Assessing the Population Structure and Invasion Risk in Suitable Areas of the Rice Pest Leptocorisa acuta (Hemiptera: Alydidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and DNA Extraction
2.2. ddRAD Sequencing and Data Processing
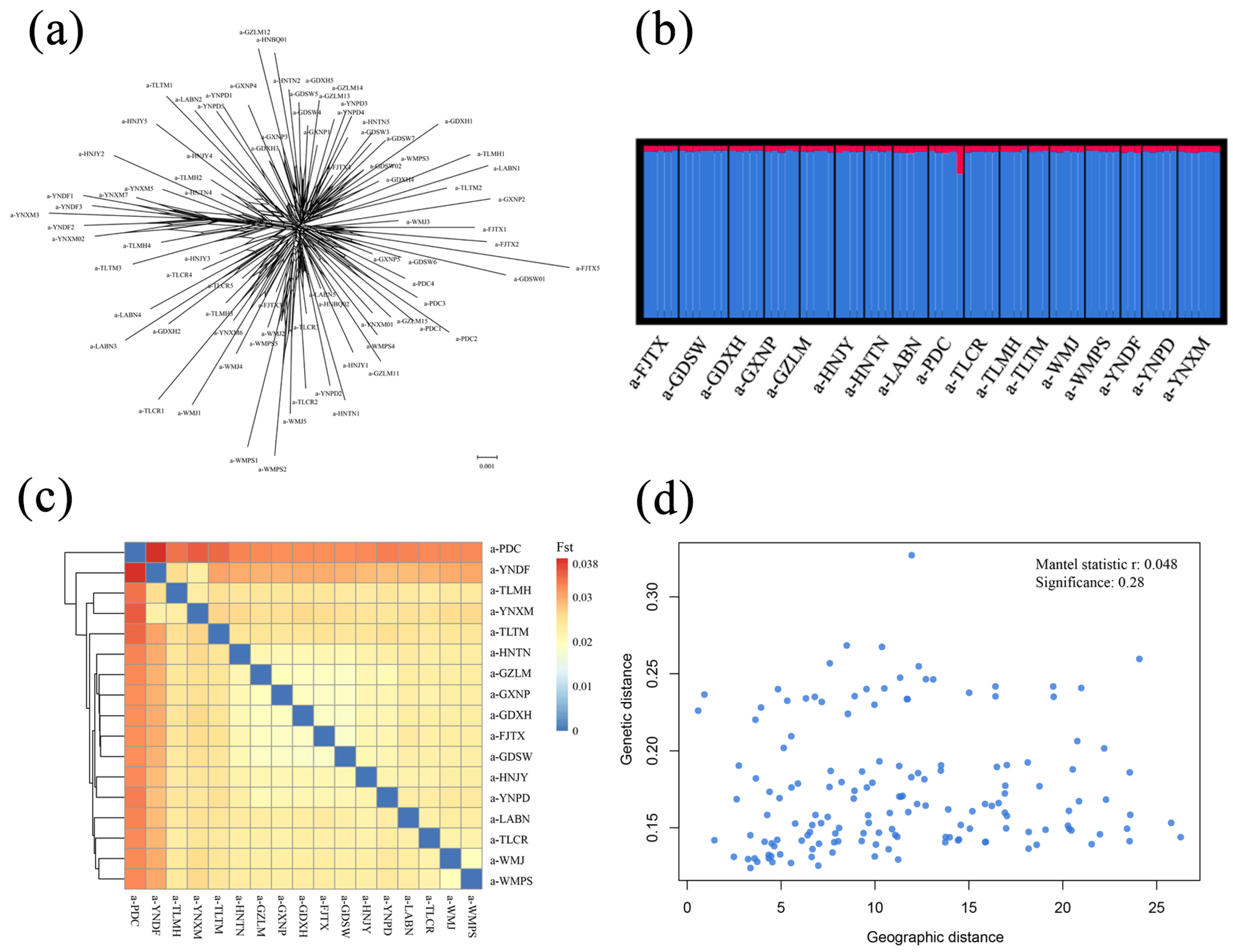
2.3. Population Genetics Analyses
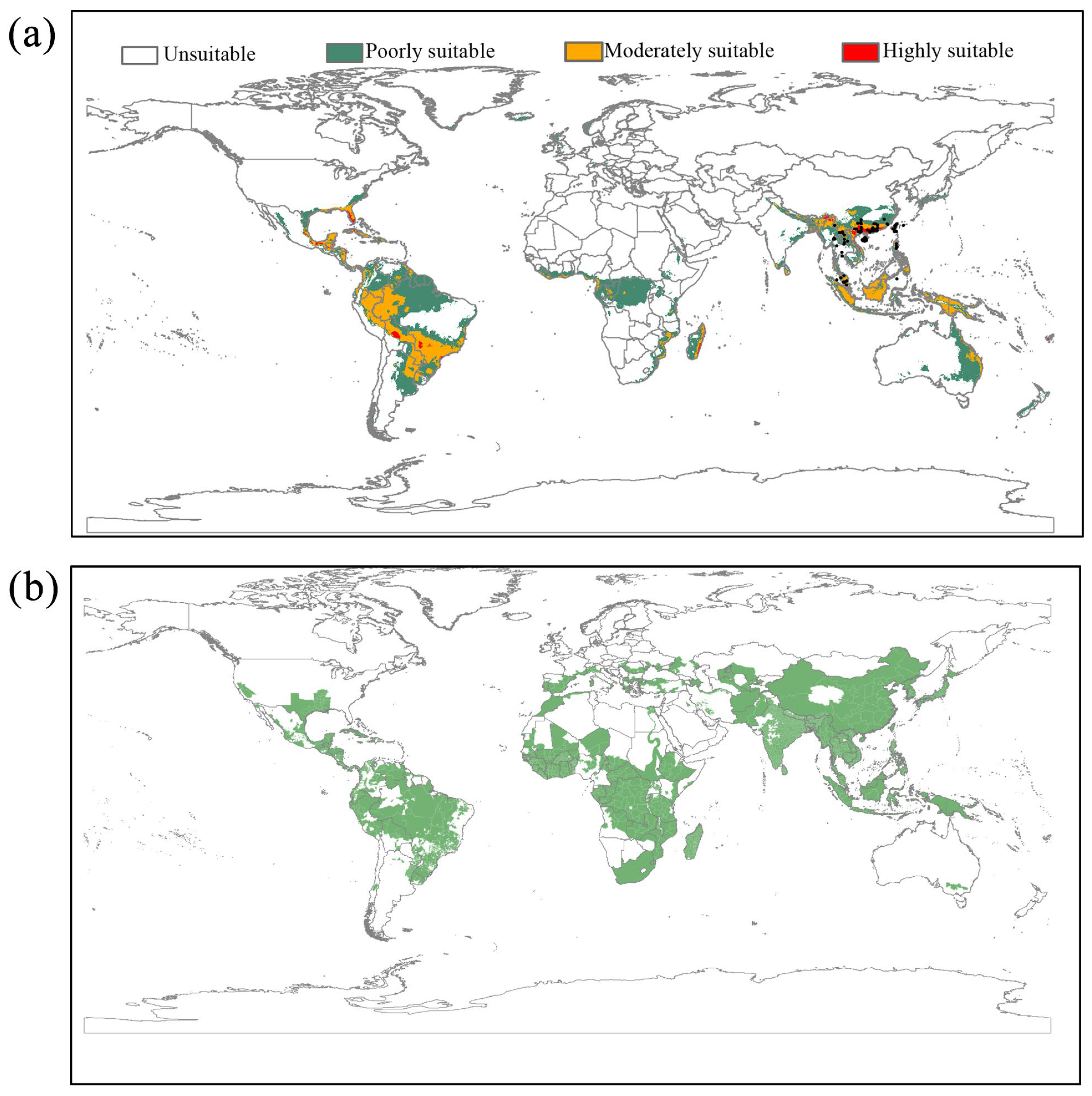
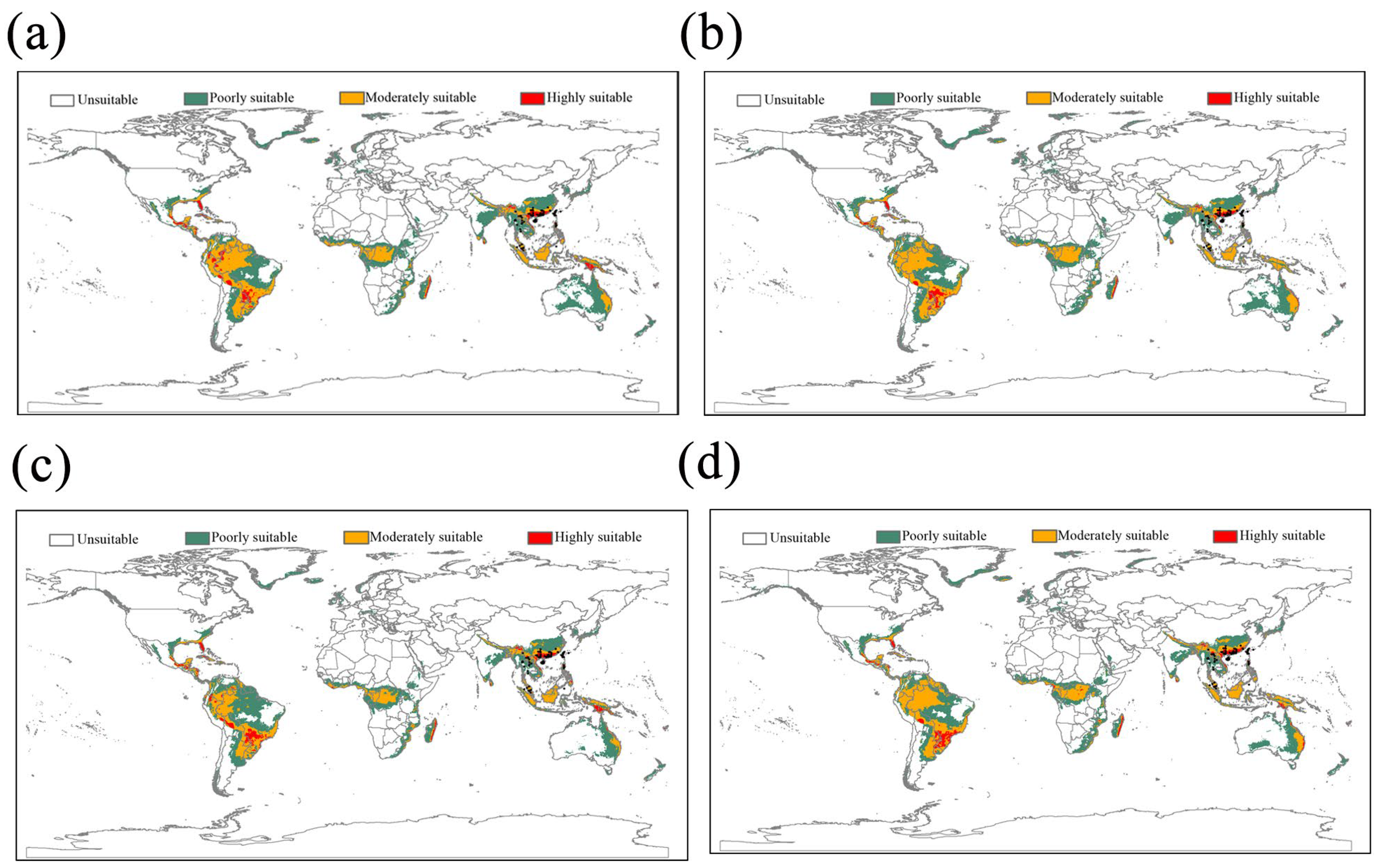
2.4. Ecological Niche Modeling
3. Results
3.1. Nuclear Genetic Diversity and the Lack of Population Structure
3.2. The Current and Future Potentially Suitable Areas for L. acuta
4. Discussion
4.1. Genetic Diversity and Population Genetic Structure
4.2. Potentially Suitable Habitat and Invasion Areas
4.3. Implications for Management
4.4. Integrated Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, X.; Kurata, N.; Wei, X.; Wang, Z.X.; Wang, A.; Zhao, Q.; Zhao, Y.; Liu, K.; Lu, H.; Li, W.; et al. A map of rice genome variation reveals the origin of cultivated rice. Nature 2012, 490, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Kalshoven, L.G.E.; Laan, P.A. Pests of Crops in Indonesia; P.T. Ichtian Bann-Van Hoeve: Jakarta, Indonesia, 1981. [Google Scholar]
- Litsinger, J.; Gyawali, B.; Wilde, G. Feeding behaviour of the rice bug Leptocorisa oratorius (F.) (Hemiptera: Alydidae). J. Plant Prot. Trop. 1998, 11, 23–35. [Google Scholar]
- Ahmad, I. The Leptocorisinae (Heteroptera: Alydidae) of the world. Bulletin of the British Museum of Natural History, 19 October 1965; Volume 5, (Entomological Supplement). [Google Scholar]
- Hasegawa, H. Distribution and taxonomy of rice bugs in Southeast Asia. Trop. Agric. 1971, 5, 229–234. [Google Scholar]
- Halteren, P.v.; Sama, S. Damage caused by the rice bug Leptocorisa acuta in South Sulawesi, Indonesia. Rice Entomol. Newsl. 1976, 4, 34. [Google Scholar]
- Velusamy, R.; Janaki, I.P.; Jayaraj, S. Control of rice gundhi bug, Leptocorisa acuta (Thunberg) (Coreidae, Hemiptera). Madras Agric. J. 1977, 64, 274. [Google Scholar]
- Chen, Z. The occurence sic of Leptocorisa acuta Thunberg and its control. Entomol. Knowl. 1992, 29, 328–331. [Google Scholar]
- Schaefer, C.W.; Panizzi, A.R. Heteroptera of Economic Importance, 1st ed.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar] [CrossRef]
- Sen, A.C.; Chaudhuri, D.P. Incidence of rice gundhy bug and steps for its control. Indian J. Entomol. 1961, 21, 287–288. [Google Scholar]
- Banerjee, P.K.; Chatterjee, P.B. Pests of hill rice in West Bengal, India. Int. Rice Res. Newsl. 1982, 7, 12–14. [Google Scholar]
- Corbett, G.H. The Bionomics and Control of Leptocorisa acuta Thunb. with Notes on Other Leptocorisa spp. in Malaya; Department of Agriculture S.S. & F.M.S.: Quezon City, Philippines, 1930; pp. 40–42. [Google Scholar]
- Sands, D.P.A. The Biology and Ecology of Leptocorisa (Hemiptera: Alydidae) in Papua New Guinea; Research Bulletin, Department of Primary Industry: Port Moresby, New Guinea, 1977; p. 104. [Google Scholar]
- Rothschild, G.H.L. Some notes on the effects of rice ear-bugs on grain yields. Trop. Agric. 1970, 47, 145–149. [Google Scholar]
- Mohiuddin, M.S.; Rao, Y.P.; Mohan, S.K.; Verma, J.P. Role of Leptocorisa acuta Thun. in the spread of bacterial blight of rice. Curr. Sci. 1976, 45, 426–427. [Google Scholar]
- Lakshmanan, P.; Mohan, S.K.; Velusamy, R. Role of earhead bug (Leptocorisa acuta) feeding on sheath rot disease caused by Sarocladium oryzae in Oryza sativa in India. Phytoparasitica 1992, 20, 107–112. [Google Scholar] [CrossRef]
- Vivekananthan, R.; Rabindran, R. Endorsement of factors associated with the development of sheath rot pathogen in rice. Madras Agric. J. 2008, 95, 61–70. [Google Scholar]
- Uichanco, L. The rice bug, Leptocorisa acuta Thunberg, in the Philippines. Philipp. Agric. Rev. 1921, 14, 87–112. [Google Scholar]
- Kay, I.R.; Brown, J.D.; Mayer, R.J. Insecticidal control of Eysarcoris trimaculatus (Distant) (Heteroptera: Pentatomidae) and Leptocorisa acuta (Thunberg) (Heteroptera: Alydidae) on rice in north Queensland, Australia. Crop Prot. 1993, 12, 310–314. [Google Scholar] [CrossRef]
- Pathak, M.D.; Khan, Z.H. Insect Pests of Rice; International Rice Research Institute (IRRI): Los Banos, Philippines, 1994; pp. 37–38. [Google Scholar]
- Reynolds, D.; Mukhopadhyay, S.; Riley, J.; Das, B.; Nath, P.; Mandal, S. Seasonal variation in windborne movement of insect pests over northeast India. Int. J. Pest Manag. 1999, 45, 195–205. [Google Scholar] [CrossRef]
- Laborte, A.G.; Gutierrez, M.A.; Balanza, J.G.; Saito, K.; Zwart, S.J.; Boschetti, M.; Murty, M.V.R.; Villano, L.; Aunario, J.K.; Reinke, R.; et al. RiceAtlas, a spatial database of global rice calendars and production. Sci. Data 2017, 4, 170074. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.K.; Weber, J.N.; Kay, E.H.; Fisher, H.S.; Hoekstra, H.E. Double digest RADseq: An inexpensive method for de novo SNP discovery and genotyping in model and non-model species. PLoS ONE 2012, 7, e37135. [Google Scholar] [CrossRef] [PubMed]
- Eaton, D.A.R.; Overcast, I. ipyrad: Interactive assembly and analysis of RADseq datasets. Bioinformatics 2020, 36, 2592–2594. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Goudet, J.; Jombart, T. R package, version 0.04-22; Hierfstat: Estimation and Tests of Hierarchical F-Statistics. 2015. Available online: https://github.com/jgx65/hierfstat (accessed on 9 September 2024).
- Wright, S. Evolution and the genetics of populations. In Experimental Results and Evolutionary Deductions; University of Chicago Press: Chicago, IL, USA, 1984. [Google Scholar]
- Slatkin, M. Gene flow and the geographic structure of natural populations. Science 1987, 236, 787–792. [Google Scholar] [CrossRef]
- Huson, D.H.; Bryant, D. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 2006, 23, 254–267. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Earl, D.A.; vonHoldt, B.M. Structure Harvester: A website and program for visualizing Structure output and implementing the Evanno method. Conserv. Genet. Resour. 2011, 4, 359–361. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software Structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Jombart, T. Adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fu, Y.X. Exploring population size changes using SNP frequency spectra. Nat. Genet. 2015, 47, 555–559. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Wisz, M.S.; Hijmans, R.J.; Li, J.; Peterson, A.T.; Graham, C.H.; Guisan, A.; NCEAS Predicting Species Distributions Working Group. Effects of sample size on the performance of species distribution models. Divers. Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- Kass, J.M.; Vilela, B.; Aiello-Lammens, M.E.; Muscarella, R.; Merow, C.; Anderson, R.P. Wallace: A flexible platform for reproducible modeling of species niches and distributions built for community expansion. Methods Ecol. Evol. 2018, 9, 1151–1156. [Google Scholar] [CrossRef]
- Liu, C.; Newell, G.; White, M. On the selection of thresholds for predicting species occurrence with presence-only data. Ecol. Evol. 2016, 6, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Lin, C.; Wang, Y.; Zhang, Y.; Ji, L. Ecological niche complexity of invasive and native cryptic species of the Bemisia tabaci species complex in China. J. Pest Sci. 2022, 95, 1245–1259. [Google Scholar] [CrossRef]
- Guisan, A.; Tingley, R.; Baumgartner, J.B.; Naujokaitis-Lewis, I.; Sutcliffe, P.R.; Tulloch, A.I.; Regan, T.J.; Brotons, L.; Mcdonald-Madden, E.; Mantyka-Pringle, C.; et al. Predicting species distributions for conservation decisions. Ecol. Lett. 2013, 16, 1424–1435. [Google Scholar] [CrossRef]
- Clark, P.U.; Dyke, A.S.; Shakun, J.D.; Carlson, A.E.; Clark, J.; Wohlfarth, B.; Mitrovica, J.X.; Hostetler, S.W.; McCabe, A.M. The last glacial maximum. Science 2009, 325, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Gutaker, R.M.; Groen, S.C.; Bellis, E.S.; Choi, J.Y.; Pires, I.S.; Bocinsky, R.K.; Slayton, E.R.; Wilkins, O.; Castillo, C.C.; Negrão, S.; et al. Genomic history and ecology of the geographic spread of rice. Nat. Plants 2020, 6, 492–502. [Google Scholar] [CrossRef]
- Emerson, K.J.; Merz, C.R.; Catchen, J.M.; Hohenlohe, P.A.; Cresko, W.A.; Bradshaw, W.E.; Holzapfel, C.M. Resolving postglacial phylogeography using high-throughput sequencing. Proc. Natl. Acad. Sci. USA 2010, 107, 16196–16200. [Google Scholar] [CrossRef]
- Rašić, G.; Filipović, I.; Weeks, A.R.; Hoffmann, A.A. Genome-wide SNPs lead to strong signals of geographic structure and relatedness patterns in the major arbovirus vector, Aedes aegypti. BMC Genom. 2014, 15, 275. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Ding, Y.; Zhou, J.Y.; Sun, K.K.; Matsukura, K.; Zhang, H.; Chen, L.; Hong, X.; Sun, J. Genetic evidence of transoceanic migration of the small brown planthopper between China and Japan. Pest Manag. Sci. 2022, 78, 2909–2920. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, L.; Foll, M.; Petit, R.J. Genetic consequences of range expansions. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 481–501. [Google Scholar] [CrossRef]
- Kerdelhué, C.; Zane, L.; Simonato, M.; Salvato, P.; Rousselet, J.; Roques, A.; Battisti, A. Quaternary history and contemporary patterns in a currently expanding species. BMC Evol. Biol. 2009, 9, 220. [Google Scholar] [CrossRef]
- Jezkova, T.; Olah-Hemmings, V.; Riddle, B.R. Niche shifting in response to warming climate after the last glacial maximum: Inference from genetic data and niche assessments in the chisel-toothed kangaroo rat (Dipodomys microps). Glob. Change Biol. 2011, 17, 3486–3502. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, W.; Luo, J.; Zhang, S.; Li, W.; Wang, C.; Lv, L.; Cui, J. Population genetic structure and expansion patterns of the cotton pest Adelphocoris fasciaticollis. J. Pest Sci. 2017, 91, 539–550. [Google Scholar] [CrossRef]
- Ye, Y.J.; Huang, J.P.; Nguyen, H.N.; Villanueva, R.J.T.; Amarga, A.K.S.; Tseng, H.-Y.; Beutel, R. Comparative Phylogeography in the Taiwan–Luzon Volcanic Belt Indicates Fast Diversification History of Pachyrhynchus Weevils (Coleoptera: Curculionidae). Insect Syst. Evol. 2022, 6, 4. [Google Scholar] [CrossRef]
- Litsinger, J.A.; Barrion, A.T.; Canapi, B.L.; Libetario, E.M.; Pantua, P.C.; Cruz, C.G.; Apostol, R.; Lumaban, M.D.; Bandon, J.; Macatula, R. Leptocorisa rice seed bugs (Hemiptera: Alydidae) in Asia: A review. Philipp. Entomol. 2015, 29, 1–103. [Google Scholar]
- Fu, X.; Liu, Y.; Li, C.; Lu, Y.; Li, Y.; Wu, K. Seasonal migration of Apolygus lucorum (Hemiptera: Miridae) over the Bohai Sea in northern China. J. Econ. Entomol. 2014, 107, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Endersby, N.M.; Mckechnie, S.W.; Vogel, H.; Gahan, L.J.; Baxter, S.W.; Ridland, P.M.; Weeks, A.R. Microsatellites isolated from diamondback moth, Plutella xylostella (L.), for studies of dispersal in Australian populations. Mol. Ecol. Notes 2005, 5, 51–53. [Google Scholar] [CrossRef]
- Lyons, J.I.; Pierce, A.A.; Barribeau, S.M.; Sternberg, E.D.; Mongue, A.J.; De Roode, J.C. Lack of genetic differentiation between monarch butterflies with divergent migration destinations. Mol. Ecol. 2012, 21, 3433–3444. [Google Scholar] [CrossRef]
- Chen, M.Z.; Cao, L.J.; Li, B.Y.; Chen, J.C.; Gong, Y.J.; Yang, Q.; Schmidt, T.L.; Yue, L.; Zhu, J.; Li, H.; et al. Migration trajectories of the diamondback moth Plutella xylostella in China inferred from population genomic variation. Pest Manag. Sci. 2021, 77, 1683–1693. [Google Scholar] [CrossRef]
- Gao, B.; Yu, L.; Qu, Y.; Song, G.; Dai, C.; Zhang, R.; Yin, Z.; Wang, K.; Gao, X.; Li, S.-H.; et al. An unstructured phylogeographic pattern with extensive gene flow in an endemic bird of south China: Collared finchbill (Spizixos semitorques). Int. J. Mol. Sci. 2011, 12, 3635–3647. [Google Scholar] [CrossRef]
- Hu, C.; Pan, T.; Wu, Y.; Zhang, C.; Chen, W.; Chang, Q. Spatial genetic structure and historical demography of East Asian wild boar. Anim. Genet. 2020, 51, 557–567. [Google Scholar] [CrossRef]
- Song, G.; Zhang, R.; Machado-Stredel, F.; Alström, P.; Johansson, U.; Irestedt, M. Great journey of Great Tits (Parus major group): Origin, diversification and historical demographics of a broadly distributed bird lineage. J. Biogeogr. 2020, 47, 1585–1598. [Google Scholar] [CrossRef]
- Weaver, A.J.; Eby, M.; Fanning, A.F.; Wiebe, E.C. Simulated influence of carbon dioxide, orbital forcing and ice sheets on the climate of the Last Glacial Maximum. Nature 1998, 394, 847–853. [Google Scholar] [CrossRef]
- Jetz, W.; McPherson, J.M.; Guralnick, R.P. Integrating biodiversity distribution knowledge: Toward a global map of life. Trends Ecol. Evol. 2012, 27, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, T.Y.; Ren, S.Z.; Zheng, L.Y. A Handbook for the Determination of the Chinese Hemiptera-Heteroptera; Science Press: Beijing, China, 1977; Volume 1, pp. 268–279. [Google Scholar]
- Hamilton, M. When rice shakes the world. In The Importance of the First Grain to World Economic and Political Stability; Advantage Media: Charleston, SC, USA, 2014. [Google Scholar]
- Lefroy, H.M. The Rice Bug (Leptocorisa varicornis, Fabr); Memoirs of the Department of Agriculture, Indian Entomology Series; Superintendent of Government Printing: Calcutta, India, 1908; Volume 2, pp. 1–13. [Google Scholar]
- Van der Goot, P. De walang sangit (Leptocorisa acuta Thunb) als viand van het rystgewas in Indonesia. Mededelingen van het Algemeen Proefstation voor den Landbouw [The “walang sangit” (rice bug, Leptocorisa acuta Thunb.), a pest of the rice crop in Indonesia.] Communications of the General Agricultural Research Station. (in Dutch, with English summary), Buitenzorg, Java. 1949; p. 66. [Google Scholar]
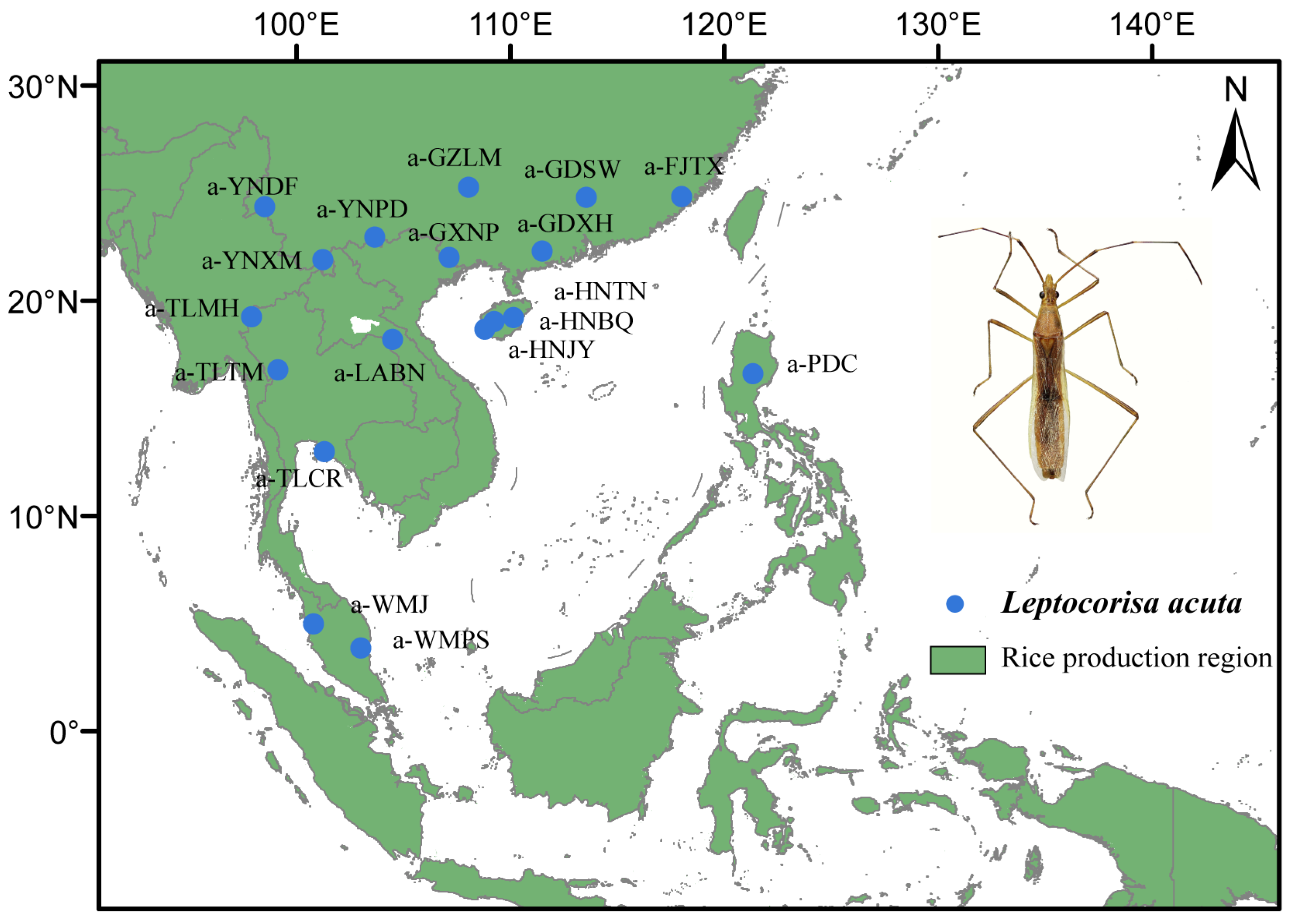
| Pop Code | N | Collection Date | Longitude (°E) | Latitude (°N) | SNP_80 Dataset | ||
|---|---|---|---|---|---|---|---|
| π | HO | HE | |||||
| a-FJTX | 5 | 25 May 2016 | 118.04 | 24.85 | 0.0234 | 0.2430 | 0.2957 |
| a-GDSW | 7 | 2 Aug 2018 | 113.57 | 24.82 | 0.0208 | 0.3526 | 0.3609 |
| a-GDXH | 5 | 17 Jul 2019 | 111.49 | 22.32 | 0.0230 | 0.2562 | 0.2915 |
| a-GXNP | 5 | 12 Aug 2020 | 107.16 | 22.04 | 0.0229 | 0.2535 | 0.2903 |
| a-GZLM | 5 | 9 Aug 2014 | 108.06 | 25.29 | 0.0223 | 0.2562 | 0.2890 |
| a-HNBQ | 2 | 28 Jul 2017 | 109.27 | 19.08 | — | — | — |
| a-HNJY | 5 | 1 Aug 2017 | 108.82 | 18.70 | 0.0236 | 0.2511 | 0.2962 |
| a-HNTN | 4 | 27 Jul 2017 | 110.17 | 19.23 | 0.0229 | 0.2960 | 0.3275 |
| a-LABN | 5 | 11 Aug 2019 | 104.51 | 18.24 | 0.0231 | 0.2507 | 0.2821 |
| a-PDC | 4 | 19 Dec 2019 | 121.35 | 16.63 | 0.0224 | 0.2907 | 0.3374 |
| a-TLCR | 5 | 10 Jul 2018 | 101.34 | 13.03 | 0.0228 | 0.2449 | 0.2849 |
| a-TLMH | 4 | 30 Aug 2018 | 97.93 | 19.29 | 0.0230 | 0.2907 | 0.3287 |
| a-TLTM | 3 | 5 Jul 2018 | 99.14 | 16.83 | 0.0241 | 0.3474 | 0.4044 |
| a-WMJ | 5 | 13 Apr 2019 | 100.81 | 5.01 | 0.0224 | 0.2520 | 0.2876 |
| a-WMPS | 5 | 19 Apr 2019 | 103.03 | 3.89 | 0.0225 | 0.2508 | 0.2905 |
| a-YNDF | 3 | 10 Jun 2016 | 98.56 | 24.39 | 0.0222 | 0.3660 | 0.4138 |
| a-YNPD | 5 | 4 Aug 2020 | 103.69 | 22.97 | 0.0229 | 0.2481 | 0.2897 |
| a-YNXM | 6 | 25 Aug 2010 | 101.27 | 21.94 | 0.0208 | 0.2165 | 0.2521 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Zhu, X.; Tang, Z.; Yi, W.; Bu, W. Assessing the Population Structure and Invasion Risk in Suitable Areas of the Rice Pest Leptocorisa acuta (Hemiptera: Alydidae). Insects 2025, 16, 949. https://doi.org/10.3390/insects16090949
Dong X, Zhu X, Tang Z, Yi W, Bu W. Assessing the Population Structure and Invasion Risk in Suitable Areas of the Rice Pest Leptocorisa acuta (Hemiptera: Alydidae). Insects. 2025; 16(9):949. https://doi.org/10.3390/insects16090949
Chicago/Turabian StyleDong, Xue, Xiuxiu Zhu, Zechen Tang, Wenbo Yi, and Wenjun Bu. 2025. "Assessing the Population Structure and Invasion Risk in Suitable Areas of the Rice Pest Leptocorisa acuta (Hemiptera: Alydidae)" Insects 16, no. 9: 949. https://doi.org/10.3390/insects16090949
APA StyleDong, X., Zhu, X., Tang, Z., Yi, W., & Bu, W. (2025). Assessing the Population Structure and Invasion Risk in Suitable Areas of the Rice Pest Leptocorisa acuta (Hemiptera: Alydidae). Insects, 16(9), 949. https://doi.org/10.3390/insects16090949






