The Courtship Behavior and the Ultrastructure of Sex Pheromone Glands in the Hind Tibiae of Male Ghost Moth Endoclita davidi (Lepidoptera: Hepialidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Source and Rearing Conditions
2.2. Observation of Courtship Behavior
2.3. The SEM Sample Preparation
2.4. Paraffin Histological Sections
2.5. TEM Sample Preparation
3. Results
3.1. The Courtship Behavior of Endoclita davidi
3.2. SEM of the Hind Tibiae and Hairpencils of the Male E. davidi
3.3. The Tissue Structure of Sex Pheromone Glands in the Hind Tibia of the Male E. davidi
3.4. The Ultrastructures of Sex Pheromone Glands in the Hind Tibia of the Male E. davidi
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, H.F.; Wang, L.Y. On the stem-borers of Chinese Heplalids (Lepidoptera:Hepialidae). Acta Entomol. Sin. 1985, 293–301+357. [Google Scholar] [CrossRef]
- Wen, T.C.; Zhu, R.C.; Kang, J.C.; Huang, M.H.; Tan, D.B.; Ariyawansha, H.; Hyde, K.D.; Liu, H. Ophiocordyceps xuefengensis sp. nov. from Larvae of Phassus nodus (Hepialidae) in Hunan Province, Southern China. Phytotaxa 2013, 123, 41. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Zhou, Q. Reproductive Behavior Rhythm of Endoclita davidi (Lepidoptera: Hepialidae), an Host Insect of Ophiocordyceps xuefengensis. J. Environ. Entomol. 2021, 43, 1273–1279. [Google Scholar] [CrossRef]
- Fung, S.Y.; Cheong, P.C.H.; Tan, N.H.; Ng, S.T.; Tan, C.S. Nutrient and Chemical Analysis of Fruiting Bodies of a Cultivar of the Chinese Caterpillar Mushroom, Ophiocordyceps sinensis (Ascomycetes). Int. J. Med. Mushrooms 2018, 20, 459–469. [Google Scholar] [CrossRef]
- Lu, Z.H.; Chen, S.J. Review on the practice and perspective of the industrial production of Ophiocordyceps sinensis. J. Environ. Entomol. 2016, 38, 24–30. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.H.; Zhang, S.B.; Wang, X.L. Preliminary Study on Production and Antimicrobial Activity of Triterpenoidsfrom Xuefeng Congcao(Ophiocordyceps xuefengensis) by Liquid Cell Culture. China TCM Sci. Technol. 2020, 27, 34–37. [Google Scholar]
- Chen, S.; Zhou, Q.; Li, G. DNA barcoding of various developmental stages of Endoclita davidi (Lepidoptera: Hepialidae) based on mtDNA COI gene sequence. Acta Entomol. Sin. 2017, 60, 681–690. [Google Scholar] [CrossRef]
- Li, G.; Zhou, Q.; Chen, S. SEM observation of antennal sensilla of adult Endoclita davidi, one host of Ophiocordyceps xuefengensis. J. Chin. Electron Microsc. Soc. 2017, 36, 63–70. [Google Scholar] [CrossRef]
- Tang, H.M.; Fan, Z.J.; Zhou, Q. Analysis and evaluation of nutrient components in wild and cultured Endoclita davidi larvae. J. Nat. Sci. Hunan Norm. Univ. 2020, 43, 30–34. [Google Scholar]
- Sánchez-Aros, L.; Queiroz, A.F.O.; Guajardo, J.; Barros-Parada, W.; Svensson, G.P.; Bergmann, J. Characterization of a Novel Male Pheromone Compound in Leucoptera sinuella (Lepidoptera: Lyonetiidae) and Its Role in Courtship Behavior. Insects 2025, 16, 32. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Abbas, S.; Abbas, A.; Abbas, M.; Hafeez, F.; Shakeel, M.; Xiao, F.; Zhao, C.R. Emerging Trends in Insect Sex Pheromones and Traps for Sustainable Management of Key Agricultural Pests in Asia: Beyond Insecticides—A Comprehensive Review. Int. J. Trop. Insect Sci. 2023, 43, 1867–1882. [Google Scholar] [CrossRef]
- Yang, J.C.; Zhang, J.P.; Wu, C.Y.; Bai, Y.; Guedes, R.N.C.; Dewer, Y.; Li, F.-Q.; Zang, L.-S. Diversity and Role of Volatile Terpene and Terpenoid Pheromones in Insects. J. Econ. Entomol. 2025, 118, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, R.N.; Shorey, H.H.; Gaston, L.K. Sex Pheromones of Noctuid Moths. X. The Morphology and Histology of the Female Sex Pheromone Gland of Trichoplusia ni (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 1966, 59, 1166–1169. [Google Scholar] [CrossRef]
- Raina, A.K.; Wergin, W.P.; Murphy, C.A.; Erbe, E.F. Structural Organization of the Sex Pheromone Gland in Helicoverpa zea in Relation to Pheromone Production and Release. Arthropod Struct. Dev. 2000, 29, 343–353. [Google Scholar] [CrossRef]
- Weatherston, J.; Percy, J.E. Studies of Physiologically Active Arthropod Secretions: I Evidence for a Sex Pheromone in Female. Can. Entomol. 1968, 100, 1065–1070. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Ren, S.; Chen, D. A Study on the Sex Pheromone-Producing Gland of the Cotton Bollworm Helicoverpa armigera Hübner (Lepidoptera: Noctuidae). Acta Entomol. Sin. 1995, 38, 184–187. [Google Scholar] [CrossRef]
- Liu, M.; Yang, M.F.; Xu, S.Y.; Yao, S.L. Calling Behavior of Adult Algedonia coclesalis (Lepidoptera: Pyralidae) and the Ultrastructure of the Sex Pheromone-Producing Glands in Its Female Adults. Acta Entomol. Sin. 2014, 57, 879–888. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, M.Y. Location and ultrastructural of the sex pheromone glands in Conogethes punctiferalis Guenée. Acta Entomol. Sin. 1990, 254–255. [Google Scholar] [CrossRef]
- Zhang, S.G.; Zhang, Y.H.; Chen, D.M. The morphology and structure of the sex pheromone glands of Tryporyza intaca Snellen and Chilo infuscatellus Snellen. Acta Entomol. Sin. 1989, 80–82+131–132. [Google Scholar] [CrossRef]
- Ostaff, D.P.; Shepherd, R.F.; Borden, J.H. Sex Attraction and Courtship Behavior in Lambdina Fiscellaria lugubrosa (Lepidoptera: Geometridae). Can. Entomol. 1974, 106, 493–501. [Google Scholar] [CrossRef]
- Ren, Z.L.; Zhao, G.; Xv, J.H.; Zhu, H.Q. Biological studies on the sex pheromone of the sophora geometrid Semiothisa cinerearia Bremer et Grey. Acta Entomol. Sin. 1991, 34, 271–277. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, X.Q.; Wu, G.Y.; Jiang, S.H. Observation for Sex-pheromone Gland Ultrastructure of Litchi Fruit Borer, Conopomor pha sinensis. Fujian J. Agric. Sci. 2013, 28, 490–493. [Google Scholar] [CrossRef]
- Hu, W.J.; Chen, W.L.; Wei, W. Comparative studies on ultrastructure of sex pheromone gland in female Scopula subpunctaria at different developmental stages. Chin. J. Appl. Entomol. 2011, 48, 1786–1791. [Google Scholar]
- Shen, F.; Yang, Y.X.; Shu, C. Mating behavior and structure of secretory body of sex pheromone of Deqin chost moth (Hepialus deqinensis). Southwest China J. Agric. Sci. 1991, 90–93. [Google Scholar] [CrossRef]
- Zhao, B.G. Studies on the Glands and the Release Mechanism of the Female Sex Pheromone in Clania Variegata Snell. J. Nanjing For. Univ. Sci. Ed. 1985, 9, 119–124. [Google Scholar] [CrossRef]
- Wojtusiak, J. A New Type of Scent Organ in Lepidoptera: A Giant Thoracic Pheromone-Disseminating Structure in a Male Incurvarioid Moth (Lepidoptera, Adelidae). J. Nat. Hist. 1999, 33, 819–824. [Google Scholar] [CrossRef]
- Grant, G.G. Morphology of the Presumed Male Pheromone Glands on the Forewings of Tortricid1 and Phycitid2 Moths. Ann. Entomol. Soc. Am. 1978, 71, 423–431. [Google Scholar] [CrossRef]
- Phelan, P.L.; Silk, P.J.; Northcott, C.J.; Tan, S.H.; Baker, T.C. Chemical Identification and Behavioral Characterization of Male Wing Pheromone of Ephestia elutella (Pyralidae). J. Chem. Ecol. 1986, 12, 135–146. [Google Scholar] [CrossRef]
- Baker, T.C.; Cardé, R.T. Courtship Behavior of the Oriental Fruit Moth (Grapholitha molesta): Experimental Analysis and Consideration of the Role of Sexual Selection in the Evolution of Courtship Pheromones in the Lepidoptera 2. Ann. Entomol. Soc. Am. 1979, 72, 173–188. [Google Scholar] [CrossRef]
- Choi, M.Y.; Ahn, S.J.; Park, K.C.; Meer, R.V.; Cardé, R.T.C.; Jurenka, R. Tarsi of Male Heliothine Moths Contain Aldehydes and Butyrate Esters as Potential Pheromone Components. J. Chem. Ecol. 2016, 42, 425–432. [Google Scholar] [CrossRef]
- Mallet, J. Sex Roles in the Ghost Moth Hepialus humuli (L.) and a Review of Mating in the Hepialidae (Lepidoptera). Zool. J. Linn. Soc. 1984, 80, 67–82. [Google Scholar] [CrossRef]
- Schulz, S.; Francke, W.; König, W.A.; Schurig, V.; Mori, K.; Kittmann, R.; Schneider, D. Male Pheromone of Swift Moth, Hepialus hecta L. (Lepidoptera: Hepialidae). J. Chem. Ecol. 1990, 16, 3511–3521. [Google Scholar] [CrossRef]
- Chen, X.; Su, X.; Qiu, Z.; Xu, Y.; Yang, Z.; Hu, P. Courtship and Mating Behavior of Endoclita signifer (Hepialidae: Lepidoptera) and the Male Sex Pheromones in Hairbrushes. J. Econ. Entomol. 2024, 117, 218–229. [Google Scholar] [CrossRef]
- Sower, L.L.; Shorey, H.H.; Gaston, L.K. Sex Pheromones of Noctuid Moths. XXI. Light: Dark Cycle Regulation and Light Inhibition of Sex Pheromone Release by Females of Trichoplusia Ni. Ann. Entomol. Soc. Am. 1970, 63, 1090–1092. [Google Scholar] [CrossRef] [PubMed]
- Levi-Zada, A.; Byers, J.A. Circadian Rhythms of Insect Pheromone Titer, Calling, Emission, and Response: A Review. Sci. Nat. 2021, 108, 35. [Google Scholar] [CrossRef] [PubMed]
- van Geffen, K.G.; van Eck, E.; de Boer, R.A.; van Grunsven, R.H.A.; Salis, L.; Berendse, F.; Veenendaal, E.M. Artificial Light at Night Inhibits Mating in a Geometrid Moth. Insect Conserv. Divers. 2015, 8, 282–287. [Google Scholar] [CrossRef]
- Wang, D.; Yang, G.; Chen, W. Diel and Circadian Patterns of Locomotor Activity in the Adults of Diamondback Moth (Plutella xylostella). Insects 2021, 12, 727. [Google Scholar] [CrossRef]
- Turner, J.R.G. The Dawn Flight of the Gold Swift Hepialus Hecta: Predator Avoidance and the Integration of Complex Lek Behaviour (Lepidoptera, Hepialidae). Biol. J. Linn. Soc. 2013, 110, 305–319. [Google Scholar] [CrossRef]
- Kan, E.; Kitajima, H.; Hidaka, T.; Nakashima, T.; Sato, T. Dusk Mating Flight in the Swift Moth, Endoclita excrescens (Butler) (Lepidoptera: Hepialidae). Appl. Entomol. Zool. 2002, 37, 147–153. [Google Scholar] [CrossRef]
- Birch, M.C.; Poppy, G.M.; Baker, T.C. Scents and Eversible Scent Structures of Male Moths. Annu. Rev. Entomol. 1990, 35, 25–54. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Hu, P. Morphological and Histological Observations on the Hair Brush of Endoclita vietnamensis (Lepidoptera, Hepialidae). Microsc. Res. Tech. 2024, 87, 3064–3072. [Google Scholar] [CrossRef]
- Andersson, S.; Rydell, J.; Svensson, M.G.E. Light, Predation and the Lekking Behaviour of the Ghost Swift Hepialus humuli (L.) (Lepidoptera, Hepialidae). Proc. R. Soc. Lond. B Biol. Sci. 1998, 265, 1345–1351. [Google Scholar] [CrossRef]
- Percy-Cunningham, J.E.E.; Macdonald, J.A. Biology and Ultrastructure of Sex Pheromone–Producing Glands. In Pheromone Biochemistry; Academic Press: Cambridge, MA, USA, 1987; pp. 27–75. [Google Scholar]
- Yang, M.H.; Zhang, J.T.; Fan, L.H.; Liu, H.X.; Luo, Y.Q.; Zong, S.X.; Cao, C.J. Ultrastructural observation of the sex pheromone communication system in Holcocerus vicarius (Walker) (Lepidoptera:Cossidae). Acta Entomol. Sin. 2011, 54, 522–530. [Google Scholar] [CrossRef]
- Hillier, N.K.; Vickers, N.J. The Role of Heliothine Hairpencil Compounds in Female Heliothis virescens (Lepidoptera: Noctuidae) Behavior and Mate Acceptance. Chem. Senses 2004, 29, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.R.S.; Schal, C.; Cardé, R.T. Sex Pheromone Gland of the Female Tiger Moth Holomelina lamae (Lepidoptera: Arctiidae). Can. J. Zool. 1991, 69, 1916–1921. [Google Scholar] [CrossRef]
- MacFarlane, J.H.; Earle, N.W. Morphology and Histology of the Female Sex Pheromone Gland of the Salt-Marsh Caterpillar, Estigmene acrea. Ann. Entomol. Soc. Am. 1970, 63, 1327–1332. [Google Scholar] [CrossRef]
- Kuenen, L.P.S.; Wagner, D.L.; Wallner, W.E.; Cardé, R.T. Female Sex Pheromone in Korscheltellus gracilis (Grote) (Lepidoptera: Hepialidae). Can. Entomol. 1994, 126, 31–41. [Google Scholar] [CrossRef]
- Allan, R.A.; Wang, Q. Mating Behaviour, and Evidence for a Female-released Sex Pheromone, in Wiseana copularis (Meyrick) (Lepidoptera: Hepialidae). N. Z. J. Zool. 2001, 28, 257–262. [Google Scholar] [CrossRef]
- Meinwald, J.; Meinwald, Y.C.; Wheeler, J.W.; Eisner, T.; Brower, L.P. Major Components in the Exocrine Secretion of a Male Butterfly (Lycorea). Science 1966, 151, 583–585. [Google Scholar] [CrossRef]
- Roscoe, L.E.; Silk, P.; Eveleigh, E.S. Evidence of Male Hair Pencil Pheromone in Choristoneura fumiferana (Lepidoptera: Tortricidae). J. Insect Sci. 2016, 16, 27. [Google Scholar] [CrossRef]
- Gnatzy, W.; Fischer, O.W.; Kiesel, A.; Vane-Wright, R.I.; Boppré, M. Diverticula in Male Lycorea halia Butterflies (Lepidoptera: Nymphalidae: Danaini: Itunina)—Support Organs for Everted Hairpencils with Unique Ultrastructure. Neotrop. Entomol. 2020, 49, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Baker, T.C.; Nishida, R.; Roelofs, W.L. Close-Range Attraction of Female Oriental Fruit Moths to Herbal Scent of Male Hairpencils. Science 1981, 214, 1359–1361. [Google Scholar] [CrossRef]
- Hirai, K. Directional Flow of Male Scent Released by Pseudaletia separata Walker (Lepidoptera: Noctuidae) and Its Repellent Effect on Adults and Larvae of Four Noctuid and One Phycitine Moth. J. Chem. Ecol. 1982, 8, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.T.; Han, Y.; Gan, Y.L.; Meng, X.Z. Location and histology of the sex pheromone-producing gland in Holcocerus insularis. Acta Entomol. Sin. 2002, 45, 430–435. [Google Scholar] [CrossRef]

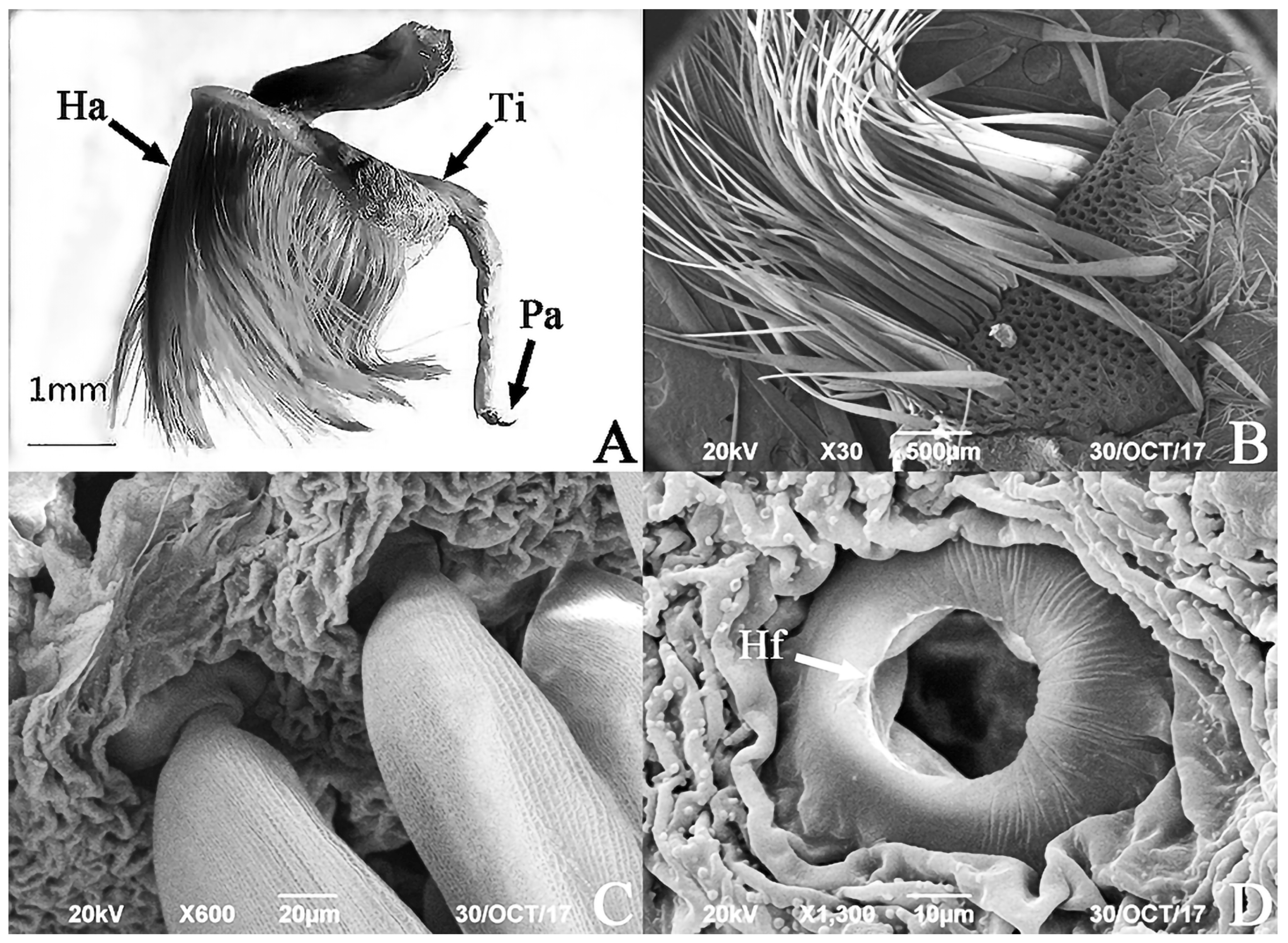
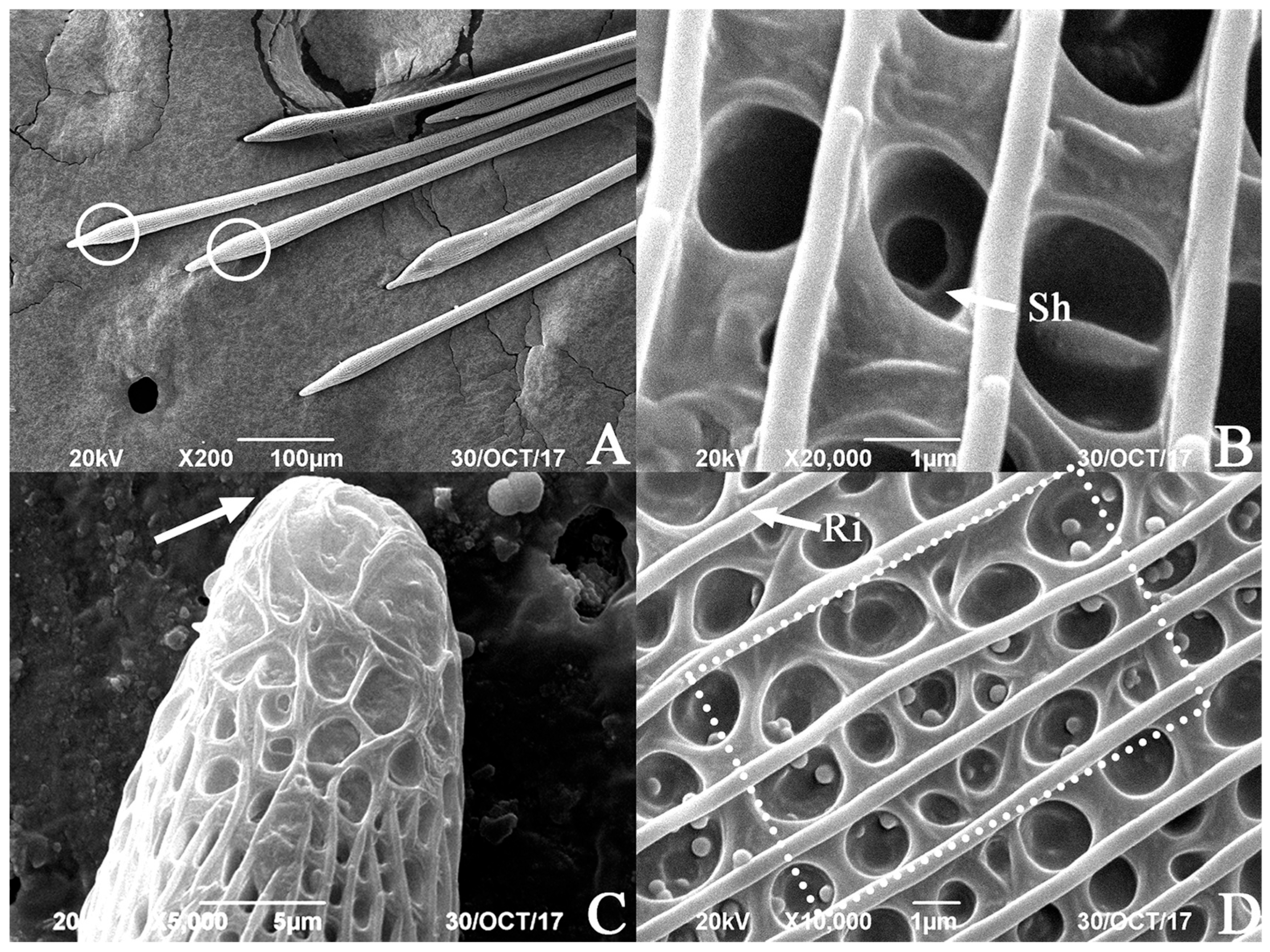
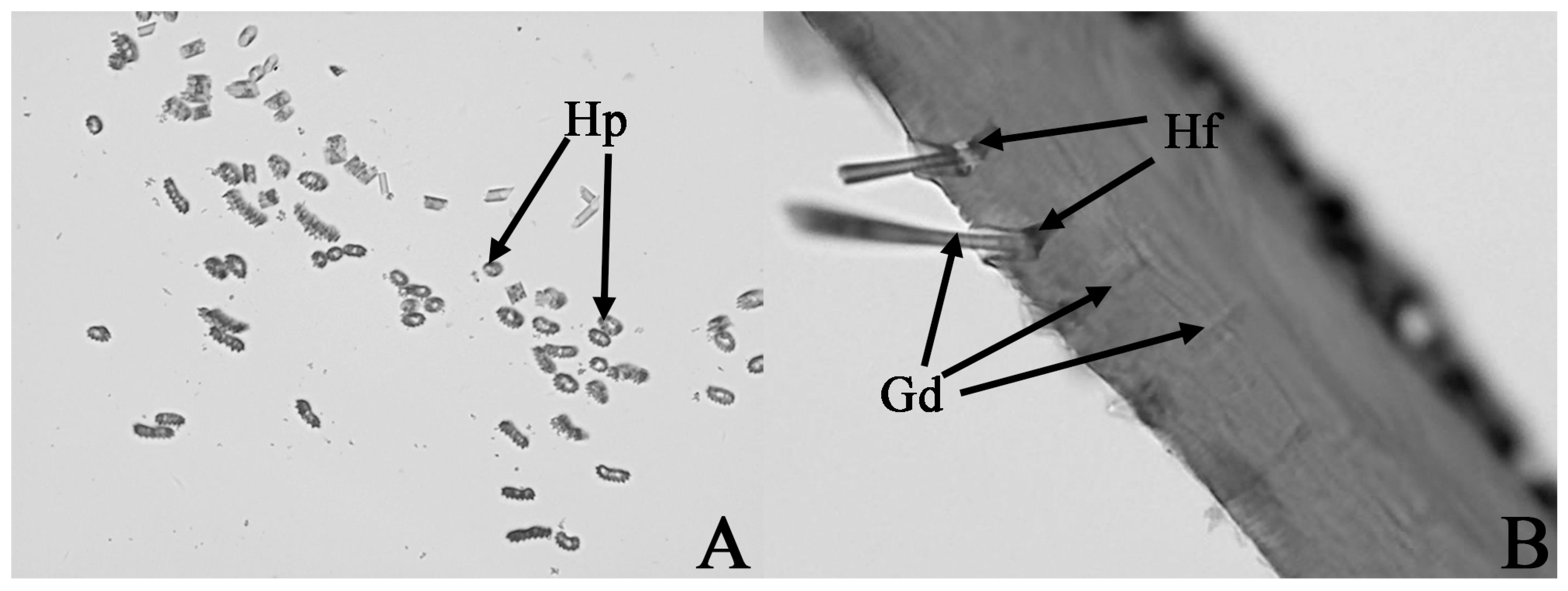

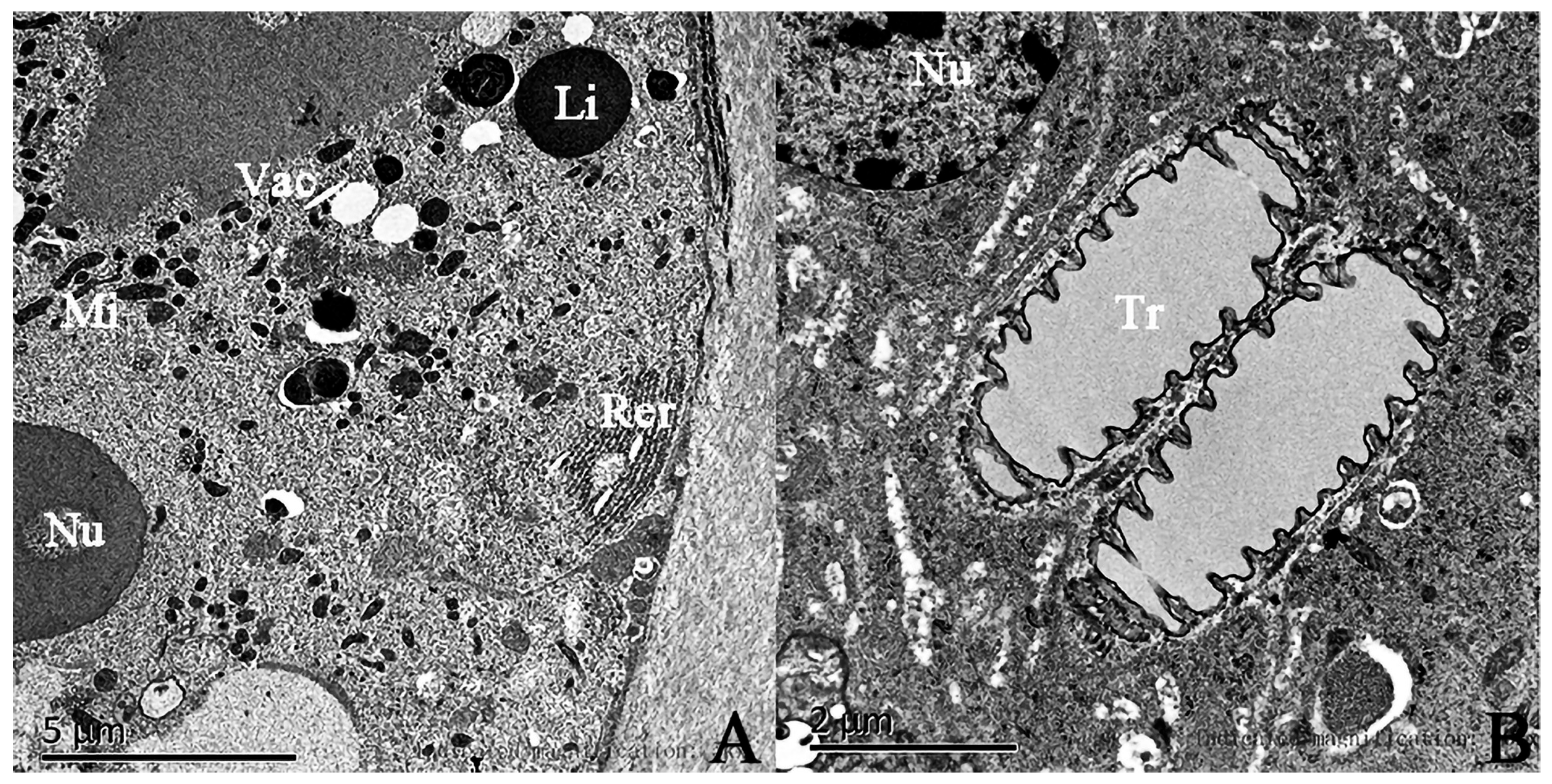
| Time | The Behavior of Male Adult E. davidi | The Behavior of Female Adult E. davidi |
|---|---|---|
| Within 5 min of entering the scotophase | The head of the hanging male moth begins to rush up and down, with its antennae gradually lifting from flat and close to its head to a more upright position. It starts flapping its wings, but the angle of wing-flapping is small (about 45°), indicating an excited state. The scent brush on the hind tibia (as indicated by the arrow in Figure 1A) has not yet been deployed. | From its resting state hanging on the top of the mesh cage, the female moth transitions into a position where its wings are slightly outstretched to the sides, forming an “eight” shape, without vibrating its wings. The abdomen slightly bends and swings both forward and backward, albeit at a small angle. |
| As the time goes on | Subsequently, the angle of the wings opening widens, and the flapping frequency increases, mimicking a flying posture. The male moth climbs towards the female on the cage top, and it can be observed that the scent brush on the hind tibia gradually unfolds and continuously vibrates. Meanwhile, the abdomen continuously draws circles or swings left and right, exhibiting a state of high excitement (Figure 1B). | The wings gradually begin to open at a small angle and vibrate as it climbs and approaches the male moth. |
| The scotophase lasts about 20 min | The male moth approaches the female, with its scent brush still unfolded, and its abdomen continues to swing left and right, adopting a mating posture. Simultaneously, it flaps its wings and uses its hind legs at a high frequency, displaying an extreme state of excitement (Figure 1C). | The female moth’s body begins to twist constantly, mainly manifested by intense bending and swinging of the abdominal tip in all directions. At the same time, she starts to flap her wings rapidly and intermittently at a high frequency. In two observed cases, before mating, the female moth ejected a stream of liquid from the abdominal tip, containing a few eggs. |
| The end of the calling | Both the male and female moths enter a stage of excitement and mutual approach, characterized by high-frequency wing flapping and abdominal twisting as they attempt to bring their abdominal tips into contact with each other (Figure 1D). At this point, the male moth approaches the female and hooks its legs onto the ventral side of the female’s chest. When the abdominal tips of the male and female moths come into contact, they enter the mating state (Figure 1E). | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Huang, X.; Chen, S.; Li, X.; Zhou, Z.; Zhou, Q. The Courtship Behavior and the Ultrastructure of Sex Pheromone Glands in the Hind Tibiae of Male Ghost Moth Endoclita davidi (Lepidoptera: Hepialidae). Insects 2026, 17, 45. https://doi.org/10.3390/insects17010045
Huang X, Chen S, Li X, Zhou Z, Zhou Q. The Courtship Behavior and the Ultrastructure of Sex Pheromone Glands in the Hind Tibiae of Male Ghost Moth Endoclita davidi (Lepidoptera: Hepialidae). Insects. 2026; 17(1):45. https://doi.org/10.3390/insects17010045
Chicago/Turabian StyleHuang, Xingrui, Shan Chen, Xing Li, Zihao Zhou, and Qiong Zhou. 2026. "The Courtship Behavior and the Ultrastructure of Sex Pheromone Glands in the Hind Tibiae of Male Ghost Moth Endoclita davidi (Lepidoptera: Hepialidae)" Insects 17, no. 1: 45. https://doi.org/10.3390/insects17010045
APA StyleHuang, X., Chen, S., Li, X., Zhou, Z., & Zhou, Q. (2026). The Courtship Behavior and the Ultrastructure of Sex Pheromone Glands in the Hind Tibiae of Male Ghost Moth Endoclita davidi (Lepidoptera: Hepialidae). Insects, 17(1), 45. https://doi.org/10.3390/insects17010045






