate immune responses in the silkworm, Bombyx mori. Invertebrate Surviv. J. 2011, 8, 59–69. [Google Scholar]
- Dunn, P.E.; Dai, W.; Kanost, M.R.; Geng, C. Soluble peptidoglycan fragments stimulate antibacterial protein synthesis by fat body from larvae of Manduca sexta. Dev. Comp. Immunol. 1985, 9, 559–568. [Google Scholar] [CrossRef]
- Kanost, M.R.; Dai, W.; Dunn, P.E. Peptidoglycan fragments elicit antibacterial protein synthesis in larvae of Manduca sexta. Arch. Insect Biochem. Physiol. 1988, 8, 147–164. [Google Scholar] [CrossRef]
- Iketani, M.; Morishima, I. Induction of antibacterial protein synthesis by soluble peptidoglycan in isolated fat body from larvae of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 1993, 23, 913–917. [Google Scholar] [CrossRef]
- Morishima, I.; Horiba, T.; Iketani, M.; Nishioka, E.; Yamano, Y. Parallel induction of cecropin and lysozyme in larvae of the silkworm, Bombyx mori. Dev. Comp. Immunol. 1995, 19, 357–363. [Google Scholar] [CrossRef]
- Rao, X.-J.; Yu, X.-Q. Lipoteichoic acid and lipopolysaccharide can activate antimicrobial peptide expression in the tobacco hornworm Manduca sexta. Dev. Comp. Immunol. 2010, 34, 1119–1128. [Google Scholar] [CrossRef]
- Ochiai, M.; Ashida, M. A pattern recognition protein for peptidoglycan. J. Biol. Chem. 1999, 274, 11854–11858. [Google Scholar] [CrossRef]
- Seitz, V.; Clermont, A.; Wedde, M.; Hummel, M.; Vilcinskas, A.; Schlatterer, K.; Podsiadlowski, L. Identification of immunorelevant genes from greater wax moth (Galleria mellonella) by a subtractive hybridization approach. Dev. Comp. Immunol. 2003, 27, 207–215. [Google Scholar] [CrossRef]
- Onoe, H.; Matsumoto, A.; Hashimoto, K.; Yamano, Y.; Morishima, I. Peptidoglycan recognition protein (PGRP) from eri-silkworm, Samia cynthia ricini; protein purification and induction of the gene expression. Comp. Biochem. Physiol. 2007, 147, 512–519. [Google Scholar] [CrossRef]
- Eum, J.H.; Seo, Y.R.; Yoe, S.M.; Kang, S.W.; Han, S.S. Analysis of the immune-inducible genes of Plutella xylostella using expressed sequence tags and cDNA microarray. Dev. Comp. Immunol. 2007, 31, 1107–1120. [Google Scholar] [CrossRef]
- Hughes, A.L. Evolution of the βGRP/GNBP/β-1,3-glucanase family of insects. Immunogenetics 2012, 64, 549–558. [Google Scholar] [CrossRef]
- Kaneko, T.; Goldman, W.E.; Mellroth, P.; Steiner, H.; Fukase, K.; Kusumoto, S.; Harley, W.; Fox, A.; Golenbock, D.; Silverman, N. Monomeric and polymeric gram-negative peptidoglycan but not purified LPS stimulate the Drosophila IMD pathway. Immunity 2004, 20, 637–649. [Google Scholar] [CrossRef]
- Tanaka, H.; Sagisaka, A.; Fujita, K.; Kaneko, Y.; Imanishi, S.; Yamakawa, M. Lipopolysaccharide elicits expression of immune-related genes in the silkworm, Bombyx mori. Insect Mol. Biol. 2009, 18, 71–75. [Google Scholar] [CrossRef]
- Ao, J.; Ling, E.; Yu, X.-Q. Drosophila C-type lectins enhance cellular encapsulation. Mol. Immunol. 2007, 44, 2541–2548. [Google Scholar] [CrossRef]
- Watanabe, A.; Miyazawa, S.; Kitami, M.; Tabunoki, H.; Ueda, K.; Sato, R. Characterization of a novel C-type lectin, Bombyx mori multibinding protein, from the B. mori hemolymph: Mechanism of wide-range microorganism recognition and role in immunity. J. Immunol. 2006, 177, 4594–4604. [Google Scholar]
- Koizumi, N.; Imai, Y.; Morozumi, A.; Imamura, M.; Kadotani, T.; Yaoi, K.; Iwahana, H.; Sato, R. Lipopolysaccharide-binding protein of Bombyx mori participates in a hemocyte-mediated defense reaction against Gram-negative bacteria. J. Insect Physiol. 1999, 45, 853–859. [Google Scholar] [CrossRef]
- Yu, X.Q.; Gan, H.; Kanost, M.R. Immulectin, an inducible C-type lectin from an insect, Manduca sexta, stimulates activation of plasma prophenoloxidase. Insect Biochem. Mol. Biol. 1999, 29, 585–597. [Google Scholar] [CrossRef]
- Yu, X.Q.; Kanost, M.R. Immulectin-2, a lipopolysaccharide-specific lectin from an insect, Manduca sexta, is induced in response to Gram-negative bacteria. J. Biol. Chem. 2000, 275, 37373–37381. [Google Scholar] [CrossRef]
- Yu, X.-Q.; Tracy, M.E.; Ling, E.; Scholz, F.R.; Trenczek, T. A novel C-type immulectin-3 from Manduca sexta is translocated from hemolymph into the cytoplasm of hemocytes. Insect Biochem. Mol. Biol. 2005, 35, 285–295. [Google Scholar] [CrossRef]
- Yu, X.-Q.; Ling, E.; Tracy, M.E.; Zhu, Y. Immulectin-4 from the tobacco hornworm Manduca sexta binds to lipopolysaccharide and lipoteichoic acid. Insect Mol. Biol. 2006, 15, 119–128. [Google Scholar] [CrossRef]
- Jiang, H.; Ma, C.; Lu, Z.-Q.; Kanost, M.R. β-1,3-Glucan recognition protein-2 (βGRP-2) from Manduca sexta: An acute-phase protein that binds β-1,3-glucan and lipoteichoic acid to aggregate fungi and bacteria and stimulate prophenoloxidase activation. Insect Biochem. Mol. Biol. 2004, 34, 89–100. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, H. Interaction of β-1,3-glucan with its recognition protein activates hemolymph proteinase 14, an initiation enzyme of the prophenoloxidase activation system in Manduca sexta. J. Biol. Chem. 2006, 281, 9271–9278. [Google Scholar] [CrossRef]
- Ashok, Y. Drosophila Toll pathway: The new model. Sci. Signal. 2009, 2, 1–2. [Google Scholar] [CrossRef]
- Hetru, C.; Hoffmann, J.A. NF-kappaB in the immune response of Drosophila. Cold Spring Harb. Perspect. Biol. 2009, 1, 1–15. [Google Scholar]
- Michel, T.; Reichhart, J.M.; Hoffmann, J.A.; Royet, J. Drosophila Toll is activated by Gram-positive bacteria through a circulating peptidoglycan recognition protein. Nature 2001, 414, 756–759. [Google Scholar] [CrossRef]
- El Chamy, L.; Leclerc, V.; Caldelari, I.; Reichhart, J.-M. Sensing of “danger signals” and pathogen-associated molecular patterns defines binary signaling pathways “upstream” of Toll. Nat. Immunol. 2008, 9, 1165–1170. [Google Scholar] [CrossRef]
- Piao, S.; Song, Y.-L.; Kim, J.H.; Park, S.Y.; Park, J.W.; Lee, B.L.; Oh, B.-H.; Ha, N.-C. Crystal structure of a clip-domain serine protease and functional roles of the clip domains. EMBO J. 2005, 24, 4404–4414. [Google Scholar] [CrossRef]
- Jang, I.-H.; Chosa, N.; Kim, S.-H.; Nam, H.-J.; Lemaitre, B.; Ochiai, M.; Kambris, Z.; Brun, S.; Hashimoto, C.; Ashida, M.; et al. A Spätzle-processing enzyme required for toll signaling activation in Drosophila innate immunity. Dev. Cell 2006, 10, 45–55. [Google Scholar] [CrossRef]
- Valanne, S.; Wang, J.-H.; Rämet, M. The Drosophila Toll signaling pathway. J. Immunol. 2011, 186, 649–656. [Google Scholar] [CrossRef]
- Jiang, H.; Vilcinskas, A.; Kanost, M.R. Immunity in lepidopteran insects. In Invertebrate Immunity; Söderhäll, K., Ed.; Springer: New York, NY, USA, 2010; pp. 181–204. [Google Scholar]
- Ao, J.; Ling, E.; Yu, X.-Q. A Toll receptor from Manduca sexta is in response to Escherichia coli infection. Mol. Immunol. 2008, 45, 543–552. [Google Scholar] [CrossRef]
- An, C.; Jiang, H.; Kanost, M.R. Proteolytic activation and function of the cytokine Spätzle in the innate immune response of a lepidopteran insect, Manduca sexta. FEBS J. 2010, 277, 148–162. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, T.; Rayaprolu, S.; Zou, Z.; Xia, Q.; Xiang, Z.; Jiang, H. Proteolytic activation of pro-Spätzle is required for the induced transcription of antimicrobial peptide genes in lepidopteran insects. Dev. Comp. Immunol. 2007, 31, 1002–1012. [Google Scholar] [CrossRef]
- Zhong, X.; Xu, X.-X.; Yi, H.-Y.; Lin, C.; Yu, X.-Q. A Toll-Spätzle pathway in the tobacco hornworm, Manduca sexta. Insect Biochem. Mol. Biol. 2012, 42, 514–524. [Google Scholar] [CrossRef]
- An, C.; Ishibashi, J.; Ragan, E.J.; Jiang, H.; Kanost, M.R. Functions of Manduca sexta hemolymph proteinases HP6 and HP8 in two innate immune pathways. J. Biol. Chem. 2009, 284, 19716–19726. [Google Scholar]
- Gertz, E.M.; Yu, Y.-K.; Agarwala, R.; Schäffer, A.A.; Altschul, S.F. Composition-based statistics and translated nucleotide searches: Improving the TBLASTN module of BLAST. BMC Biol. 2006, 4, 41–54. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 1–6. [Google Scholar]
- Goujon, M.; McWilliam, H.; Li, W.; Valentin, F.; Squizzato, S.; Paern, J.; Lopez, R. A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Res. 2010, 38, 695–699. [Google Scholar] [CrossRef]
- Felsenstein, J. PHYLIP-Phylogeny Inference Package (Version 3.2). Cladistics 1989, 5, 164–166. [Google Scholar]
- Gilbert, D. Phylodendron version 0.8d beta. Available online: http://iubio.bio.indiana.edu/treeapp/ (accessed on 26 May 2013).
- Choe, K.-M.; Lee, H.; Anderson, K.V. Drosophila peptidoglycan recognition protein LC (PGRP-LC) acts as a signal-transducing innate immune receptor. Proc. Natl. Acad. Sci. USA 2005, 102, 1122–1126. [Google Scholar] [CrossRef]
- Schmidt, R.L.; Trejo, T.R.; Plummer, T.B.; Platt, J.L.; Tang, A.H. Infection-induced proteolysis of PGRP-LC controls the IMD activation and melanization cascades in Drosophila. FASEB J. 2008, 22, 918–929. [Google Scholar]
- Kaneko, T.; Yano, T.; Aggarwal, K.; Lim, J.-H.; Ueda, K.; Oshima, Y.; Peach, C.; Erturk-Hasdemir, D.; Goldman, W.E.; Oh, B.-H.; et al. PGRP-LC and PGRP-LE have essential yet distinct functions in the Drosophila immune response to monomeric DAP-type peptidoglycan. Nat. Immunol. 2006, 7, 715–723. [Google Scholar]
- Takehana, A.; Yano, T.; Mita, S.; Kotani, A.; Oshima, Y.; Kurata, S. Peptidoglycan recognition protein (PGRP)-LE and PGRP-LC act synergistically in Drosophila immunity. EMBO J. 2004, 23, 4690–4700. [Google Scholar] [CrossRef]
- Leulier, F.; Vidal, S.; Saigo, K.; Ueda, R.; Lemaitre, B. Inducible expression of double-stranded RNA reveals a role for dFADD in the regulation of the antibacterial response in Drosophila adults. Curr. Biol. 2002, 12, 996–1000. [Google Scholar] [CrossRef]
- Leulier, F.; Rodriguez, A.; Khush, R.S.; Abrams, J.M.; Lemaitre, B. The Drosophila caspase Dredd is required to resist Gram-negative bacterial infection. EMBO Rep. 2000, 1, 353–358. [Google Scholar] [CrossRef]
- Valanne, S.; Kallio, J.; Kleino, A.; Rämet, M. Large-scale RNAi screens add both clarity and complexity to Drosophila NF-κB signaling. Dev. Comp. Immunol. 2012, 37, 9–18. [Google Scholar] [CrossRef]
- Kleino, A.; Valanne, S.; Ulvila, J.; Kallio, J.; Myllymäki, H.; Enwald, H.; Stöven, S.; Poidevin, M.; Ueda, R.; Hultmark, D.; et al. Inhibitor of apoptosis 2 and TAK1-binding protein are components of the Drosophila Imd pathway. EMBO J. 2005, 24, 3423–3434. [Google Scholar] [CrossRef]
- Zhuang, Z.-H.; Sun, L.; Kong, L.; Hu, J.-H.; Yu, M.-C.; Reinach, P.; Zang, J.-W.; Ge, B.-X. Drosophila TAB2 is required for the immune activation of JNK and NF-kappaB. Cell. Signal. 2006, 18, 964–970. [Google Scholar] [CrossRef]
- Gesellchen, V.; Kuttenkeuler, D.; Steckel, M.; Pelte, N.; Boutros, M. An RNA interference screen identifies Inhibitor of Apoptosis Protein 2 as a regulator of innate immune signalling in Drosophila. EMBO Rep. 2005, 6, 979–984. [Google Scholar] [CrossRef]
- Valanne, S.; Kleino, A.; Myllymäki, H.; Vuoristo, J.; Rämet, M. Iap2 is required for a sustained response in the Drosophila Imd pathway. Dev. Comp. Immunol. 2007, 31, 991–1001. [Google Scholar] [CrossRef]
- Kleino, A.; Myllymäki, H.; Kallio, J.; Vanha-aho, L.-M.; Oksanen, K.; Ulvila, J.; Hultmark, D.; Valanne, S.; Rämet, M. Pirk is a negative regulator of the Drosophila Imd pathway. J. Immunol. 2008, 180, 5413–5422. [Google Scholar]
- Cheng, T.-C.; Zhang, Y.-L.; Liu, C.; Xu, P.-Z.; Gao, Z.-H.; Xia, Q.-Y.; Xiang, Z.-H. Identification and analysis of Toll-related genes in the domesticated silkworm, Bombyx mori. Dev. Comp. Immunol. 2008, 32, 464–475. [Google Scholar] [CrossRef]
- Park, J.-A.; Kim, Y. Eicosanoid biosynthesis is activated via Toll, but not Imd signal pathway in response to fungal infection. J. Invertebr. Pathol. 2012, 110, 382–388. [Google Scholar] [CrossRef]
- Johnson, J.A.; Bitra, K.; Zhang, S.; Wang, L.; Lynn, D.E.; Strand, M.R. The UGA-CiE1 cell line from Chrysodeixis includens exhibits characteristics of granulocytes and is permissive to infection by two viruses. Insect Biochem. Mol. Biol. 2010, 40, 394–404. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Casanova-Torres, Á.M.; Goodrich-Blair, H. Immune Signaling and Antimicrobial Peptide Expression in Lepidoptera. Insects 2013, 4, 320-338. https://doi.org/10.3390/insects4030320
Casanova-Torres ÁM, Goodrich-Blair H. Immune Signaling and Antimicrobial Peptide Expression in Lepidoptera. Insects. 2013; 4(3):320-338. https://doi.org/10.3390/insects4030320
Chicago/Turabian StyleCasanova-Torres, Ángel M., and Heidi Goodrich-Blair. 2013. "Immune Signaling and Antimicrobial Peptide Expression in Lepidoptera" Insects 4, no. 3: 320-338. https://doi.org/10.3390/insects4030320
APA StyleCasanova-Torres, Á. M., & Goodrich-Blair, H. (2013). Immune Signaling and Antimicrobial Peptide Expression in Lepidoptera. Insects, 4(3), 320-338. https://doi.org/10.3390/insects4030320




