Taxonomic Revision of D. carlosvilelai Vela and Rafael, 2001, and Updated Record of D. paraguayensis Duda, 1927 Registry in Ecuador †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
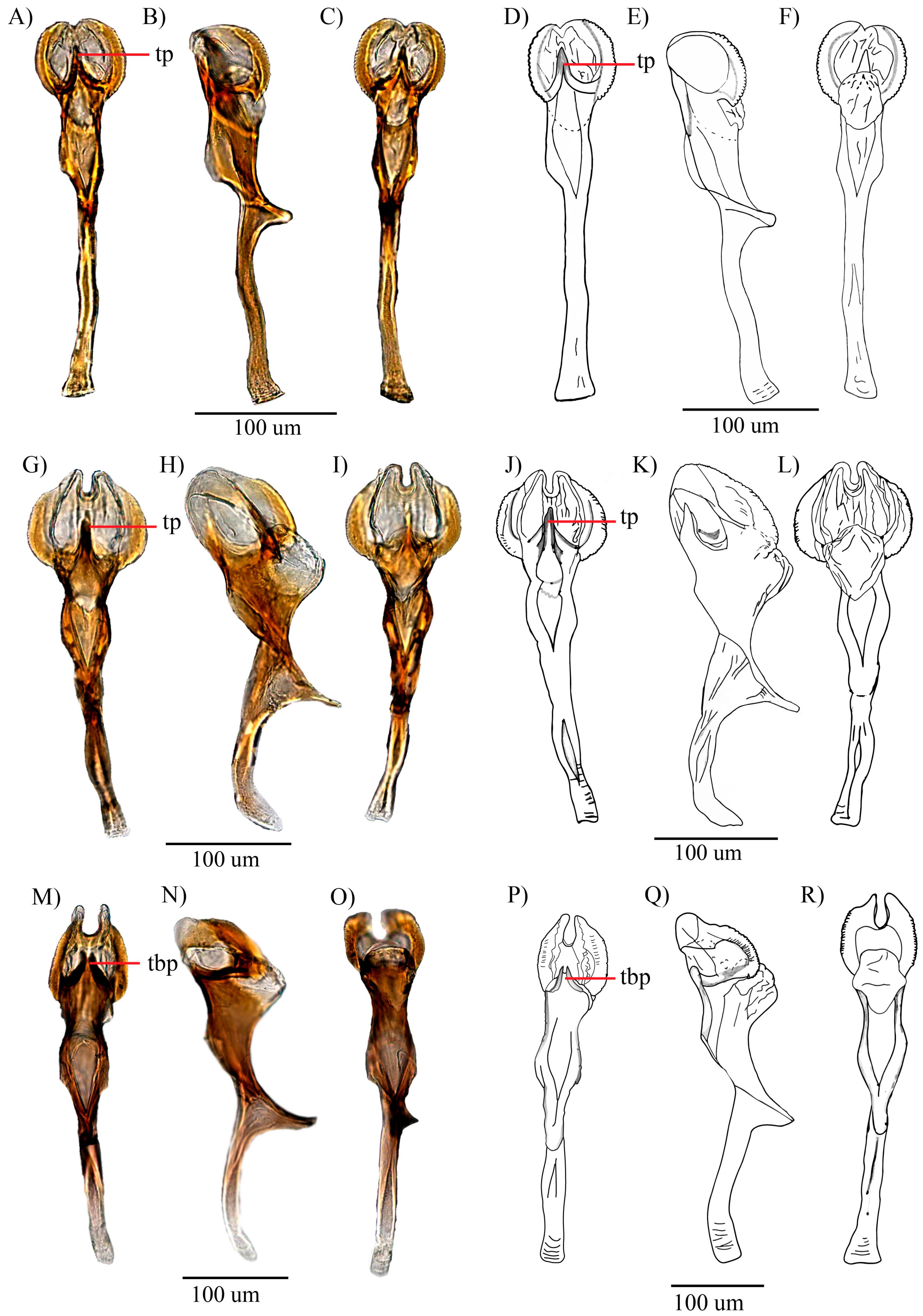
3.1. Re-Description of Drosophila carlosvilelai Vela and Rafael, 2001
 (dissected, terminalia in microtube, dry-mounted), Ecuador, Napo, Laguna de Papallacta, 3400 masl, 00°22′52.6″ S 78°09′44.4″ W, Nov. 2016, K. Casal et al., V. Rafael, A. Peñafiel, and C. Suárez det. (QCAZ-I 261143). Allotype
(dissected, terminalia in microtube, dry-mounted), Ecuador, Napo, Laguna de Papallacta, 3400 masl, 00°22′52.6″ S 78°09′44.4″ W, Nov. 2016, K. Casal et al., V. Rafael, A. Peñafiel, and C. Suárez det. (QCAZ-I 261143). Allotype  (dissected, terminalia in microtubes, dry-mounted), Ecuador, Carchi, Reserva Ecológica el Ángel, 3762 masl, 00°47′22.1″ N 77°54′03.2″ W, Jul. 2016, A. Peñafiel col., V. Rafael, A. Peñafiel, and C. Suárez det. (QCAZ-I 260751).
(dissected, terminalia in microtubes, dry-mounted), Ecuador, Carchi, Reserva Ecológica el Ángel, 3762 masl, 00°47′22.1″ N 77°54′03.2″ W, Jul. 2016, A. Peñafiel col., V. Rafael, A. Peñafiel, and C. Suárez det. (QCAZ-I 260751). (dissected, terminalia in microtubes, dry-mounted), Ecuador, Napo, Laguna de Papallacta, 3400 masl, same data as the neotype, Nov. 2016, K. Casal col., V. Rafael, A. Peñafiel, and C. Suárez det. (QCAZ-I 261144-261170). 3
(dissected, terminalia in microtubes, dry-mounted), Ecuador, Napo, Laguna de Papallacta, 3400 masl, same data as the neotype, Nov. 2016, K. Casal col., V. Rafael, A. Peñafiel, and C. Suárez det. (QCAZ-I 261144-261170). 3  (dissected, terminalia in microtubes, dry-mounted, from an isofemale line), Ecuador, Carchi, Reserva Ecológica el Ángel, 3762 masl, same data as the allotype, A. Peñafiel col., V. Rafael, A. Peñafiel, and C. Suárez det. (QCAZ-I 260752-260754).
(dissected, terminalia in microtubes, dry-mounted, from an isofemale line), Ecuador, Carchi, Reserva Ecológica el Ángel, 3762 masl, same data as the allotype, A. Peñafiel col., V. Rafael, A. Peñafiel, and C. Suárez det. (QCAZ-I 260752-260754).3.1.1. Diagnosis
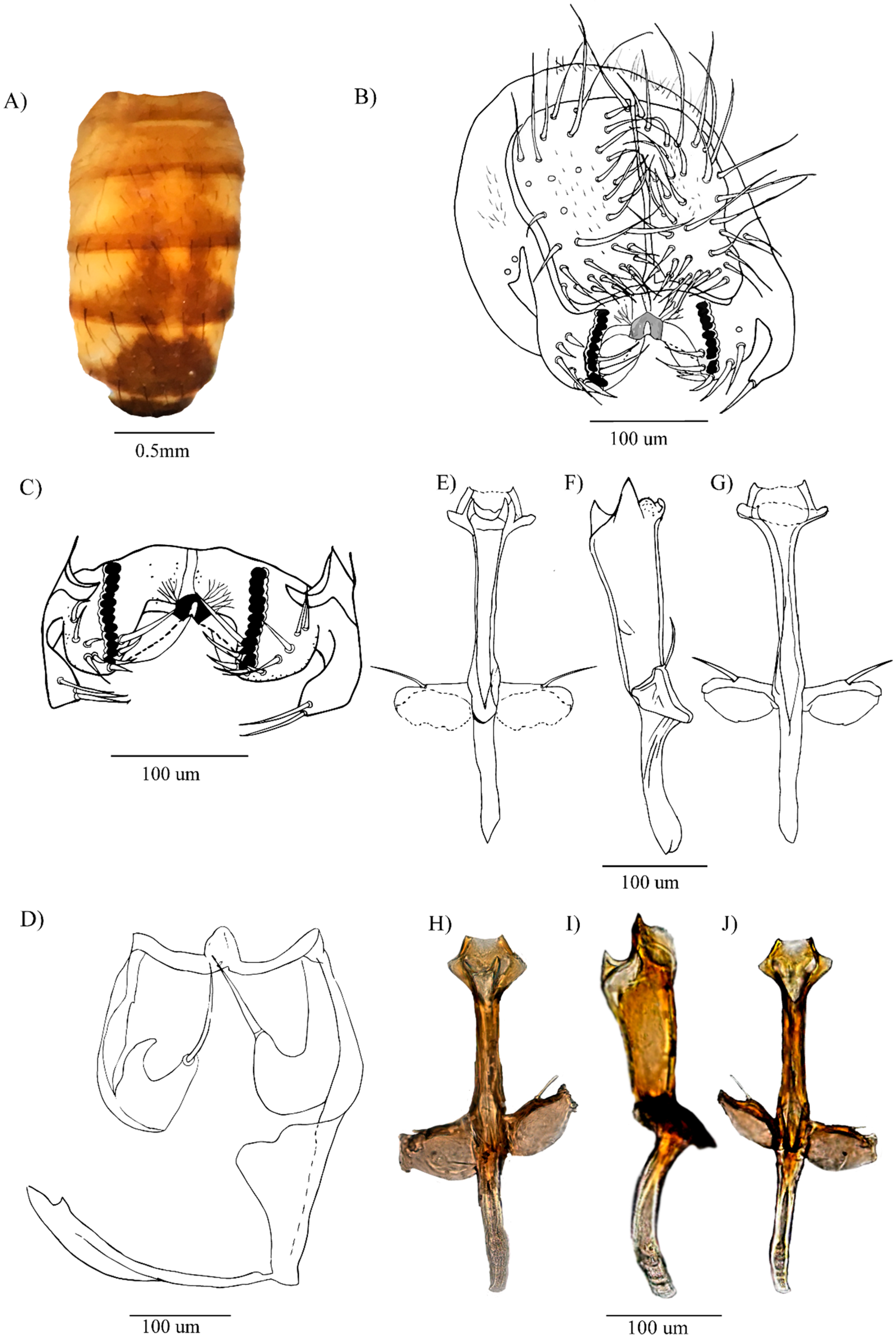
3.1.2. Re-Description of Male
3.1.3. Description of the Female
3.1.4. Distribution
3.1.5. Biology
3.1.6. Note
3.2. Description of Drosophila paraloewi sp. nov.
 (dissected, terminalia in microtube, dry-mounted), Ecuador, Pichincha, Pasochoa, 3260–3310 masl, 00°28′ S 78°29′ W, July 1997, D. Vela col., V. Rafael, D. Vela, and C. Suárez det. (QCAZ-I 261171).
(dissected, terminalia in microtube, dry-mounted), Ecuador, Pichincha, Pasochoa, 3260–3310 masl, 00°28′ S 78°29′ W, July 1997, D. Vela col., V. Rafael, D. Vela, and C. Suárez det. (QCAZ-I 261171). (dissected, terminalia in microtubes, dry-mounted), Ecuador, Pichincha, Pasochoa, 3260–3310 masl, same data as the holotype, D. Vela col., V. Rafael, D. Vela, and C. Suárez det. (QCAZ-I 261172-261183).
(dissected, terminalia in microtubes, dry-mounted), Ecuador, Pichincha, Pasochoa, 3260–3310 masl, same data as the holotype, D. Vela col., V. Rafael, D. Vela, and C. Suárez det. (QCAZ-I 261172-261183).3.2.1. Diagnosis
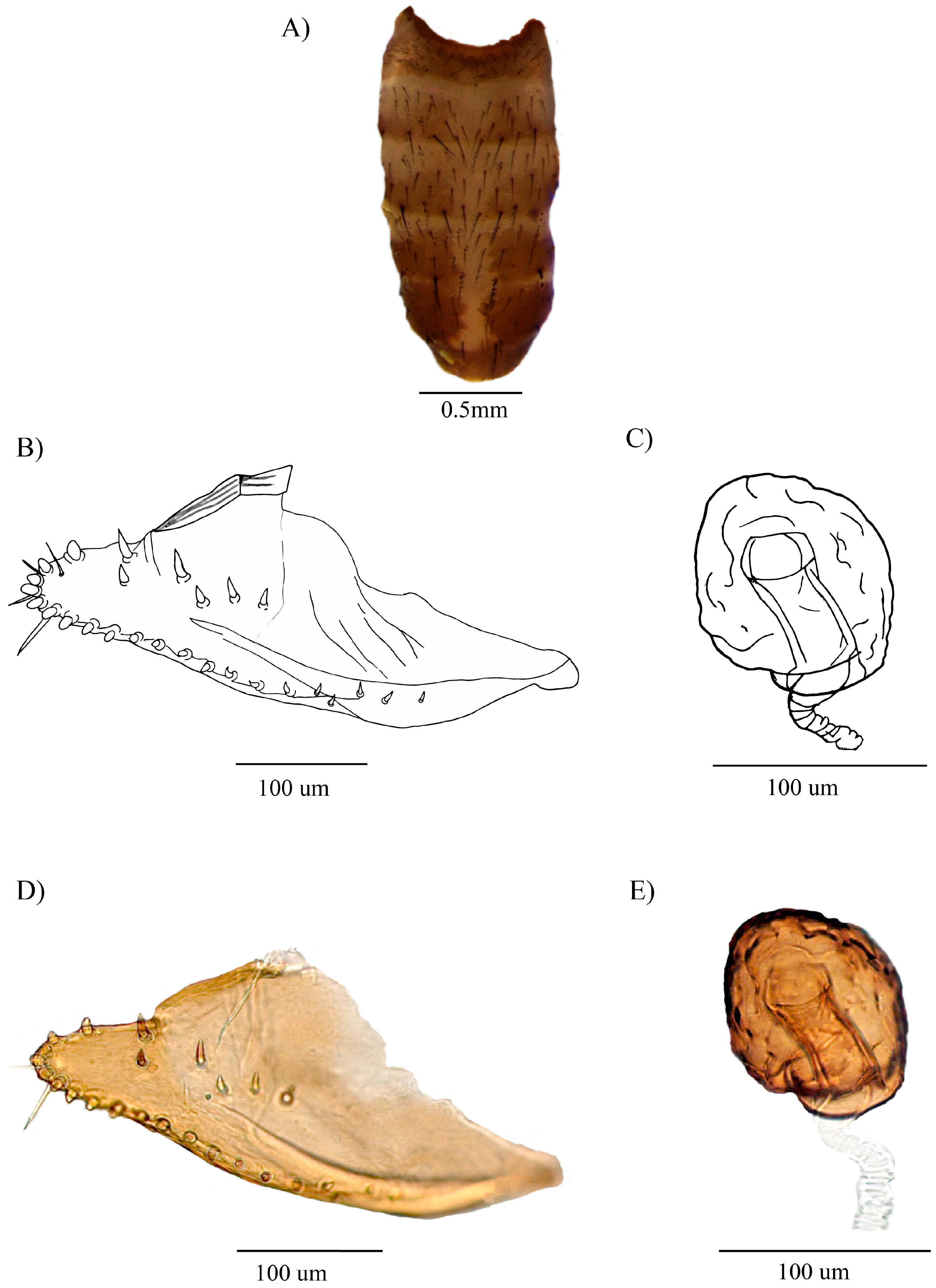
3.2.2. Description of the Male
3.2.3. Distribution
3.2.4. Biology
3.2.5. Etymology
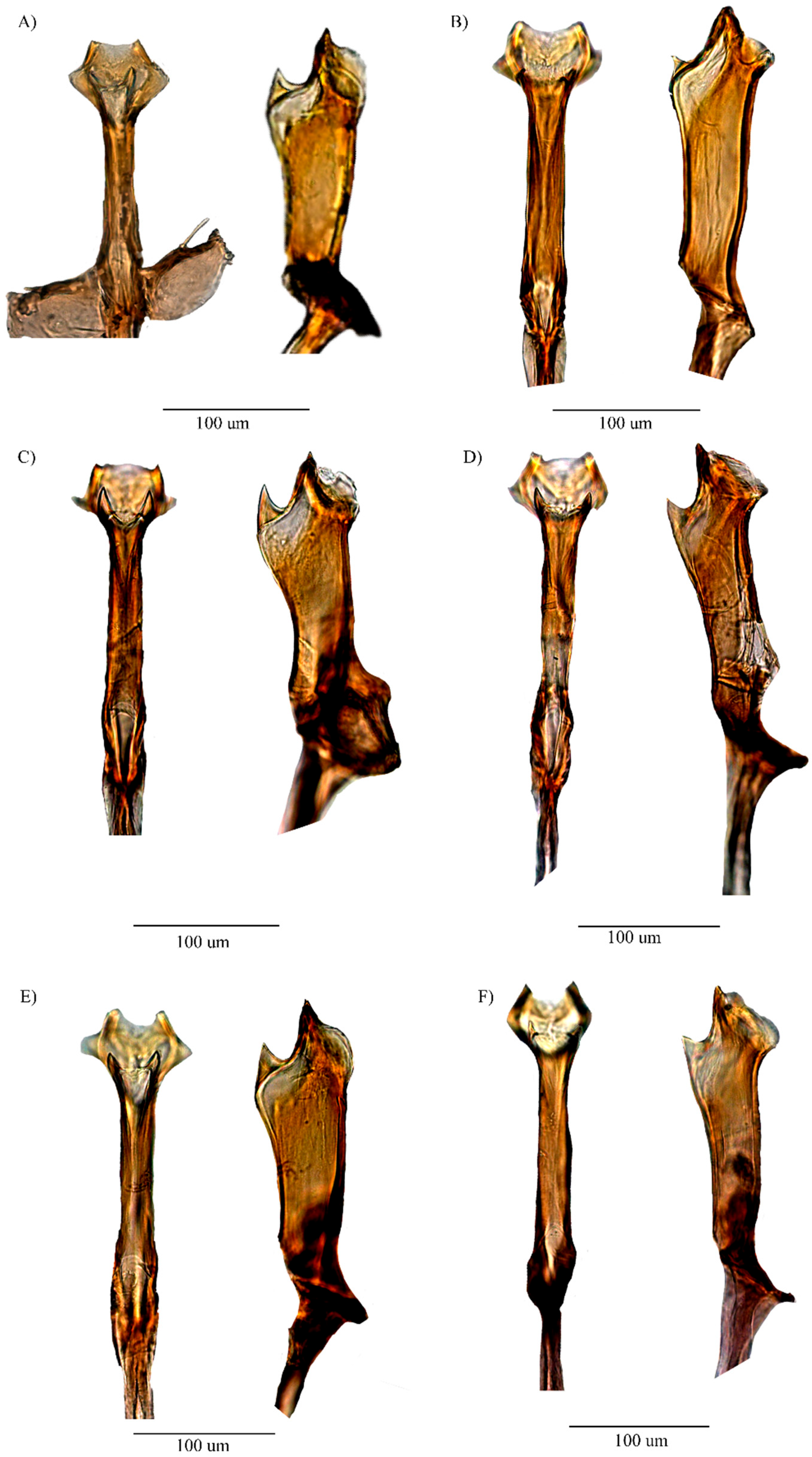
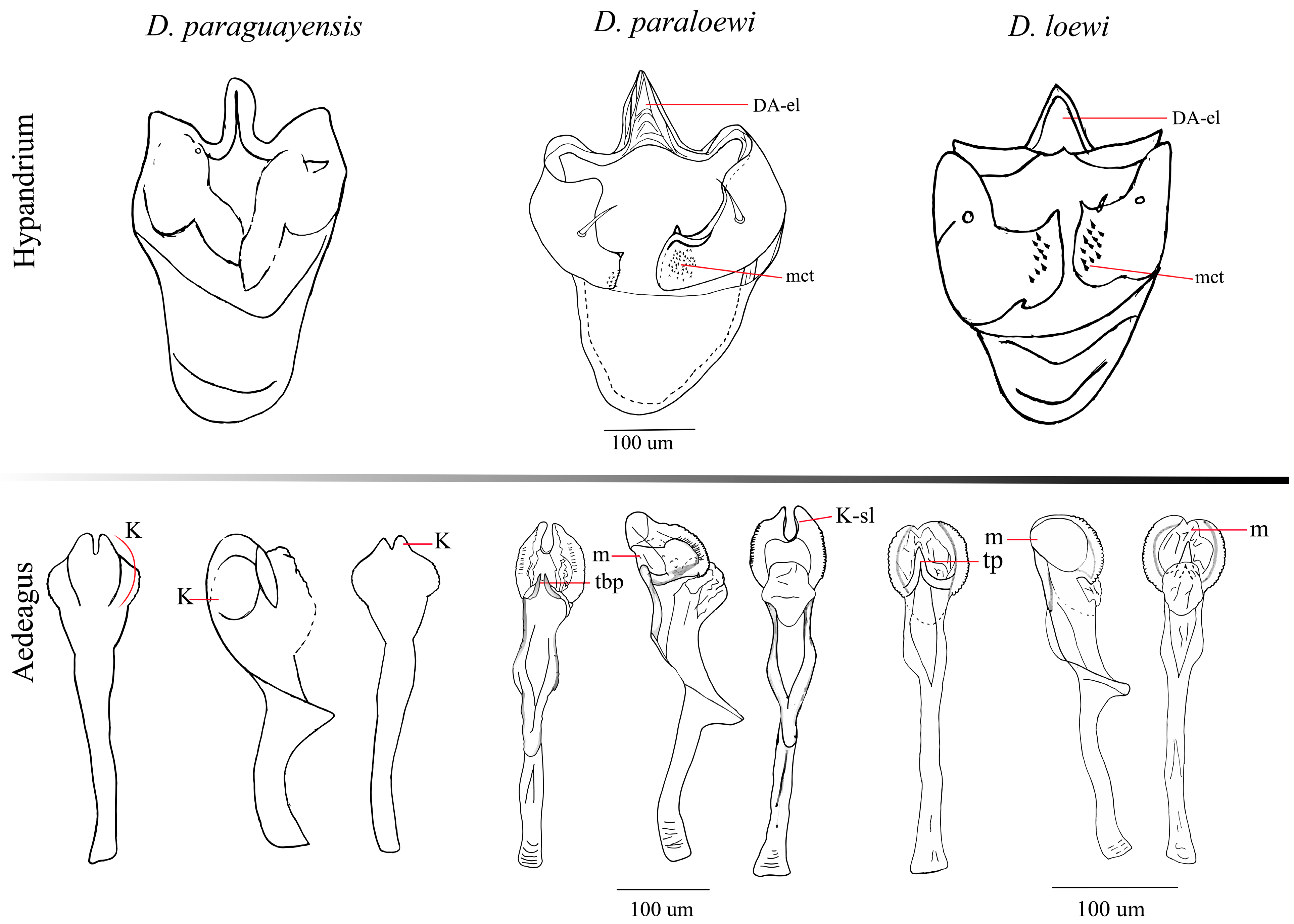
3.2.6. Relationship

4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MASL | Meters Above Sea Level |
| QCAZ-I | Quito Católica Zoología Sección Invertebrados |
| TPB | Triangular and bifurcated shape |
| P | Paraphysis |
| A-el | Elongated Dorsal Arc |
| K-sl | Slightly Keratinized |
| M | Membranous |
| MCT | Microtrichous |
| K | Keratinized |
| TP | Triangular projection |
References
- Bächli, G. TaxoDros Print Species List: Genus, Subgenus, Group. Available online: https://www.taxodros.uzh.ch/search/prt_rawfile.php?prt=SPECIES-LIST_GR_SR (accessed on 10 August 2025).
- Báez, S.; Jaramillo, L.; Cuesta, F.; Donoso, D.A. Effects of Climate Change on Andean Biodiversity: A Synthesis of Studies Published until 2015. Neotrop. Biodivers. 2016, 2, 18. [Google Scholar] [CrossRef]
- Dangles, O.; Barragán, A.; Cárdenas, R.E.; Onore, G.; Keil, C. Entomology in Ecuador: Recent Developments and Future Challenges. Ann. Soc. Entomol. Fr. 2009, 45, 424–436. [Google Scholar] [CrossRef][Green Version]
- Llangarí Arizo, L.M.; Tamayo Gudiño, M.I.; Peñafiel Vinueza, A.D.; Rafael, V. Descripción de Tres Especies Nuevas Del Género Drosophila (Diptera: Drosophilidae) En La Reserva Natural Chamanapamba, Tungurahua, Ecuador. Rev. Ecuat. Med. Cienc. Biol. 2022, 43, 37–53. [Google Scholar] [CrossRef]
- Vela, D.; Rafael, V. Ocho Nuevas Especies Del Grupo Tripunctata, Género Drosophila (Diptera, Drosophilidae), y El Registro de Drosophila Paraguayensis En El Bosque Protector Pasochoa, Pichincha-Ecuador. Rev. Pontif. Univ. Catól. Ecuad. 2001, 66, 92–120. [Google Scholar]
- Bächli, G.; Viljoen, F.; Escher, S.A.; Saura, A. The Drosophilidae (Diptera) of Fennoscandia and Denmark; Brill: Leiden, The Netherlands, 2004; Volume 39. [Google Scholar]
- Vilela, C.R.; Bächli, G. Taxonomic Studies on Neotropical Species of Seven Genera of Drosophilidae (Diptera). Mitt. Schweiz. Entomol. Ges. 1990, 63, 1–332. [Google Scholar]
- Duda, O. Die Südamerikanischen Drosophilidae (Dipteren) Unter Berücksichtigung Auch Der Anderen Neotropischen Sowie Nearktischen Arten. Naturgesch 1927, 91A, 1–228. [Google Scholar]
- Sierra, R. Propuesta Preliminar de Un Sistema de Clasificación de Vegetación Para El Ecuador Continental; Proyecto INEFAN/GEF-BIRF y Ecociencia: Quito, Ecuador, 1999. [Google Scholar]
- Ministerio del Ambiente (MAE). Naturaleza, senderismo y asombrosos paisajes en la Reserva Ecológica “El Ángel”. Available online: https://www.turismo.gob.ec/naturaleza-senderismo-y-asombrosos-paisajes-en-la-reserva-ecologica-el-angel/ (accessed on 24 January 2024).
- Cabezas, M.B. Diversidad Del Género Drosophila (Díptera, Drosophilidae) En Dos Bosques Nublados de Las Estribaciones Occidentales Ecuatorianas, Estación Científica Río Guajalito (Santo Domingo de Los Tsáchilas, Eciador) y Reserva Intillacta (Pichincha, Ecuador); Pontificia Universidad Católica del Ecuador: Quito, Ecuador, 2012; p. 156. [Google Scholar]
- Rafael, V.; Vela, D. Drosophila Distribution in Ecuador. Rev. Pontif. Univ. Catól. Ecuador 2000, 83, 85–88. [Google Scholar]
- Suárez, C.; Peñafiel-Vinueza, A.D.; Rafael, V.; Tamayo Gudiño, M.I. Descripción de Ocho Especies Nuevas Del Grupo D. Tripunctata (Diptera: Drosophilidae), En Tres Bosques Andinos de Napo, Ecuador. Rev. Ecuat. Med. Cienc. Biol. 2024, 45, 30–49. [Google Scholar] [CrossRef]
- Vilela, C.R.; Bächli, G. Five New Species of Neotropical Drosophila (Diptera, Drosophilidae). Mitt. Schweiz. Entomol. Ges. 2000, 73, 49–65. [Google Scholar]
- Bächli, G.; Vilela, C.R.; Ratcov, V. Morphological Differences among Drosophila Paraguayensis Duda, 1927 and Its Close Relatives (Diptera, Drosophilidae). Mitt. Schweiz. Entomol. Ges. = Bull. Soc. Entomol. Suisse 2000, 73, 67–92. [Google Scholar]
| Structure | Drosophila loewi | Drosophila hemiloewi | Drosophila paraloewi sp. nov. | |
|---|---|---|---|---|
| External terminalia | Ventral lobe | Rectangular shaped with one inner seta | Rounded shaped with two inner setae | Rectangular shaped with two inner setae |
| Surstylus | Three setae at the base on both sides | Single seta at the base, on the right side and three on the left side. | Single seta at the base, on the right side and two on the left side. | |
| Internal terminalia | Hypandrium | Gonopod merged to paraphysis | Gonopod merged to paraphysis | Gonopod merged to paraphysis |
| Lateral roughness on the submedial external surface of the gonopod | Lack lateral roughness on the submedial external surface of the gonopod | Lack lateral roughness on the submedial external surface of the gonopod | ||
| Aedeagus apex | Head elongated in the dorsal view but flattened in the lateral view | Head ear-shaped chitinized latero-distal membranes projected in the dorso-subapical view | Head elongated in the dorsal view but flattened in the lateral view | |
| Triangular projection between the two keratinized, serrated laminae | Triangular projection between the two keratinized, serrated laminae | Triangular bifurcated projection between the two keratinized, serrated laminae |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suárez, C.E.; Peñafiel-Vinueza, A.; Rafael, V.; Tamayo, M.I.; Vela, D. Taxonomic Revision of D. carlosvilelai Vela and Rafael, 2001, and Updated Record of D. paraguayensis Duda, 1927 Registry in Ecuador. Insects 2025, 16, 944. https://doi.org/10.3390/insects16090944
Suárez CE, Peñafiel-Vinueza A, Rafael V, Tamayo MI, Vela D. Taxonomic Revision of D. carlosvilelai Vela and Rafael, 2001, and Updated Record of D. paraguayensis Duda, 1927 Registry in Ecuador. Insects. 2025; 16(9):944. https://doi.org/10.3390/insects16090944
Chicago/Turabian StyleSuárez, Coraima Elizabeth, Ana Peñafiel-Vinueza, Violeta Rafael, María Isabel Tamayo, and Doris Vela. 2025. "Taxonomic Revision of D. carlosvilelai Vela and Rafael, 2001, and Updated Record of D. paraguayensis Duda, 1927 Registry in Ecuador" Insects 16, no. 9: 944. https://doi.org/10.3390/insects16090944
APA StyleSuárez, C. E., Peñafiel-Vinueza, A., Rafael, V., Tamayo, M. I., & Vela, D. (2025). Taxonomic Revision of D. carlosvilelai Vela and Rafael, 2001, and Updated Record of D. paraguayensis Duda, 1927 Registry in Ecuador. Insects, 16(9), 944. https://doi.org/10.3390/insects16090944





