Sublethal Effects of Neonicotinoids: How Physiological and Behavioral Disruptions in Non-Target Insects Threaten Biodiversity and Ecosystem Services
Simple Summary
Abstract
1. Introduction
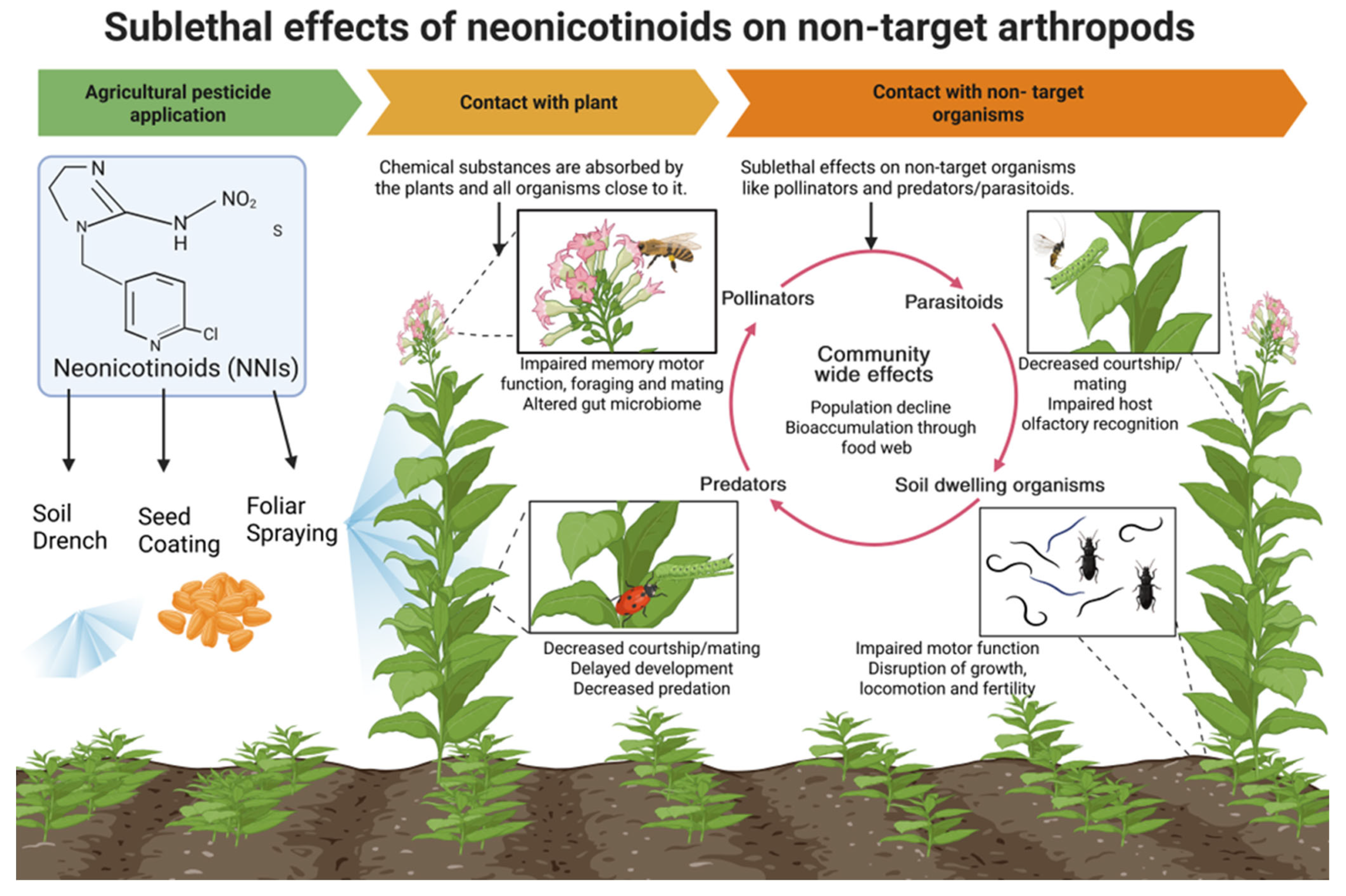
2. How Do Neonicotinoids Reach Non-Target Organisms
3. Physiological Effects
4. Behavioral Effects
5. Reproductive Effects
6. Community-Wide Effects
7. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seifert, J. Neonicotinoids. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2005; pp. 196–200. [Google Scholar] [CrossRef]
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the Status and Global Strategy for Neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908. [Google Scholar] [CrossRef]
- Goulson, D. REVIEW: An Overview of the Environmental Risks Posed by Neonicotinoid Insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. Systemic Insecticides (Neonicotinoids and Fipronil): Trends, Uses, Mode of Action and Metabolites. Environ. Sci. Pollut. Res. 2015, 22, 5–34. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.J.; Goulson, D. The Environmental Risks of Neonicotinoid Pesticides: A Review of the Evidence Post 2013. Environ. Sci. Pollut. Res. 2017, 24, 17285–17325. [Google Scholar] [CrossRef] [PubMed]
- Todey, S.A.; Fallon, A.M.; Arnold, W.A. Neonicotinoid Insecticide Hydrolysis and Photolysis: Rates and Residual Toxicity. Environ. Toxicol. Chem. 2018, 37, 2797–2809. [Google Scholar] [CrossRef]
- Elbert, A.; Haas, M.; Springer, B.; Thielert, W.; Nauen, R. Applied Aspects of Neonicotinoid Uses in Crop Protection. Pest Manag. Sci. 2008, 64, 1099–1105. [Google Scholar] [CrossRef]
- Main, A.R.; Webb, E.B.; Goyne, K.W.; Mengel, D. Neonicotinoid Insecticides Negatively Affect Performance Measures of Non-target Terrestrial Arthropods: A Meta-analysis. Ecol. Appl. 2018, 28, 1232–1244. [Google Scholar] [CrossRef]
- Hladik, M.L.; Main, A.R.; Goulson, D. Environmental Risks and Challenges Associated with Neonicotinoid Insecticides. Environ. Sci. Technol. 2018, 52, 3329–3335. [Google Scholar] [CrossRef]
- Malhotra, N.; Chen, K.H.C.; Huang, J.C.; Lai, H.T.; Uapipatanakul, B.; Roldan, M.J.M.; Macabeo, A.P.G.; Ger, T.R.; Hsiao, C.D. Physiological Effects of Neonicotinoid Insecticides on Non-Target Aquatic Animals—An Updated Review. Int. J. Mol. Sci. 2021, 22, 9591. [Google Scholar] [CrossRef]
- de França, S.M.; Breda, M.O.; Barbosa, D.R.S.; Araujo, A.M.N.; Guedes, C.A.; de França, S.M.; Breda, M.O.; Barbosa, D.R.S.; Araujo, A.M.N.; Guedes, C.A. The Sublethal Effects of Insecticides in Insects. In Biological Control of Pest and Vector Insects; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Van Der Sluijs, J.P.; Amaral-Rogers, V.; Belzunces, L.P.; Bijleveld Van Lexmond, M.F.; Bonmatin, J.M.; Chagnon, M.; Downs, C.A.; Furlan, L.; Gibbons, D.W.; Giorio, C.; et al. Conclusions of the Worldwide Integrated Assessment on the Risks of Neonicotinoids and Fipronil to Biodiversity and Ecosystem Functioning. Environ. Sci. Pollut. Res. 2015, 22, 148–154. [Google Scholar] [CrossRef]
- Losey, J.E.; Vaughn, M. The Economic Value of Ecological Services Provided by Insects. Bioscience 2006, 56, 311–323. [Google Scholar] [CrossRef]
- Ejomah, A. Effects of Neonicotinoids Through Food Web in Agroecosystem and Adjacent Habitats. 2025. Available online: https://BioRender.com/ol4lp4z (accessed on 18 December 2025).
- Williamson, S.M.; Willis, S.J.; Wright, G.A. Exposure to Neonicotinoids Influences the Motor Function of Adult Worker Honeybees. Ecotoxicology 2014, 23, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Colin, M.E.; Bonmatin, J.M.; Moineau, I.; Gaimon, C.; Brun, S.; Vermandere, J.P. A Method to Quantify and Analyze the Foraging Activity of Honey Bees: Relevance to the Sublethal Effects Induced by Systemic Insecticides. Arch. Environ. Contam. Toxicol. 2004, 47, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Christen, V.; Grossar, D.; Charrière, J.D.; Eyer, M.; Jeker, L. Correlation between Increased Homing Flight Duration and Altered Gene Expression in the Brain of Honey Bee Foragers after Acute Oral Exposure to Thiacloprid and Thiamethoxam. Front. Insect Sci. 2021, 1, 765570. [Google Scholar] [CrossRef] [PubMed]
- Hesselbach, H.; Scheiner, R. The Novel Pesticide Flupyradifurone (Sivanto) Affects Honeybee Motor Abilities. Ecotoxicology 2019, 28, 354–366. [Google Scholar] [CrossRef]
- Lambin, M.; Armengaud, C.; Raymond, S.; Gauthier, M. Imidacloprid-Induced Facilitation of the Proboscis Extension Reflex Habituation in the Honeybee. Arch. Insect Biochem. Physiol. 2001, 48, 129–134. [Google Scholar] [CrossRef]
- Medrzycki, P.; Montanari, R.; Bortolotti, L.; Sabatini, A.G.; Maini, S.; Porrini, C. Effects of Imidacloprid Administered in Sub-Lethal Doses on Honey Bee Behaviour. Lab. Tests. Bull. Insectol. 2003, 56, 59–62. [Google Scholar]
- Suchail, S.; Guez, D.; Belzunces, L.P. Discrepancy between Acute and Chronic Toxicity Induced by Imidacloprid and Its Metabolites in Apis Mellifera. Environ. Toxicol. Chem. 2001, 20, 2482–2486. [Google Scholar] [CrossRef]
- Tosi, S.; Nieh, J.C. A Common Neonicotinoid Pesticide, Thiamethoxam, Alters Honey Bee Activity, Motor Functions, and Movement to Light. Sci. Rep. 2017, 7, 15132. [Google Scholar] [CrossRef]
- Kenna, D.; Cooley, H.; Pretelli, I.; Ramos Rodrigues, A.; Gill, S.D.; Gill, R.J. Pesticide Exposure Affects Flight Dynamics and Reduces Flight Endurance in Bumblebees. Ecol. Evol. 2019, 9, 5637–5650. [Google Scholar] [CrossRef]
- Sargent, C.; Ebanks, B.; Hardy, I.C.W.; Davies, T.G.E.; Chakrabarti, L.; Stöger, R. Acute Imidacloprid Exposure Alters Mitochondrial Function in Bumblebee Flight Muscle and Brain. Front. Insect Sci. 2021, 1, 765179. [Google Scholar] [CrossRef] [PubMed]
- Crall, J.D.; Switzer, C.M.; Oppenheimer, R.L.; Ford Versypt, A.N.; Dey, B.; Brown, A.; Eyster, M.; Guérin, C.; Pierce, N.E.; Combes, S.A.; et al. Neonicotinoid Exposure Disrupts Bumblebee Nest Behavior, Social Networks, and Thermoregulation. Science 2018, 362, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Motta, J.V.d.O.; Gomes, D.S.; da Silva, L.L.; de Oliveira, M.S.; Bastos, D.S.S.; Resende, M.T.C.S.; Alvim, J.R.L.; Reis, A.B.; de Oliveira, L.L.; Afzal, M.B.S.; et al. Effects of Sublethal Concentration of Thiamethoxam Formulation on the Wild Stingless Bee, Partamona helleri Friese (Hymenoptera: Apidae): Histopathology, Oxidative Stress and Behavioral Changes. Sci. Total Environ. 2024, 957, 177626. [Google Scholar] [CrossRef] [PubMed]
- Crispim, P.D.; de Oliveira, V.E.S.; Batista, N.R.; Nocelli, R.C.F.; Antonialli-Junior, W.F. Lethal and Sublethal Dose of Thiamethoxam and Its Effects on the Behavior of a Non-Target Social Wasp. Neotrop. Entomol. 2023, 52, 422–430. [Google Scholar] [CrossRef]
- Jacob, C.R.d.O.; Zanardi, O.Z.; Malaquias, J.B.; Souza Silva, C.A.; Yamamoto, P.T. The Impact of Four Widely Used Neonicotinoid Insecticides on Tetragonisca angustula (Latreille) (Hymenoptera: Apidae). Chemosphere 2019, 224, 65–70. [Google Scholar] [CrossRef]
- Penn, H.J.; Dale, A.M. Imidacloprid Seed Treatments Affect Individual Ant Behavior and Community Structure but Not Egg Predation, Pest Abundance or Soybean Yield. Pest Manag. Sci. 2017, 73, 1625–1632. [Google Scholar] [CrossRef]
- Kunkel, B.A.; Held, D.W.; Potter, D.A. Lethal and Sublethal Effects of Bendiocarb, Halofenozide, and Imidacloprid on Harpalus Pennsylvanicus (Coleoptera: Carabidae) Following Different Modes of Exposure in Turfgrass. J. Econ. Entomol. 2001, 94, 60–67. [Google Scholar] [CrossRef]
- Cavallaro, M.C.; Hladik, M.L.; McMurry, S.S.; Hittson, S.; Boyles, L.K.; Hoback, W.W. Neonicotinoid Exposure Causes Behavioral Impairment and Delayed Mortality of the Federally Threatened American Burying Beetle, Nicrophorus Americanus. PLoS ONE 2025, 20, e0314243. [Google Scholar] [CrossRef]
- Tooming, E.; Merivee, E.; Must, A.; Merivee, M.I.; Sibul, I.; Nurme, K.; Williams, I.H. Behavioural Effects of the Neonicotinoid Insecticide Thiamethoxam on the Predatory Insect Platynus assimilis. Ecotoxicology 2017, 26, 902–913. [Google Scholar] [CrossRef]
- Hunn, J.G.; Macaulay, S.J.; Matthaei, C.D. Food Shortage Amplifies Negative Sublethal Impacts of Low-Level Exposure to the Neonicotinoid Insecticide Imidacloprid on Stream Mayfly Nymphs. Water 2019, 11, 2142. [Google Scholar] [CrossRef]
- Bradford, B.R.; Whidden, E.; Gervasio, E.D.; Checchi, P.M.; Raley-Susman, K.M. Neonicotinoid-Containing Insecticide Disruption of Growth, Locomotion, and Fertility in Caenorhabditis elegans. PLoS ONE 2020, 15, e0238637. [Google Scholar] [CrossRef]
- Catae, A.F.; Roat, T.C.; Pratavieira, M.; da Silva Menegasso, A.R.; Palma, M.S.; Malaspina, O. Exposure to a Sublethal Concentration of Imidacloprid and the Side Effects on Target and Nontarget Organs of Apis mellifera (Hymenoptera, Apidae). Ecotoxicology 2018, 27, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Christen, V.; Mittner, F.; Fent, K. Molecular Effects of Neonicotinoids in Honey Bees (Apis mellifera). Environ. Sci. Technol. 2016, 50, 4071–4081. [Google Scholar] [CrossRef] [PubMed]
- Miotelo, L.; Maloni, G.; Grella, T.C.; Nocelli, R.C.F.; Ferro, M.; Malaspina, O. Thiamethoxam-Induced Stress Responses in Melipona scutellaris: Insights into the Toxicological Effects on Malpighian Tubules. Apidologie 2025, 56, 74. [Google Scholar] [CrossRef]
- Maloni, G.; Miotelo, L.; Otero, I.V.R.; de Souza, F.C.; Nocelli, R.C.F.; Malaspina, O. Acute Toxicity and Sublethal Effects of Thiamethoxam on the Stingless Bee Scaptotrigona postica: Survival, Neural Morphology, and Enzymatic Responses. Environ. Pollut. 2025, 369, 125864. [Google Scholar] [CrossRef]
- Wei, F.; Wang, D.; Li, H.; Xia, P.; Ran, Y.; You, J. Toxicogenomics Provides Insights to Toxicity Pathways of Neonicotinoids to Aquatic Insect, Chironomus dilutus. Environ. Pollut. 2020, 260, 114011. [Google Scholar] [CrossRef]
- Decourtye, A.; Armengaud, C.; Renou, M.; Devillers, J.; Cluzeau, S.; Gauthier, M.; Pham-Delègue, M.H. Imidacloprid Impairs Memory and Brain Metabolism in the Honeybee (Apis mellifera L.). Pestic. Biochem. Physiol. 2004, 78, 83–92. [Google Scholar] [CrossRef]
- Piiroinen, S.; Goulson, D. Chronic Neonicotinoid Pesticide Exposure and Parasite Stress Differentially Affects Learning in Honeybees and Bumblebees. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160246. [Google Scholar] [CrossRef]
- Tan, K.; Chen, W.; Dong, S.; Liu, X.; Wang, Y.; Nieh, J.C. A Neonicotinoid Impairs Olfactory Learning in Asian Honey Bees (Apis cerana) Exposed as Larvae or as Adults. Sci. Rep. 2015, 5, 10989. [Google Scholar] [CrossRef]
- Smith, D.B.; Arce, A.N.; Rodrigues, A.R.; Bischoff, P.H.; Burris, D.; Ahmed, F.; Gill, R.J. Insecticide Exposure during Brood or Early-Adult Development Reduces Brain Growth and Impairs Adult Learning in Bumblebees. Proc. R. Soc. B 2020, 287, 20192442. [Google Scholar] [CrossRef]
- Muth, F.; Francis, J.S.; Leonard, A.S. Modality-Specific Impairment of Learning by a Neonicotinoid Pesticide. Biol. Lett. 2019, 15, 20190359. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, F.E.; Tibbetts, E.A. Field-Realistic Exposure to Neonicotinoid and Sulfoximine Insecticides Impairs Visual and Olfactory Learning and Memory in Polistes Paper Wasps. J. Exp. Biol. 2023, 226, 246083. [Google Scholar] [CrossRef] [PubMed]
- Wickramasingha, P.D.; Morrissey, C.A.; Phillips, I.D.; Crane, A.L.; Ferrari, M.C.O.; Chivers, D.P. Exposure to the Insecticide, Imidacloprid, Impairs Predator-Recognition Learning in Damselfly Larvae. Environ. Pollut. 2024, 342, 123085. [Google Scholar] [CrossRef]
- Tackenberg, M.C.; Giannoni-Guzmán, M.A.; Sanchez-Perez, E.; Doll, C.A.; Agosto-Rivera, J.L.; Broadie, K.; Moore, D.; McMahon, D.G. Neonicotinoids Disrupt Circadian Rhythms and Sleep in Honey Bees. Sci. Rep. 2020, 10, 17929. [Google Scholar] [CrossRef] [PubMed]
- Tasman, K.; Rands, S.A.; Hodge, J.J.L. The Neonicotinoid Insecticide Imidacloprid Disrupts Bumblebee Foraging Rhythms and Sleep. iScience 2020, 23, 101827. [Google Scholar] [CrossRef]
- Siviter, H.; Folly, A.J.; Brown, M.J.F.; Leadbeater, E. Individual and Combined Impacts of Sulfoxaflor and Nosema bombi on Bumblebee (Bombus terrestris) Larval Growth. Proc. R. Soc. B Biol. Sci. 2020, 287, 20200935. [Google Scholar] [CrossRef]
- Rosa, A.d.S.; Teixeira, J.S.G.; Vollet-Neto, A.; Queiroz, E.P.; Blochtein, B.; Pires, C.S.S.; Imperatriz-Fonseca, V.L. Consumption of the Neonicotinoid Thiamethoxam during the Larval Stage Affects the Survival and Development of the Stingless Bee, Scaptotrigona Aff. depilis. Apidologie 2016, 47, 729–738. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Z.; Yu, X.; Ma, D.; Yu, C.; Liu, F.; Mu, W. Influence of Lethal and Sublethal Exposure to Clothianidin on the Seven-Spotted Lady Beetle, Coccinella septempunctata L. (Coleoptera: Coccinellidae). Ecotoxicol. Environ. Saf. 2018, 161, 208–213. [Google Scholar] [CrossRef]
- You, Y.; Zeng, Z.; Zheng, J.; Zhao, J.; Luo, F.; Chen, Y.; Xie, M.; Liu, X.; Wei, H. The Toxicity Response of Coccinella septempunctata L. (Coleoptera: Coccinellidae) after Exposure to Sublethal Concentrations of Acetamiprid. Agriculture 2022, 12, 1642. [Google Scholar] [CrossRef]
- Su, Y.; Ren, X.; Ma, X.; Wang, D.; Hu, H.; Song, X.; Cui, J.; Ma, Y.; Yao, Y. Evaluation of the Toxicity and Sublethal Effects of Acetamiprid and Dinotefuran on the Predator Chrysopa pallens (Rambur) (Neuroptera: Chrysopidae). Toxics 2022, 10, 309. [Google Scholar] [CrossRef]
- Ray, A.; Gadratagi, B.G.; Rana, D.K.; Ullah, F.; Adak, T.; Govindharaj, G.P.P.; Patil, N.B.; Mahendiran, A.; Desneux, N.; Rath, P.C. Multigenerational Insecticide Hormesis Enhances Fitness Traits in a Key Egg Parasitoid, Trichogramma chilonis Ishii. Agronomy 2022, 12, 1392. [Google Scholar] [CrossRef]
- Rix, R.R.; Cutler, G.C. Low Doses of a Neonicotinoid Stimulate Reproduction in a Beneficial Predatory Insect. J. Econ. Entomol. 2020, 113, 2179–2186. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Gadratagi, B.G.; Budhlakoti, N.; Rana, D.K.; Adak, T.; Govindharaj, G.P.P.; Patil, N.B.; Mahendiran, A.; Rath, P.C. Functional Response of an Egg Parasitoid, Trichogramma chilonis Ishii to Sublethal Imidacloprid Exposure. Pest Manag. Sci. 2023, 79, 3656–3665. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.B.; Mannion, C.M.; Klein, M.G.; Moyseenko, J.J.; Bishop, B. Effect of Insecticides on Tiphia vernalis (Hymenoptera: Tiphiidae) Oviposition and Survival of Progeny to Cocoon Stage When Parasitizing Popillia japonica (Coleoptera: Scarabaeidae) Larvae. J. Econ. Entomol. 2005, 98, 694–703. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, P.; Yang, X.; Ruan, C.; Biondi, A.; Desneux, N.; Zang, L. Selectivity of Novel and Traditional Insecticides Used for Management of Whiteflies on the Parasitoid Encarsia formosa. Pest Manag. Sci. 2019, 75, 2716–2724. [Google Scholar] [CrossRef]
- Alberoni, D.; Favaro, R.; Baffoni, L.; Angeli, S.; Di Gioia, D. Neonicotinoids in the Agroecosystem: In-Field Long-Term Assessment on Honeybee Colony Strength and Microbiome. Sci. Total Environ. 2021, 762, 144116. [Google Scholar] [CrossRef]
- Morfin, N.; Goodwin, P.H.; Correa-Benitez, A.; Guzman-Novoa, E. Sublethal Exposure to Clothianidin during the Larval Stage Causes Long-Term Impairment of Hygienic and Foraging Behaviours of Honey Bees. Apidologie 2019, 50, 595–605. [Google Scholar] [CrossRef]
- Schneider, C.W.; Tautz, J.; Grünewald, B.; Fuchs, S. RFID Tracking of Sublethal Effects of Two Neonicotinoid Insecticides on the Foraging Behavior of Apis mellifera. PLoS ONE 2012, 7, e30023. [Google Scholar] [CrossRef]
- Tison, L.; Duer, A.; Púčiková, V.; Greggers, U.; Menzel, R. Detrimental Effects of Clothianidin on Foraging and Dance Communication in Honey Bees. PLoS ONE 2020, 15, e0241134. [Google Scholar] [CrossRef]
- Tison, L.; Hahn, M.L.; Holtz, S.; Rößner, A.; Greggers, U.; Bischoff, G.; Menzel, R. Honey Bees’ Behavior Is Impaired by Chronic Exposure to the Neonicotinoid Thiacloprid in the Field. Environ. Sci. Technol. 2016, 50, 7218–7227. [Google Scholar] [CrossRef]
- Kessler, S.C.; Tiedeken, E.J.; Simcock, K.L.; Derveau, S.; Mitchell, J.; Softley, S.; Stout, J.C.; Wright, G.A. Bees Prefer Foods Containing Neonicotinoid Pesticides. Nature 2015, 521, 74–76. [Google Scholar] [CrossRef]
- Arce, A.N.; Rodrigues, A.R.; Yu, J.; Colgan, T.J.; Wurm, Y.; Gill, R.J. Foraging Bumblebees Acquire a Preference for Neonicotinoid-Treated Food with Prolonged Exposure. Proc. R. Soc. B 2018, 285, 20180655. [Google Scholar] [CrossRef] [PubMed]
- Leza, M.; Watrous, K.M.; Bratu, J.; Woodard, S.H. Effects of Neonicotinoid Insecticide Exposure and Monofloral Diet on Nest-Founding Bumblebee Queens. Proc. R. Soc. B Biol. Sci. 2018, 285, 20180761. [Google Scholar] [CrossRef]
- Muth, F.; Leonard, A.S. A Neonicotinoid Pesticide Impairs Foraging, but Not Learning, in Free-Flying Bumblebees. Sci. Rep. 2019, 9, 4764. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.A.; Raine, N.E. Chronic Exposure to a Neonicotinoid Pesticide Alters the Interactions between Bumblebees and Wild Plants. Funct. Ecol. 2016, 30, 1132–1139. [Google Scholar] [CrossRef]
- Schöfer, N.; Ackermann, J.; Hoheneder, J.; Hofferberth, J.; Ruther, J. Sublethal Effects of Four Insecticides Targeting Cholinergic Neurons on Partner and Host Finding in the Parasitic Wasp Nasonia vitripennis. Environ. Toxicol. Chem. 2023, 42, 2400–2411. [Google Scholar] [CrossRef]
- Tappert, L.; Pokorny, T.; Hofferberth, J.; Ruther, J. Sublethal Doses of Imidacloprid Disrupt Sexual Communication and Host Finding in a Parasitoid Wasp. Sci. Rep. 2017, 7, srep42756. [Google Scholar] [CrossRef]
- Boff, S.; Friedel, A.; Mussury, R.M.; Lenis, P.R.; Raizer, J. Changes in Social Behavior Are Induced by Pesticide Ingestion in a Neotropical Stingless Bee. Ecotoxicol. Environ. Saf. 2018, 164, 548–553. [Google Scholar] [CrossRef]
- Stapel, J.O.; Cortesero, A.M.; Lewis, W.J. Disruptive Sublethal Effects of Insecticides on Biological Control: Altered Foraging Ability and Life Span of a Parasitoid after Feeding on Extrafloral Nectar of Cotton Treated with Systemic Insecticides. Biol. Control 2000, 17, 243–249. [Google Scholar] [CrossRef]
- Palmer, M.J.; Moffat, C.; Saranzewa, N.; Harvey, J.; Wright, G.A.; Connolly, C.N. Cholinergic Pesticides Cause Mushroom Body Neuronal Inactivation in Honeybees. Nat. Commun. 2013, 4, 1634. [Google Scholar] [CrossRef]
- Favaro, R.; Roved, J.; Haase, A.; Angeli, S. Impact of Chronic Exposure to Two Neonicotinoids on Honey Bee Antennal Responses to Flower Volatiles and Pheromonal Compounds. Front. Insect Sci. 2022, 2, 821145. [Google Scholar] [CrossRef] [PubMed]
- Thiel, S.; Köhler, H.R. A Sublethal Imidacloprid Concentration Alters Foraging and Competition Behaviour of Ants. Ecotoxicology 2016, 25, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, R.F.; Lester, P.J.; Miller, A.S.; Ryan, K.G. A Neurotoxic Pesticide Changes the Outcome of Aggressive Interactions between Native and Invasive Ants. Proc. R. Soc. B Biol. Sci. 2013, 280, 20132157. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, F.; Reitz, S.; Jalali, M.A.; Ziaaddini, M.; Izadi, H. Lethal and Sublethal Effects of Two Commercial Insecticides on Egg Parasitoids (Hymenoptera: Scelionidae) of Green Stink Bugs (Hemiptera: Pentatomidae). J. Econ. Entomol. 2021, 114, 33–39. [Google Scholar] [CrossRef]
- Ranjbar, F.; Reitz, S.; Sardary, A.E.; Jalali, M.A.; Ziaaddini, M.; Izadi, H. Assessment of Toxicity Risk of Selected Insecticides Used in Pistachio Ecosystem on Two Egg Parasitoids (Hymenoptera: Scelionidae) of Stink Bugs (Hemiptera: Pentatomidae). J. Econ. Entomol. 2021, 114, 1588–1596. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Z.; Yu, X.; Yu, C.; Liu, F.; Mu, W. Sublethal and Transgenerational Effects of Thiamethoxam on the Demographic Fitness and Predation Performance of the Seven-Spot Ladybeetle Coccinella septempunctata L. (Coleoptera: Coccinellidae). Chemosphere 2019, 216, 168–178. [Google Scholar] [CrossRef]
- Fernandes, M.E.; avia Alves, F.M.; Pereira, R.C.; Aquino, L.A.; Fernandes, F.L.; Zanuncio, J.C. Lethal and Sublethal Effects of Seven Insecticides on Three Beneficial Insects in Laboratory Assays and Field Trials. Chemosphere 2016, 156, 45–55. [Google Scholar] [CrossRef]
- Yao, F.L.; Zheng, Y.; Zhao, J.W.; Desneux, N.; He, Y.X.; Weng, Q.Y. Lethal and Sublethal Effects of Thiamethoxam on the Whitefly Predator Serangium japonicum (Coleoptera: Coccinellidae) through Different Exposure Routes. Chemosphere 2015, 128, 49–55. [Google Scholar] [CrossRef]
- He, Y.; Zhao, J.; Zheng, Y.; Desneux, N.; Wu, K. Lethal Effect of Imidacloprid on the Coccinellid Predator Serangium japonicum and Sublethal Effects on Predator Voracity and on Functional Response to the Whitefly Bemisia tabaci. Ecotoxicology 2012, 21, 1291–1300. [Google Scholar] [CrossRef]
- Zhang, L.; Lv, H.; Li, X.; Wan, H.; He, S.; Li, J.; Ma, K. Sublethal Effects of Acetamiprid and Afidopyropen on Harmonia axyridis: Insights from Transcriptomics Analysis. Ecotoxicol. Environ. Saf. 2023, 262, 115203. [Google Scholar] [CrossRef]
- Pearsons, K.A.; Tooker, J.F. Acute Toxicity of Neonicotinoid Insecticides to Ground Beetles (Coleoptera: Carabidae) from Pennsylvania. Environ. Entomol. 2025, 54, 574–584. [Google Scholar] [CrossRef]
- Martinou, A.F.; Seraphides, N.; Stavrinides, M.C. Lethal and Behavioral Effects of Pesticides on the Insect Predator Macrolophus Pygmaeus. Chemosphere 2014, 96, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.X.; Zhu, Y.; Li, J.J.; Wang, N.M.; Yu, Q.T.; Xue, C.B. Acute Lethal and Sublethal Effects of Four Insecticides on the Lacewing (Chrysoperla sinica Tjeder). Chemosphere 2020, 250, 12632110. [Google Scholar] [CrossRef] [PubMed]
- Korenko, S.; Saska, P.; Kysilková, K.; Řezáč, M.; Heneberg, P. Prey Contaminated with Neonicotinoids Induces Feeding Deterrent Behavior of a Common Farmland Spider. Sci. Rep. 2019, 9, 15895. [Google Scholar] [CrossRef] [PubMed]
- Řezáč, M.; Řezáčová, V.; Heneberg, P. Contact Application of Neonicotinoids Suppresses the Predation Rate in Different Densities of Prey and Induces Paralysis of Common Farmland Spiders. Sci. Rep. 2019, 9, 5724. [Google Scholar] [CrossRef]
- Forfert, N.; Troxler, A.; Retschnig, G.; Gauthier, L.; Straub, L.; Moritz, R.F.A.; Neumann, P.; Williams, G.R. Neonicotinoid Pesticides Can Reduce Honeybee Colony Genetic Diversity. PLoS ONE 2017, 12, e0186109. [Google Scholar] [CrossRef]
- Williams, G.R.; Troxler, A.; Retschnig, G.; Roth, K.; Yañez, O.; Shutler, D.; Neumann, P.; Gauthier, L. Neonicotinoid Pesticides Severely Affect Honey Bee Queens. Sci. Rep. 2015, 5, 14621. [Google Scholar] [CrossRef]
- Kremer, A.N.; King, B.H. A Neonicotinoid Affects the Mating Behavior of Spalangia endius (Hymenoptera: Pteromalidae), a Biological Control Agent of Filth Flies. Environ. Entomol. 2019, 48, 489–495. [Google Scholar] [CrossRef]
- Korenko, S.; Sýkora, J.; Řezáč, M.; Heneberg, P. Neonicotinoids Suppress Contact Chemoreception in a Common Farmland Spider. Sci. Rep. 2020, 10, 7019. [Google Scholar] [CrossRef]
- Straub, L.; Minnameyer, A.; Camenzind, D.; Kalbermatten, I.; Tosi, S.; Van Oystaeyen, A.; Wäckers, F.; Neumann, P.; Strobl, V. Thiamethoxam as an Inadvertent Anti-Aphrodisiac in Male Bees. Toxicol. Rep. 2022, 9, 36–45. [Google Scholar] [CrossRef]
- Strobl, V.; Albrecht, M.; Villamar-Bouza, L.; Tosi, S.; Neumann, P.; Straub, L. The Neonicotinoid Thiamethoxam Impairs Male Fertility in Solitary Bees, Osmia cornuta. Environ. Pollut. 2021, 284, 117106. [Google Scholar] [CrossRef]
- Whitehorn, P.R.; O’Connor, S.; Wackers, F.L.; Goulson, D. Neonicotinoid Pesticide Reduces Bumble Bee Colony Growth and Queen Production. Science 2012, 336, 351–352. [Google Scholar] [CrossRef] [PubMed]
- Laycock, I.; Lenthall, K.M.; Barratt, A.T.; Cresswell, J.E. Effects of Imidacloprid, a Neonicotinoid Pesticide, on Reproduction in Worker Bumble Bees (Bombus terrestris). Ecotoxicology 2012, 21, 1937–1945. [Google Scholar] [CrossRef] [PubMed]
- Baron, G.L.; Jansen, V.A.A.; Brown, M.J.F.; Raine, N.E. Pesticide Reduces Bumblebee Colony Initiation and Increases Probability of Population Extinction. Nat. Ecol. Evol. 2017, 1, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Siviter, H.; Brown, M.J.F.; Leadbeater, E. Sulfoxaflor Exposure Reduces Bumblebee Reproductive Success. Nature 2018, 561, 109–112. [Google Scholar] [CrossRef]
- Willis Chan, D.S.; Raine, N.E. Population Decline in a Ground-Nesting Solitary Squash Bee (Eucera pruinosa) Following Exposure to a Neonicotinoid Insecticide Treated Crop (Cucurbita pepo). Sci. Rep. 2021, 11, 4241. [Google Scholar] [CrossRef]
- Whitehorn, P.R.; Cook, N.; Blackburn, C.V.; Gill, S.M.; Green, J.; Shuker, D.M. Sex Allocation Theory Reveals a Hidden Cost of Neonicotinoid Exposure in a Parasitoid Wasp. Proc. R. Soc. B Biol. Sci. 2015, 282, 201503089. [Google Scholar] [CrossRef]
- Majidpour, M.; Maroofpour, N.; Ghane-Jahromi, M. Potential Demographic Impact of the Insecticide Mixture between Thiacloprid and Deltamethrin on the Cotton Aphid and Two of Its Natural Enemies. Bull. Entomol. Res. 2023, 113, 37–48. [Google Scholar] [CrossRef]
- Schläppi, D.; Kettler, N.; Straub, L.; Glauser, G.; Neumann, P. Long-Term Effects of Neonicotinoid Insecticides on Ants. Commun. Biol. 2020, 3, 335. [Google Scholar] [CrossRef]
- Gontijo, P.C.; Moscardini, V.F.; Michaud, J.P.; Carvalho, G.A. Non-Target Effects of Chlorantraniliprole and Thiamethoxam on Chrysoperla carnea When Employed as Sunflower Seed Treatments. J. Pest Sci. 2014, 87, 711–719. [Google Scholar] [CrossRef]
- Fogel, M.N.; Schneider, M.I.; Desneux, N.; González, B.; Ronco, A.E. Impact of the Neonicotinoid Acetamiprid on Immature Stages of the Predator Eriopis connexa (Coleoptera: Coccinellidae). Ecotoxicology 2013, 22, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Agudo, M.; González-Cabrera, J.; Picó, Y.; Calatayud-Vernich, P.; Urbaneja, A.; Dicke, M.; Tena, A. Neonicotinoids in Excretion Product of Phloem-Feeding Insects Kill Beneficial Insects. Proc. Natl. Acad. Sci. USA 2019, 116, 16817–16822. [Google Scholar] [CrossRef] [PubMed]
- Quesada, C.R.; Scharf, M.E. Whiteflies Can Excrete Insecticide-Tainted Honeydew on Tomatoes. Environ. Pollut. 2023, 337, 122527. [Google Scholar] [CrossRef] [PubMed]
- Grafton-Cardwell, E.E.; Lee, J.E.; Robillard, S.M.; Gorden, J.M. Role of Imidacloprid in Integrated Pest Management of California Citrus. J. Econ. Èntomol. 2008, 101, 451–460. [Google Scholar] [CrossRef]
- Douglas, M.R.; Rohr, J.R.; Tooker, J.F. Neonicotinoid Insecticide Travels through a Soil Food Chain, Disrupting Biological Control of Non-Target Pests and Decreasing Soya Bean Yield. J. Appl. Ecol. 2015, 52, 250–260. [Google Scholar] [CrossRef]
- Sur, R.; Stork, A. Uptake, Translocation and Metabolism of Imidacloprid in Plants. Bull. Insectol. 2003, 56, 35–40. [Google Scholar]
- EPA. 2010 U.S. Environmental Protection Agency (EPA) Decontamination Research and Development Conference; EPA: Washington, DC, USA, 2011. [Google Scholar]
- Morrissey, C.A.; Mineau, P.; Devries, J.H.; Sanchez-Bayo, F.; Liess, M.; Cavallaro, M.C.; Liber, K. Neonicotinoid Contamination of Global Surface Waters and Associated Risk to Aquatic Invertebrates: A Review. Environ. Int. 2015, 74, 291–303. [Google Scholar] [CrossRef]
- Schaafsma, A.; Limay-Rios, V.; Xue, Y.; Smith, J.; Baute, T. Field-scale Examination of Neonicotinoid Insecticide Persistence in Soil as a Result of Seed Treatment Use in Commercial Maize (Corn) Fields in Southwestern Ontario. Environ. Toxicol. Chem. 2016, 35, 295–302. [Google Scholar] [CrossRef]
- Bredeson, M.M.; Lundgren, J.G. Neonicotinoid Insecticidal Seed-Treatment on Corn Contaminates Interseeded Cover Crops Intended as Habitat for Beneficial Insects. Ecotoxicology 2019, 28, 222–228. [Google Scholar] [CrossRef]
- Weichel, L.; Nauen, R. Uptake, Translocation and Bioavailability of Imidacloprid in Several Hop Varieties. Pest Manag. Sci. 2004, 60, 440–446. [Google Scholar] [CrossRef]
- Bonmatin, J.M.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; Long, E.; Marzaro, M.; Mitchell, E.A.; et al. Environmental Fate and Exposure; Neonicotinoids and Fipronil. Environ. Sci. Pollut. Res. 2015, 22, 35–67. [Google Scholar] [CrossRef] [PubMed]
- Girolami, V.; Mazzon, L.; Squartini, A.; Mori, N.; Marzaro, M.; Bernardo, A.D.; Greatti, M.; Giorio, C.; Tapparo, A. Translocation of Neonicotinoid Insecticides from Coated Seeds to Seedling Guttation Drops: A Novel Way of Intoxication for Bees. J. Econ. Entomol. 2009, 102, 1808–1815. [Google Scholar] [CrossRef] [PubMed]
- van de Veire, M.; Tirry, L. Side Effects of Pesticides on Four Species of Beneficials Used in IPM in Glasshouse Vegetable Crops: “Worst Case” Laboratory Tests. Pestic. Benef. Org. 2003, 26, 41–50. [Google Scholar]
- Tomizawa, M.; Yamamoto, I. Structure-Activity Relationships of Nicotinoids and Imidacloprid Analogs. J. Pestic. Sci. 1993, 18, 91–98. [Google Scholar] [CrossRef]
- Tomizawa, M.; Casida, J.E. Molecular Recognition of Neonicotinoid Insecticides: The Determinants of Life or Death. Acc. Chem. Res. 2009, 42, 260–269. [Google Scholar] [CrossRef]
- Anadón, A.; Ares, I.; Martínez, M.; Martínez-Larrañaga, M.R.; Martínez, M.A. Neurotoxicity of Neonicotinoids. In Advances in Neurotoxicology; Aschner, M., Costa, L.G., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 4, pp. 167–207. [Google Scholar]
- Martelli, F.; Zhongyuan, Z.; Wang, J.; Wong, C.O.; Karagas, N.E.; Roessner, U.; Rupasinghe, T.; Venkatachalam, K.; Perry, T.; Bellen, H.J.; et al. Low Doses of the Neonicotinoid Insecticide Imidacloprid Induce ROS Triggering Neurological and Metabolic Impairments in Drosophila. Proc. Natl. Acad. Sci. USA 2020, 117, 25840–25850. [Google Scholar] [CrossRef]
- Zuščíková, L.; Bažány, D.; Greifová, H.; Knížatová, N.; Kováčik, A.; Lukáč, N.; Jambor, T. Screening of Toxic Effects of Neonicotinoid Insecticides with a Focus on Acetamiprid: A Review. Toxics 2023, 11, 598. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Tennekes, H.A. Time-Cumulative Toxicity of Neonicotinoids: Experimental Evidence and Implications for Environmental Risk Assessments. Int. J. Environ. Res. Public Health 2020, 17, 1629. [Google Scholar] [CrossRef]
- Schroeder, M.E.; Flattum, R.F. The Mode of Action and Neurotoxic Properties of the Nitromethylene Heterocycle Insecticides. Pestic. Biochem. Physiol. 1984, 22, 148–160. [Google Scholar] [CrossRef]
- Nauen, R.; Ebbinghaus-Kintscher, U.; Schmuck, R. Toxicity and Nicotinic Acetylcholine Receptor Interaction of Imidacloprid and Its Metabolites in Apis mellifera (Hymenoptera: Apidae). Pest Manag. Sci. 2001, 57, 577–586. [Google Scholar] [CrossRef]
- Grünewald, B.; Siefert, P. Acetylcholine and Its Receptors in Honeybees: Involvement in Development and Impairments by Neonicotinoids. Insects 2019, 10, 420. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Wells, J.; Brooke, B.D.; Bermudez, I.; Jones, A.K. The Neonicotinoid Imidacloprid, and the Pyrethroid Deltamethrin, Are Antagonists of the Insect Rdl GABA Receptor. J. Neurochem. 2015, 135, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Tasman, K.; Hidalgo, S.; Zhu, B.; Rands, S.A.; Hodge, J.J.L. Neonicotinoids Disrupt Memory, Circadian Behaviour and Sleep. Sci. Rep. 2021, 11, 2061. [Google Scholar] [CrossRef] [PubMed]
- Lelito, K.R.; Shafer, O.T. Reciprocal Cholinergic and GABAergic Modulation of the Small Ventrolateral Pacemaker Neurons of Drosophila’s Circadian Clock Neuron Network. J. Neurophysiol. 2012, 107, 2096–2108. [Google Scholar] [CrossRef]
- Shi, X.; Shi, J.; Yu, L.; Wu, X. Metabolic Profiling of Apis mellifera Larvae Treated with Sublethal Acetamiprid Doses. Ecotoxicol. Environ. Saf. 2023, 254, 114716. [Google Scholar] [CrossRef]
- Chen, X.; Li, A.; Yin, L.; Ke, L.; Dai, P.; Liu, Y.-J. Early-Life Sublethal Thiacloprid Exposure to Honey Bee Larvae: Enduring Effects on Adult Bee Cognitive Abilities. Toxics 2024, 12, 18. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Z.; Li, M.; Fang, Y.; Qu, J.; Mao, T.; Chen, J.; Li, F.; Sun, H.; Li, B. Responses of Detoxification Enzymes in the Midgut of Bombyx mori after Exposure to Low-Dose of Acetamiprid. Chemosphere 2020, 251, 126438. [Google Scholar] [CrossRef]
- Mattson, M.P. Hormesis Defined. Ageing Res. Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef]
- Agathokleous, E.; Blande, J.D.; Masui, N.; Calabrese, E.J.; Zhang, J.; Sicard, P.; Guedes, R.N.C.; Benelli, G. Sublethal Chemical Stimulation of Arthropod Parasitoids and Parasites of Agricultural and Environmental Importance. Environ. Res. 2023, 237, 116876. [Google Scholar] [CrossRef]
- Tatarko, A.R.; Leonard, A.S.; Mathew, D. A Neonicotinoid Pesticide Alters Drosophila Olfactory Processing. Sci. Rep. 2023, 13, 10606. [Google Scholar] [CrossRef]
- Parkinson, R.H.; Gray, J.R. Neural Conduction, Visual Motion Detection, and Insect Flight Behaviour Are Disrupted by Low Doses of Imidacloprid and Its Metabolites. NeuroToxicology 2019, 72, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Prouty, C.; Bartlett, L.J.; Krischik, V.; Altizer, S. Adult Monarch Butterflies Show High Tolerance to Neonicotinoid Insecticides. Ecol. Entomol. 2023, 48, 531–543. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Isaac, N.J.B.; Bullock, J.M.; Roy, D.B.; Garthwaite, D.G.; Crowe, A.; Pywell, R.F. Impacts of Neonicotinoid Use on Long-Term Population Changes in Wild Bees in England. Nat. Commun. 2016, 7, 12459. [Google Scholar] [CrossRef]
- Thompson, H.M.; Maus, C. The Relevance of Sublethal Effects in Honey Bee Testing for Pesticide Risk Assessment. Pest Manag. Sci. 2007, 63, 1058–1061. [Google Scholar] [CrossRef]
- Jackson, D.E.; Ratnieks, F.L.W. Communication in Ants. Curr. Biol. 2006, 16, R570–R574. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, R.H.; Zhang, S.; Gray, J.R. Neonicotinoid and Sulfoximine Pesticides Differentially Impair Insect Escape Behavior and Motion Detection. Proc. Natl. Acad. Sci. USA 2020, 117, 5510–5515. [Google Scholar] [CrossRef]
- Oliver, T.H.; Heard, M.S.; Isaac, N.J.B.; Roy, D.B.; Procter, D.; Eigenbrod, F.; Freckleton, R.; Hector, A.; Orme, C.D.L.; Petchey, O.L.; et al. Biodiversity and Resilience of Ecosystem Functions. Trends Ecol. Evol. 2015, 30, 673–684. [Google Scholar] [CrossRef]
- O’Gorman, E.J.; Yearsley, J.M.; Crowe, T.P.; Emmerson, M.C.; Jacob, U.; Petchey, O.L. Loss of Functionally Unique Species May Gradually Undermine Ecosystems. Proc. R. Soc. B Biol. Sci. 2011, 278, 1886–1893. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, L.; Chen, J. Sublethal Effect of Imidacloprid on Solenopsis invicta (Hymenoptera: Formicidae) Feeding, Digging, and Foraging Behavior. Environ. Entomol. 2015, 44, 1544–1552. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, L.; Chen, J. Impact of Imidacloprid on New Queens of Imported Fire Ants, Solenopsis invicta (Hymenoptera: Formicidae). Sci. Rep. 2015, 5, 17938. [Google Scholar] [CrossRef]
- Pan, F.; Lu, Y.; Wang, L. Toxicity and Sublethal Effects of Sulfoxaflor on the Red Imported Fire Ant, Solenopsis invicta. Ecotoxicol. Environ. Saf. 2017, 139, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.L. Insect Declines in the Anthropocene. Annu. Rev. Entomol. 2020, 65, 457–480. [Google Scholar] [CrossRef] [PubMed]
- Stork, N.E. How Many Species of Insects and Other Terrestrial Arthropods Are There on Earth? Annu. Rev. Entomol. 2018, 63, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Stork, N.E.; McBroom, J.; Gely, C.; Hamilton, A.J. New Approaches Narrow Global Species Estimates for Beetles, Insects, and Terrestrial Arthropods. Proc. Natl. Acad. Sci. USA 2015, 112, 7519–7523. [Google Scholar] [CrossRef]
- Frank, S.D.; Tooker, J.F. Neonicotinoids Pose Undocumented Threats to Food Webs. Proc. Natl. Acad. Sci. USA 2020, 117, 22609–22613. [Google Scholar] [CrossRef]
- Tooker, J.F.; Pearsons, K.A. Newer Characters, Same Story: Neonicotinoid Insecticides Disrupt Food Webs through Direct and Indirect Effects. Curr. Opin. Insect Sci. 2021, 46, 50–56. [Google Scholar] [CrossRef]
- Humann-Guilleminot, S.; Clément, S.; Desprat, J.; Binkowski, Ł.J.; Glauser, G.; Helfenstein, F. A Large-Scale Survey of House Sparrows Feathers Reveals Ubiquitous Presence of Neonicotinoids in Farmlands. Sci. Total Environ. 2019, 660, 1091–1097. [Google Scholar] [CrossRef]
- Pelosi, C.; Bertrand, C.; Daniele, G.; Coeurdassier, M.; Benoit, P.; Nélieu, S.; Lafay, F.; Bretagnolle, V.; Gaba, S.; Vulliet, E.; et al. Residues of Currently Used Pesticides in Soils and Earthworms: A Silent Threat? Agric. Ecosyst. Environ. 2021, 305, 107167. [Google Scholar] [CrossRef]
- Beketov, M.A.; Kefford, B.J.; Schäfer, R.B.; Liess, M. Pesticides Reduce Regional Biodiversity of Stream Invertebrates. Proc. Natl. Acad. Sci. USA 2013, 110, 11039–11043. [Google Scholar] [CrossRef]
- Pisa, L.W.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Downs, C.A.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; Mcfield, M.; et al. Effects of Neonicotinoids and Fipronil on Non-Target Invertebrates. Environ. Sci. Pollut. Res. 2014, 22, 68–102. [Google Scholar] [CrossRef]
- Chagnon, M.; Kreutzweiser, D.; Mitchell, E.A.D.; Morrissey, C.A.; Noome, D.A.; Van der Sluijs, J.P. Risks of Large-Scale Use of Systemic Insecticides to Ecosystem Functioning and Services. Environ. Sci. Pollut. Res. 2015, 22, 119–134. [Google Scholar] [CrossRef]
- Cavallaro, M.C.; Main, A.R.; Liber, K.; Phillips, I.D.; Headley, J.V.; Peru, K.M.; Morrissey, C.A. Neonicotinoids and Other Agricultural Stressors Collectively Modify Aquatic Insect Communities. Chemosphere 2019, 226, 945–955. [Google Scholar] [CrossRef]
- Barmentlo, H.S.; Schrama, M.; De Snoo, G.R.; Van Bodegom, P.M.; Van Nieuwenhuijzen, A.; Vijver, M.G. Experimental Evidence for Neonicotinoid Driven Decline in Aquatic Emerging Insects. Proc. Natl. Acad. Sci. USA 2021, 118, e2105692118. [Google Scholar] [CrossRef]
- Szczepaniec, A.; Creary, S.F.; Laskowski, K.L.; Nyrop, J.P.; Raupp, M.J. Neonicotinoid Insecticide Imidacloprid Causes Outbreaks of Spider Mites on Elm Trees in Urban Landscapes. PLoS ONE 2011, 6, e20018. [Google Scholar] [CrossRef]
- Szczepaniec, A.; Raupp, M.J.; Parker, R.D.; Kerns, D.; Eubanks, M.D. Neonicotinoid Insecticides Alter Induced Defenses and Increase Susceptibility to Spider Mites in Distantly Related Crop Plants. PLoS ONE 2013, 8, e62620. [Google Scholar] [CrossRef]
- Lima, A.F.; Aguirre, N.M.; Carvalho, G.A.; Grunseich, J.M.; Helms, A.M.; Peñaflor, M.F.G.V. Effects of Neonicotinoid Seed Treatment on Maize Anti-Herbivore Defenses Vary across Plant Genotypes. J. Pest Sci. 2024, 97, 199–212. [Google Scholar] [CrossRef]
| Effect | Organism | Reference |
|---|---|---|
| Physiological Effects | ||
| Impaired motor function | Apis mellifera | Williamson et al., 2014 [15], Colin et al., 2004 [16], Christen et al., 2021 [17], Hesselbach and Scheiner 2019 [18], Lambin et al., 2001 [19], Medrzycki et al., 2003 [20], Suchail et al., 2001 [21], Tosi and Nieh 2017 [22] |
| Bombus terrestris | Kenna et al., 2019 [23], Sargent et al., 2021 [24] | |
| Bombus impatiens | Crall et al., 2018 [25] | |
| Partamona helleri | Motta et al., 2024 [26] | |
| Protopolybia exigua | Crispim et al., 2023 [27] | |
| Tetragonisca angustula | Jacob et al., 2019 [28] | |
| Tetramorium caespitum | Penn & Dale 2017 [29] | |
| Harpalus pennsylvanicus | Kunkel et al., 2001 [30] | |
| Nicrophorus americanus | Cavallaro et al., 2025 [31] | |
| Platynus assimilis | Tooming et al., 2017 [32] | |
| Deleatidium spp. | Hunn et al., 2019 [33] | |
| Caenorhabditis elegans | Bradford et al., 2020 [34] | |
| Impaired cellular processes | Apis mellifera | Catae et al., 2018 [35], Christen et al., 2016 [36] |
| Melipona scutellaris | Miotelo et al., 2025 [37] | |
| Scaptorigona postica | Maloni et al., 2025 [38] | |
| Chironomus dilutus | Wei et al., 2020 [39] | |
| Impaired learning or memory | Apis mellifera | Decourtye et al., 2004 [40], Piiroinen & Goulson 2016 [41] |
| Apis cerana | Tan et al., 2015 [42] | |
| Bombus terrestris | Smith et al., 2020 [43] | |
| Bombus impatiens | Muth et al., 2019 [44] | |
| Polistes fuscatus | Corcoran & Tibbetts 2023 [45] | |
| Lestes congener | Wickramasingha et al., 2024 [46] | |
| Impaired sleep or circadian rhythm | Apis mellifera | Tackenberg et al., 2020 [47] |
| Bombus terrestris | Tasman et al., 2020 [48] | |
| Delayed development | Bombus terrestris | Siviter et al., 2020 [49] |
| Scaptorigona aff. depilis | Rosa et al., 2016 [50] | |
| Coccinella septempunctata | Jiang et al., 2018 [51] | |
| You et al., 2022 [52] | ||
| Chrysopa pallens | Su et al., 2022 [53] | |
| Hormesis: Stimulation of reproduction | Trichogramma chilonis Ishii | Ray et al., 2022 [54] |
| Podisus maculiventris | Rix and Cutler 2020 [55] | |
| Hormesis: Increased predation and host finding | Trichogramma chilonis Ishii | Ray et al., 2023 [56] |
| Tiphia vernalis | Oliver et al., 2005 [57] | |
| Encarsia formosa | Wang et al., 2019 [58] | |
| Altered gut microbiome | Apis mellifera | Alberoni et al., 2021 [59] |
| Impaired foraging | Apis mellifera | Morfin et al., 2019 [60], Schneider et al., 2012 [61], Tison et al., 2020 [62], Tison et al., 2016 [63] |
| Bombus terrestris | Kessler et al., 2015 [64], Arce et al., 2018 [65] | |
| Bombus impatiens | Leza et al., 2018 [66], Muth & Leonard 2019 [67], Stanley & Raine 2016 [68] | |
| Nasonia vitripennis | Schöfer et al., 2023 [69], Tappert et al., 2017 [70] | |
| Melipona quadrifasciata | Boff et al., 2018 [71] | |
| Microplitis croceipes | Stapel et al., 2000 [72] | |
| Olfactory recognition | Apis mellifera | Palmer et al., 2013 [73] |
| Favaro et al., 2022 [74] | ||
| Apis cerana | Tan et al., 2015 [42] | |
| Nasonia vitripennis | Schöfer et al., 2023 [69] | |
| Increased aggression | Lasius flavus | Thiel & Kohler. 2016 [75] |
| Decreased aggression | Monomorium antarcticum | Barbieri et al., 2013 [76] |
| Decreased predation | Psix saccharicola | Ranjbar, Reitz, Jalali, et al., 2021 [77], Ranjbar, Reitz, Sardary, et al., 2021 [78] |
| Trissolcus semistriatus | Ranjbar, Reitz, Jalali, et al., 2021 [77], Ranjbar, Reitz, Sardary, et al., 2021 [78] | |
| Tiphia vernalis | Oliver et al., 2005 [57] | |
| Coccinella septempunctata | Jiang et al., 2019 [79] | |
| Cycloneda sanguinea | Fernandes et al., 2016 [80] | |
| Chauliognathus flavipes | Fernandes et al., 2016 [80] | |
| Serangium japonicum | Yao et al., 2015 [81], He et al., 2012 [82] | |
| Platynus assimilis | Tooming et al., 2017 [32] | |
| Harmonia axyridis | Zhang et al., 2023 [83] | |
| Carabidae spp. | Pearsons & Tooker 2025 [84] | |
| Orius insidiosus | Fernandes et al., 2016 [80] | |
| Macrolophus pygmaeus | Martinou et al., 2014 [85] | |
| Chrysoperla sinica | Shan et al., 2020 [86] | |
| Pardosa agrestis | Korenko et al., 2019 [87] | |
| Pardosa lugubris | Řezáč et al., 2019 [88] | |
| Philodromus cespitum | Řezáč et al., 2019 [88] | |
| Reproductive Effects | ||
| Impaired courtship and mating | Apis mellifera | Forfert et al., 2017 [89], Williams et al., 2015 [90] |
| Spalangia endius | Kremer & King 2019 [91] | |
| Nasonia vitripennis | Schöfer et al., 2023 [69], Tappert et al., 2017 [70] | |
| Pardosa agrestis | Korenko et al., 2020 [92] | |
| Decreased sperm viability | Apis mellifera | Williams et al., 2015 [90] |
| Bombus terrestris | Straub et al., 2022 [93] | |
| Osmia cornuta | Strobl et al., 2021 [94] | |
| Decreased fecundity | Bombus terrestris | Whitehorn et al., 2012 [95], Laycock et al., 2012 [96], Baron et al., 2017 [97], Siviter et al., 2018 [98] |
| Bombus impatiens | Leza et al., 2018 [66] | |
| Crall et al., 2018 [25] | ||
| Eucera pruinosa | Willis Chan and Raine 2021 [99] | |
| Nasonia vitripennis | Whitehorn et al., 2015 [100] | |
| Aphidius flaviventris | Majidpour et al., 2022 [101] | |
| Lasius niger | Schläppi et al., 2020 [102] | |
| Chrysoperla carnea | Gontijo et al., 2014 [103] | |
| Coccinella septempunctata | Jiang et al., 2018 [51] | |
| Caenorhabditis elegans | Bradford et al., 2020 [34] | |
| Reduced egg viability | Coccinella septempunctata | Jiang et al., 2019 [79] |
| Eriopis connexa | Fogel et al., 2013 [104] | |
| Harmonia axyridis | Zhang et al., 2023 [83] | |
| Community Effects | ||
| Beneficial insect exposure through parasitism and predation | Anagyrus pseudococci | Calvo-Agudo et al., 2019 [105] |
| Quesada & Scharf 2023 [106] | ||
| Aphytis melinus | Grafton-Cardwell et al., 2008 [107] | |
| Comperiella bifasciata | Grafton-Cardwell et al., 2008 [107] | |
| Chlaenius tricolor | Douglas et al., 2015 [108] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Spence, S.K.; Alharbi, S.A.M.; Ejomah, A.; Maleki, F.A.; Wolfin, M.S.; Kersch-Becker, M.F. Sublethal Effects of Neonicotinoids: How Physiological and Behavioral Disruptions in Non-Target Insects Threaten Biodiversity and Ecosystem Services. Insects 2026, 17, 26. https://doi.org/10.3390/insects17010026
Spence SK, Alharbi SAM, Ejomah A, Maleki FA, Wolfin MS, Kersch-Becker MF. Sublethal Effects of Neonicotinoids: How Physiological and Behavioral Disruptions in Non-Target Insects Threaten Biodiversity and Ecosystem Services. Insects. 2026; 17(1):26. https://doi.org/10.3390/insects17010026
Chicago/Turabian StyleSpence, Sarah K., Shorooq A. M. Alharbi, Afure Ejomah, Feizollah A. Maleki, Michael S. Wolfin, and Mônica F. Kersch-Becker. 2026. "Sublethal Effects of Neonicotinoids: How Physiological and Behavioral Disruptions in Non-Target Insects Threaten Biodiversity and Ecosystem Services" Insects 17, no. 1: 26. https://doi.org/10.3390/insects17010026
APA StyleSpence, S. K., Alharbi, S. A. M., Ejomah, A., Maleki, F. A., Wolfin, M. S., & Kersch-Becker, M. F. (2026). Sublethal Effects of Neonicotinoids: How Physiological and Behavioral Disruptions in Non-Target Insects Threaten Biodiversity and Ecosystem Services. Insects, 17(1), 26. https://doi.org/10.3390/insects17010026





