Advances in the Molecular Mechanisms of Resistance in Chilo suppressalis
Simple Summary
Abstract
1. Introduction: The Occurrence and Damage of Chilo suppressalis
2. Advances in Insecticide Resistance in C. suppressalis
2.1. Evolution of Resistance to Nereistoxin Insecticides in C. suppressalis
2.2. Evolution of Resistance to Organophosphate Insecticides in C. suppressalis
2.3. Evolution of Resistance to Phenylpyrazole Insecticides in C. suppressalis
2.4. Evolution of Resistance to Macrolide Insecticides in C. suppressalis
2.5. Evolution of Resistance to Diacylhydrazine Insecticides in C. suppressalis
2.6. Evolution of Resistance to Diamide Insecticides in C. suppressalis
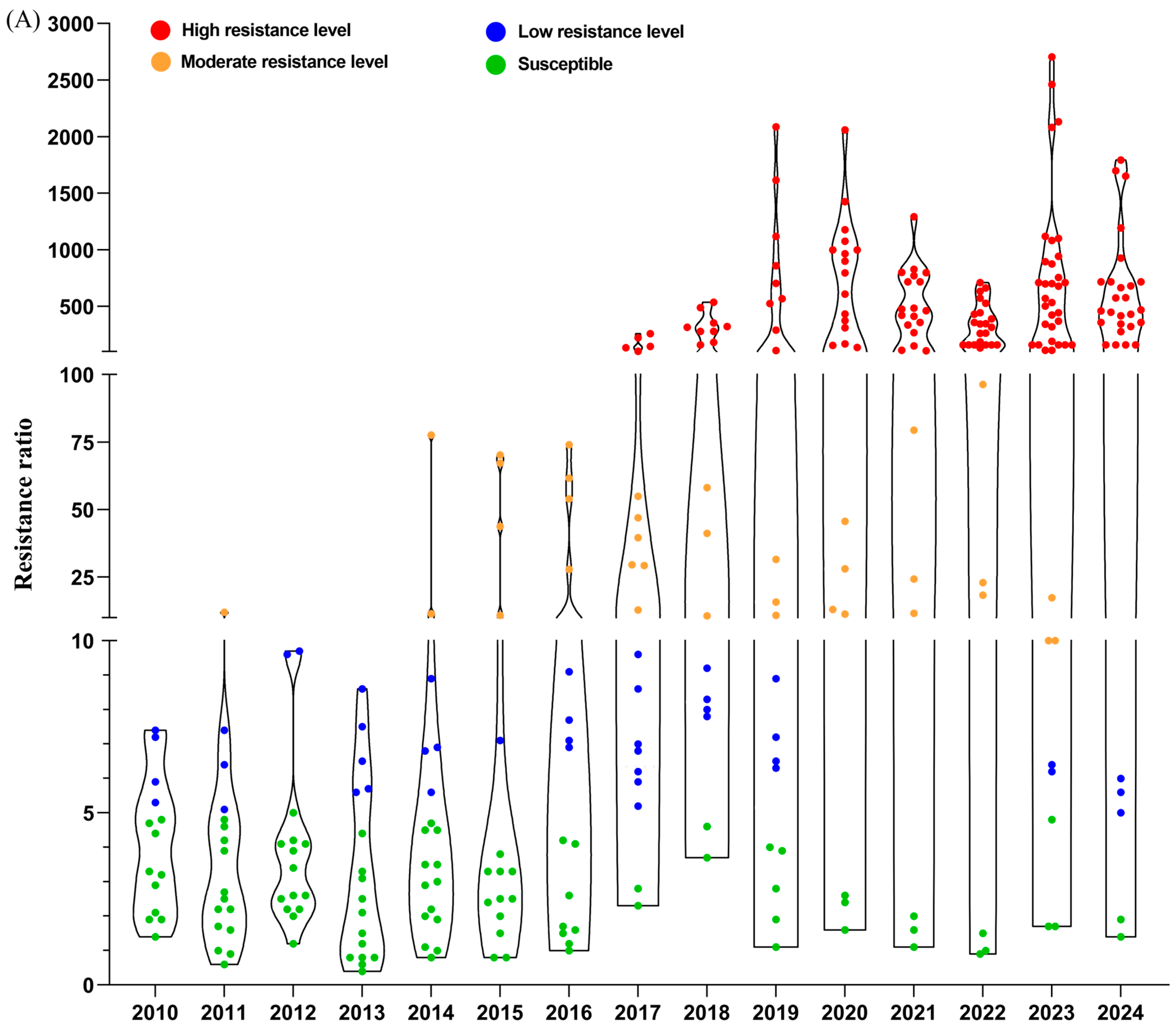
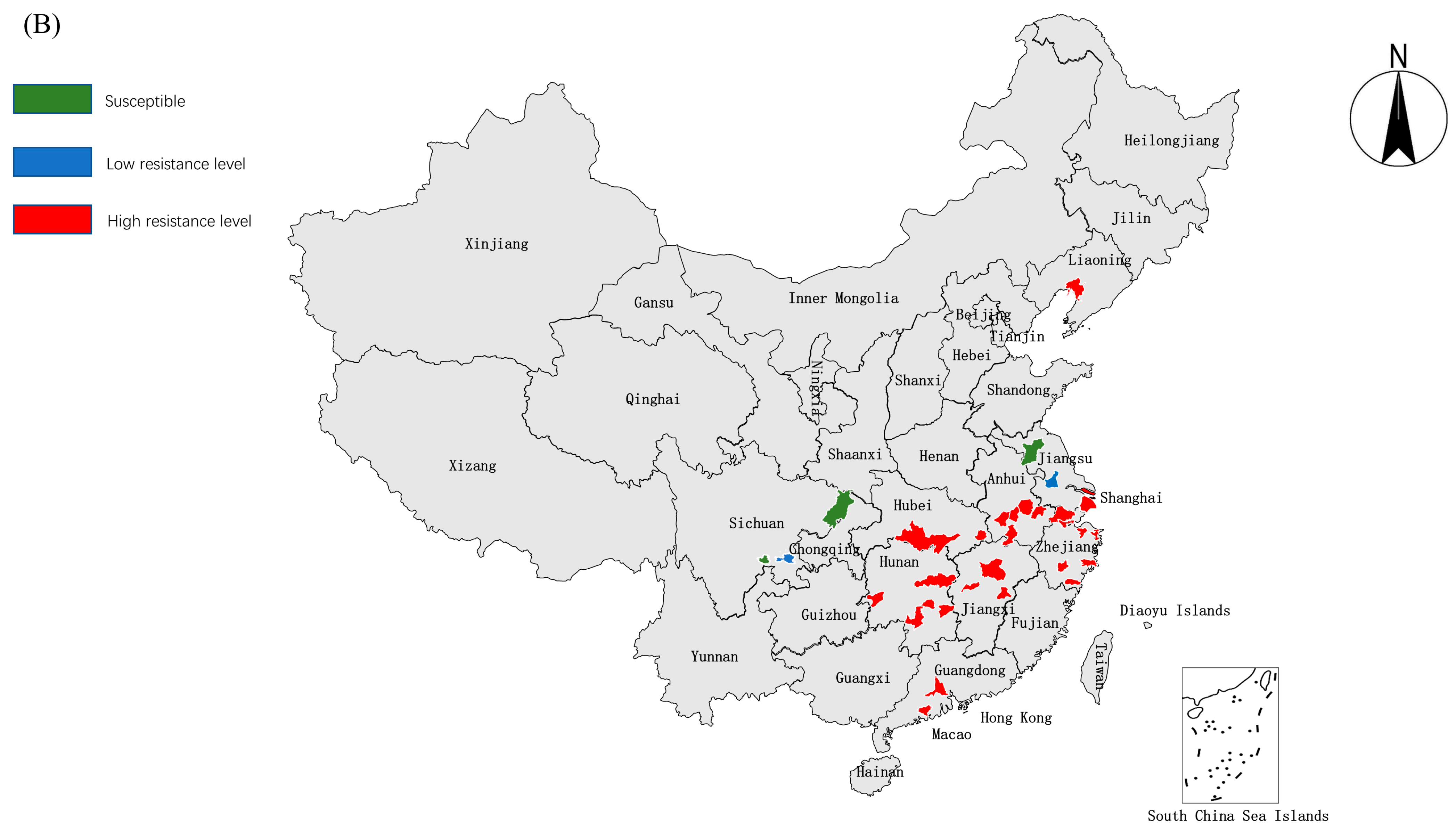
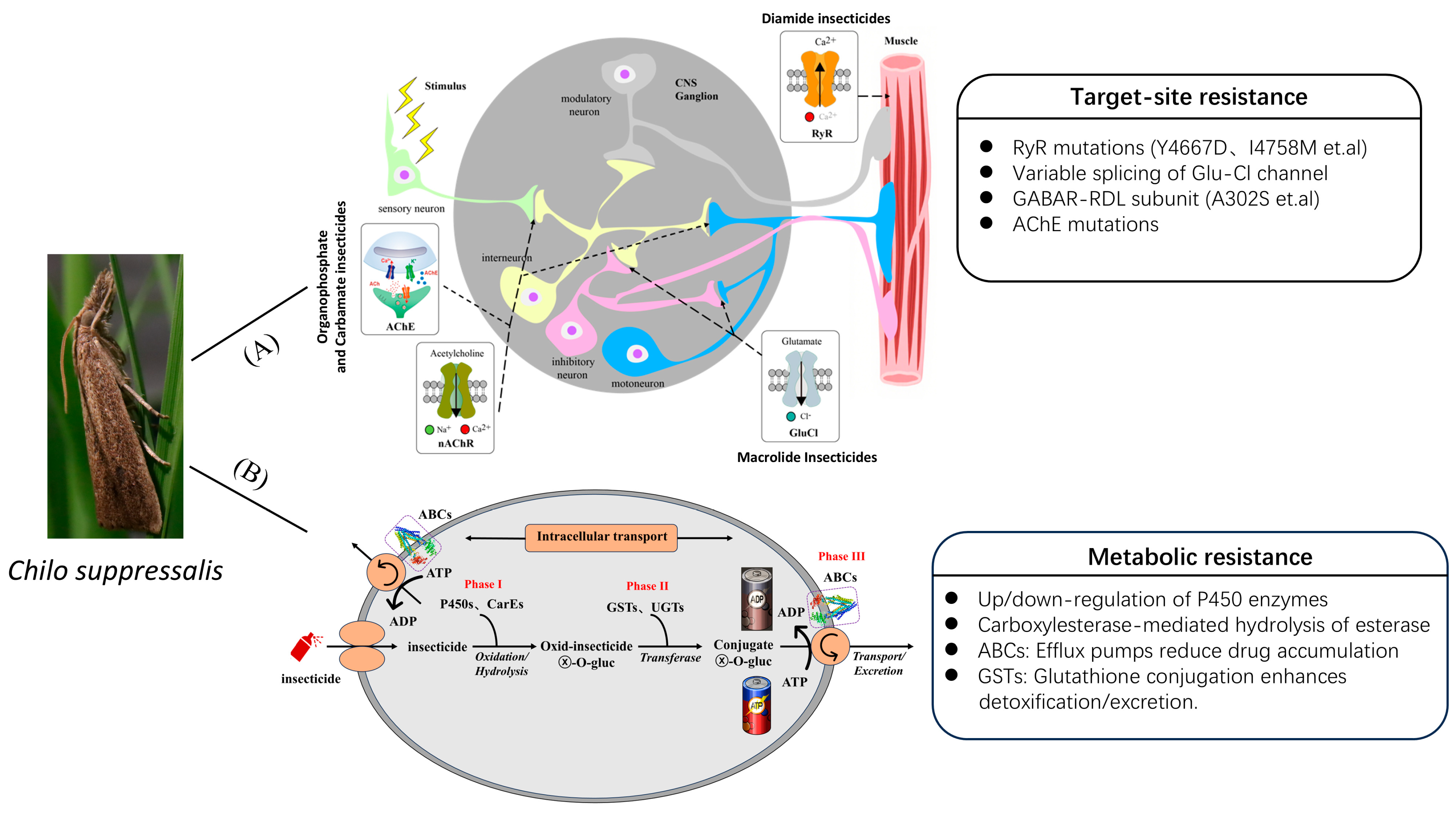
3. The Resistance Mechanisms of C. suppressalis
3.1. Target-Site Resistance
3.1.1. Acetylcholinesterase (AChE)
3.1.2. Ryanodine Receptor (RyR)
3.1.3. Glutamate-Gated Chloride Channels (GluCls)
3.2. Metabolic Resistance
3.2.1. Cytochrome P450 Monooxygenases
3.2.2. Carboxylesterases
3.2.3. Glutathione S-Transferases
3.2.4. UDP-Glycosyltransferases
3.2.5. ATP-Binding Cassette
3.2.6. Flavin-Containing Monooxygenases
| Insecticides | Resistance Ratios | Target-Site Resistance | Metabolic Resistance | Functional Validation | References |
|---|---|---|---|---|---|
| Triazophos | 68.7 | _ | CYP324A12, CYP321F3 and CYP9A68 | Synergism experiment and qRT-PCR | [15] |
| Carbofuran | >1000 | E101D A314S F402V R667Q H668P | _ | Enzyme kinetics and inhibition assays | [52,53] |
| Methoxyfenozide | >100 | _ | CYP321F3 | Synergism experiment, qRT-PCR, and transgenic expression in Drosophila melanogaster | [39] |
| Chlorantraniliprole | 82.37 | _ | CYP6CV5, CYP9A68, CYP321F3 and CYP324A12 | RNAi | [71] |
| Chlorantraniliprole | 44.32 | _ | UGT40AL11 and UGT33AG3 | RNAi | [77] |
| Chlorantraniliprole | 77.6 | G4910E | Bioassay and sequencing of CsRyR | [44] | |
| Fubendiamide | 42.6 | G4910E | Bioassay and sequencing of CsRyR | [44] | |
| Chlorantraniliprole | 249.6 | Y4667D Y4667C I4758M | Bioassay and sequencing of CsRyR | [56] | |
| Chlorantraniliprole | 102.9–536.8 | Y4667D/C I4758M G4915E Y4891F | Bioassay, sequencing of CsRyR and CRISPR/Cas9 genome-modified Drosophila melanogaster | [46] | |
| Chlorantraniliprole | 109.6–2087.5 | I4758M and Y4667C | Bioassay, sequencing of CsRyR and CRISPR/Cas9 genome-modified Drosophila melanogaster | [47] | |
| Tetraniliprole | 27.7–806.8 | Y4667D/C I4758M G4915E Y4891F | Bioassay, sequencing of CsRyR and CRISPR/Cas9 genome-modified Drosophila melanogaster | [57] | |
| Chlorantraniliprole | 111.6–2706.4 | Y4667D | Bioassay, introgression of the CsRyR 4667D allele into the susceptible strain and molecular docking | [48] |
4. C. suppressalis Resistance Management
5. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sheng, C.F.; Wang, H.T.; Sheng, S.Y.; Gao, L.D.; Xuan, W.J. Pest status and loss assess ment of crop damage caused by the rice borers Chilo suppressalis and Tryporyza incertulas in China. Entomol. Knowl. 2003, 40, 289–294. [Google Scholar]
- Yi, B.J. Occurrence Regularity and Control Technology of Chilo suppressalis in Rice. North. Rice 2014, 44, 52–53. [Google Scholar]
- Sheng, C.F.; Wang, H.T.; Gao, L.D.; Xuan, W.J. Current Status, Loss Estimation and Control Strategies of Major Outbreaks of Rice Stem Borers in China. Plant Prot. 2003, 29, 37–39. [Google Scholar]
- Xiang, Y.Y.; Zhang, F.; Xia, B.W.; Guo, R. Occurrence Status and Control Countermeasures of Rice Stem Borers in China. China Plant Prot. 2011, 31, 20–23. [Google Scholar]
- Ye, G.Y.; Fang, Q.; Xu, H.X.; Wu, S.F.; Teng, Z.W.; Xu, G.; Dang, C.; Xiong, S.J. Research Advances on the Occurrence, Damage and Management of Rice Stem Borers in China. Plant Prot. 2023, 49, 167–180. [Google Scholar]
- Wang, H. Occurrence and Control of Chilo suppressalis in Rice. North. Rice 2011, 41, 55. [Google Scholar]
- Wu, L.Y.; Chen, J.G. Investigation on Occurrence Regularity and Forecasting Methods of Chilo suppressalis in Cold Region Rice. China Plant Prot. 2009, 29, 19–20. [Google Scholar]
- Jiang, Q.; Zhan, H.Y.; Deng, J.Q. Occurrence Characteristics and Control Strategies of Chilo suppressalis in Rice in Changde City. China Plant Prot. 2023, 43, 51–55. [Google Scholar]
- Wang, Z.J.; Ren, Z.J.; Song, X.D.; Wang, C.R.; Zhang, Q.F.; Yu, H.C. The Frequency of Occurrence of Chilo suppressalis in Harbin. Chin. J. Appl. Entomol. 2023, 60, 913–921. [Google Scholar]
- Yang, Z.; Tang, G.P.; Han, R.C.; Zhu, S.; Huang, R.L. Effects of Different Foods on the Growth and Reproduction of the Rice Stem Borer Chilo suppressalis. Chin. J. Appl. Entomol. 2025, 62, 172–178. [Google Scholar]
- Lin, K.J.; Hou, M.L.; Han, L.Z.; Liu, Y.D. Research Progress in Host Selection and Underlying Mechanisms, and Factors Affecting Population Dynamics of Chilo suppressalis. Plant Prot. 2008, 22–28. [Google Scholar]
- He, S.C.; Zhou, S.X.; Li, L.J.; Gao, Y.B.; Mao, G.; Liu, J.; Sun, K.N.; Li, G.X.; Lu, X. Loss of Rice Crops Caused by the Stemborer, Chilo suppressalis. Chin. J. Appl. Entomol. 2025, 61, 157–161. [Google Scholar]
- Che, L.; Jiang, Q.H.; Wang, Y.; Li, C.G.; Yan, S. Comparative Analysis of Occurrence and Control of Pests in Five Rice-Producing Regions in China. Plant Prot. 2022, 48, 233–241. [Google Scholar]
- Zhong, Z.H.; Wang, X.M.; Liu, L.; Xiao, H.; Zhong, L. Progress and Strategy Optimization on Chemical Control and Integrated Management of Striped Rice Stemborer, Chilo suppressalis (Walker). Mod. Agrochem. 2023, 22, 17–21. [Google Scholar]
- Zhao, X.; Xu, X.; Wang, X.G.; Yin, Y.; Li, M.Y.; Wu, Y.Q.; Liu, Y.H.; Cheng, Q.H.; Gong, C.W.; Shen, L.T. Mechanisms for multiple resistances in field populations of rice stem borer, Chilo suppressalis (Lepidoptera: Crambidae) from Sichuan Province, China. Pestic. Biochem. Physiol. 2021, 171, 104720. [Google Scholar] [CrossRef]
- Wang, S.; Liu, C.; Qiao, S.T.; Guo, F.R.; Xie, Y.; Sun, H.; Liu, Y.; Zhao, S.Q.; Zhou, L.Q.; He, L.F.; et al. The Evolution and Mechanisms of Multiple-Insecticide Resistance in Rice Stem Borer, Chilo suppressalis Walker (Lepidoptera: Crambidae). J. Agric. Food Chem. 2024, 72, 26475–26490. [Google Scholar] [CrossRef]
- Su, J.Y.; Zhang, Z.Z.; Wu, M.; Gao, C.F. Geographic susceptibility of Chilo suppressalis Walker (Lepidoptera: Crambidae), to chlorantraniliprole in China. Pest Manag. Sci. 2014, 70, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.S.; Zhou, L.J.; Hu, M.Y. Study on the Mechanism and Resistance to Pesticides of Nerextoxion. Pestic. Sci. Admin. 2001, 22, 30–32. [Google Scholar]
- Su, J.K.; Chu, B. Resistance of the Rice Stem Borer Chilo suppressalis Walker to Monosultap in Yangzhou District. J. Yangzhou Univ. 2004, 25, 76–78. [Google Scholar]
- Jiang, X.H.; Zhang, Q.H.; Hu, S.M.; Xie, S.J.; Xu, X.G. The Status of Pesticide Resistance of Rice Stalk Borer in Zhejiang Province and Their Management Tactics. China Plant Prot. 2001, 21, 27–29. [Google Scholar]
- Xiong, J.M.; Zhu, X.F.; Cheng, L.X. The Current Status of Resistance of Chilo suppressalis in Nanchang Area and the Management Strategies. Biol. Disaster Sci. 2004, 27, 3–4. [Google Scholar]
- Zhou, L.Q. Resistance Monitoring and Resistance Molecular Mechanism to Chlorantraniliprole in Chilo suppressalis (Walker). Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2017. [Google Scholar]
- Cao, M.Z.; Shen, J.L.; Zhang, J.Z.; Lü, M.; Liu, X.Y.; Zhou, W.J. Monitoring of Insecticide Resistance and Inheritance Analysis of Triazophos Resistance in the Striped Stem Borer (Lepidoptera: Pyralidae). Chin. J. Rice Sci. 2004, 18, 75–81. [Google Scholar]
- Qu, M.J.; Han, Z.J.; Xu, X.J.; Shao, X.L.; Tian, X.Z.; Fu, M.L. Dynamics and Risk Assessment of Triazophos Resistance in Rice Stem Borer (Chilo suppressalis Walker). J. Nanjing Agric. Univ. 2005, 28, 38–42. [Google Scholar]
- Huang, C.H. Studies on Differential Susceptibility and Its Mechanisms to Fipronil and Triazophosphos of Rice Stem Borers, Chilo suppressalis and Sesamia inferens. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2006. [Google Scholar]
- Xiong, J.M.; Huang, X.Y.; Zhu, X.F.; Xiao, H.J. Test of resistance to insecticides in rice stem borer, Chilo suppressalis. Biol. Disaster Sci. 2006, 29, 175–177. [Google Scholar]
- Ying, Z.L. Monitoring of Resistance of Chilo suppressalis to Fipronil and Other Esticides and the Research Advance Management. Master’s Thesis, Hunan Agricultural University, Changsha, China, 2007. [Google Scholar]
- Hu, J. Monitoring of Insecticide Resistance and Resistance Risk Assessment for JS118 in the Rice Stem Borer, Chilo suppressalis (Walker). Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2010. [Google Scholar]
- Mao, K.K.; Li, W.H.; Liao, X.; Liu, C.Y.; Qin, Y.; Ren, Z.J.; Qin, X.Y.; Wan, H.; Sheng, F.; Li, J.H. Dynamics of Insecticide Resistance in Different Geographical Populations of Chilo suppressalis (Lepidoptera: Crambidae) in China 2016–2018. J. Econ. Entomol. 2019, 112, 1866–1874. [Google Scholar] [CrossRef]
- Meng, H.R.; Huang, R.; Wan, H.; Li, J.H.; Li, J.K.; Zhang, X.L. Insecticide resistance monitoring in field populations of Chilo suppressalis Walker (Lepidoptera: Crambidae) from central China. Front. Physiol. 2022, 13, 1029319. [Google Scholar] [CrossRef]
- Su, C.Y.; Li, C.Y.; Cai, T.W.; Xie, Y.L.; He, S.; Ma, K.S.; Wan, H.; Zhou, H.Z.; Li, J.H. Resistance monitoring in field populations of Chilo suppressalis (Lepidoptera: Crambidae) in Hubei Province to four insecticides in 2019–2022. J. Appl. Entomol. 2024, 148, 272–278. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.P.; Shao, Z.R.; Guo, J.Q. Monitoring and Management of Insecticide Resistance of Chilo suppressalis in China. Plant Prot. 2011, 37, 141–144. [Google Scholar]
- Xiong, J.M.; Zhu, X.F.; Xiao, H.J. Monitoring and Administering of Resistance of Rice Stem Borer, Chilo suppressalis to 4 Conventional Insecticides in Nanchang Region. Acta Agric. Univ. 2006, 28, 877–880. [Google Scholar]
- Lv, L.; Chen, Q.Z.; Zhang, S.; Yang, X.L.; Chang, X.Q. Mensuration and Analysis of Insecticide Resistance of Chilo suppressalis (Walker) and Tryporyza incertulas (Walker). J. Huazhong Agric. Univ. 2008, 27, 213–216. [Google Scholar]
- Zhao, D.D. Resistance Monitoring to Insecticides in Chilo suppressalis (Walker) and Cloning and Expression Analysis of Ryanodine Receptor Genes in Sesamia inferens (Walker). Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2013. [Google Scholar]
- Zhang, S. Results of National Agricultural Pest Resistance Monitoring in 2020 and Suggestions for Scientific Pesticide Use. China Plant Prot. 2021, 41, 71–78. [Google Scholar]
- Wang, S.; Huang, J.M.; Guo, F.R.; Liu, C.; Xie, Y.; Qiao, S.T.; Chen, Y.X.; Wu, S.F.; Bass, C.; Gao, C.F. Flavin-Dependent Monooxgenase Confers Resistance to Chlorantraniliprole and Spinetoram in the Rice Stem Borer Chilo suppressalis Walker (Lepidoptera: Crambidae). J. Agric. Food Chem. 2024, 72, 26943–26956. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.H.; Wang, G.R.; Zhong, L.Q.; Zhang, F.C.; Bai, Q.; Zheng, X.S.; Lu, Z.X. Resistance monitoring of Chilo suppressalis (Walker) (Lepidoptera: Crambidae) to chlorantraniliprole in eight field populations from east and central China. Crop Prot. 2017, 100, 196–202. [Google Scholar] [CrossRef]
- Guo, F.R.; Wang, S.C.; Liu, Y.; Wang, S.; Huang, J.M.; Sun, H.; He, L.F.; Xie, Y.; Qiao, S.T.; Yang, F.X.; et al. CYP321F3 mediates metabolic resistance to methoxyfenozide in rice stem borer, Chilo suppressalis. Pestic. Biochem. Physiol. 2025, 210, 106383. [Google Scholar] [CrossRef]
- Zhang, Z.Z. Monitoring of Insecticide Resistance and Baseline Susceptibility of Chilo suppressalis (Lepidoptera: Pyralidae) to Chlorantraniliprole. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2012. [Google Scholar]
- Gao, C.F.; Yao, R.; Zhang, Z.Z.; Wu, M.; Zhang, S.; Su, J.Y. Susceptibility Baseline and Chlorantraniliprole Resistance Monitoring in Chilo suppressalis (Lepidoptera: Pyralidae). J. Econ. Entomol. 2013, 106, 2190–2194. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, S.; Yao, R.; Wu, S.F.; Su, J.Y.; Gao, C.F. Susceptibility of the Rice Stem Borer, Chilo suppressalis (Lepidoptera: Crambidae), to Flubendiamide in China. J. Econ. Entomol. 2014, 107, 1250–1255. [Google Scholar] [CrossRef]
- Su, J.Y.; Zhang, Z.Z.; Wu, M.; Gao, C.F. Changes in Insecticide Resistance of the Rice Striped Stem Borer (Lepidoptera: Crambidae). J. Econ. Entomol. 2014, 107, 333–341. [Google Scholar] [CrossRef]
- Yao, R.; Zhao, D.D.; Zhang, S.; Zhou, L.Q.; Wang, X.; Gao, C.F.; Wu, S.F. Monitoring and mechanisms of insecticide resistance in Chilo suppressalis (Lepidoptera: Crambidae), with special reference to diamides. Pest Manag. Sci. 2017, 73, 1169–1178. [Google Scholar] [CrossRef]
- Zhao, D.D.; Zhou, L.Q.; Zhang, S.; Yao, R.; Qiu, Y.X.; Gao, C.F. Resistance Monitoring and Cross-resistance to the Diamides in the Rice Stem Borer, Chilo suppressalis (Lepidoptera: Pyralidae). Chin. J. Rice Sci. 2017, 31, 307–314. [Google Scholar]
- Huang, J.M.; Rao, C.; Wang, S.; He, L.F.; Zhao, S.Q.; Zhou, L.Q.; Zhao, Y.X.; Yang, F.X.; Gao, C.F.; Wu, S.F. Multiple target-site mutations occurring in lepidopterans confer resistance to diamide insecticides. Insect Biochem. Mol. Biol. 2020, 121, 103367. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.M.; Sun, H.; He, L.F.; Liu, C.; Ge, W.C.; Ni, H.; Gao, C.F.; Wu, S.F. Double ryanodine receptor mutations confer higher diamide resistance in rice stem borer, Chilo suppressalis. Pest Manag. Sci. 2021, 77, 4971–4979. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Qiao, S.T.; Li, P.Z.; Xie, Y.; Guo, F.R.; Liu, J.W.; Hu, W.K.; Gao, M.Y.; Zheng, L.J.; Yang, F.X.; et al. Y4667D Mutation in the Ryanodine Receptor Confers High Level Resistance to Diamide Insecticides in the Rice Stem Borer, Chilo suppressalis Walker (Lepidoptera: Crambidae). J. Agric. Food Chem. 2025, 73, 9920–9931. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Peng, Y.C.; Han, Z.J. Cloning and Polymorphism Analysis of Acetylcholinesterase Genes from Sesamia inferens. J. Nanjing Agric. Univ. 2019, 42, 1050–1058. [Google Scholar]
- Reyes-Espinosa, F.; Méndez-Alvarez, D.; Pérez-Rodríguez, M.A.; Herrera-Mayorga, V.; Juarez-Saldivar, A.; Cruz-Hernandez, M.A.; Rivera, G. In Silico Study of the Resistance to Organophosphorus Pesticides Associated with Point Mutations in Acetylcholinesterase of Lepidoptera: B. mandarina, B. mori, C. auricilius, C. suppressalis, C. pomonella, H. armigera, P. xylostella, S. frugiperda, and S. litura. Int. J. Mol. Sci. 2019, 20, 2404. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Chen, C.K.; Han, Z.J.; Wang, Y.C.; Tian, Z.H. Resistance Measurement of Chilo suppressalis from Jiangsu Province and its Resistance Mechanism to Methamidophos. J. Plant Prot. 2001, 28, 173–177. [Google Scholar]
- Dai, S.M.; Chang, C.; Huang, X.Y. Distinct contributions of A314S and novel R667Q substitutions of acetylcholinesterase 1 to carbofuran resistance of Chilo suppressalis Walker. Pest Manag. Sci. 2016, 72, 1421–1426. [Google Scholar] [CrossRef]
- Chang, C.; Cheng, X.; Huang, X.Y.; Dai, S.M. Amino acid substitutions of acetylcholinesterase associated with carbofuran resistance in Chilo suppressalis. Pest Manag. Sci. 2014, 70, 1930–1935. [Google Scholar] [CrossRef]
- Huang, C.H.; Fang, Q.; Ye, G.Y.; Yao, H.W.; Guo, J.Y.; Cheng, J.A. Tissue and subcellular distribution of insecticide–Resistance related enzymes in larvae of striped stem borer, Chilo suppressalis (Walker). J. South. Agric. 2009, 40, 153–158. [Google Scholar]
- Richardson, E.B.; Troczka, B.J.; Gutbrod, O.; Davies, T.G.E.; Nauen, R. Diamide resistance: 10 years of lessons from lepidopteran pests. J. Pest Sci. 2020, 93, 911–928. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, L.; Chen, Q.; Qin, W.J.; Huang, S.J.; Jiang, Y.; Qin, H.G. Chlorantraniliprole resistance and its biochemical and new molecular target mechanisms in laboratory and field strains of Chilo suppressalis (Walker). Pest Manag. Sci. 2018, 74, 1416–1423. [Google Scholar] [CrossRef]
- Sun, H.; Wang, S.; Liu, C.; Hu, W.K.; Liu, J.W.; Zheng, L.J.; Gao, M.Y.; Guo, F.R.; Qiao, S.T.; Liu, J.L.; et al. Risk assessment, fitness cost, cross-resistance, and mechanism of tetraniliprole resistance in the rice stem borer, Chilo suppressalis. Insect Sci. 2023, 31, 835–846. [Google Scholar] [CrossRef]
- Ma, R.F.; Haji-Ghassemi, O.; Ma, D.; Jiang, H.; Lin, L.Y.; Xu, T.; Murayama, T.; Moussian, B.; Van Petegem, F.; Yuchi, Z.G. Structural Basis for Diamide Modulation of Ryanodine Receptor: Calcium Signaling and Excitation-Contraction in Cardiac, Skeletal and Smooth Muscle. Nat. Chem. Biol. 2020, 16, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Mo, W.J.; Lu, Z.X.; Ullah, F.; Zheng, X.S.; Xu, H.X.; Lu, Y.H. Csu-miR-375 and Csu-miR-11631 regulate ryanodine receptor to induce chlorantraniliprole resistance in Chilo suppressalis. Entomol. Gen. 2025, 45, 285–294. [Google Scholar] [CrossRef]
- Wolstenholme, A.J. Glutamate-gated Chloride Channels. J. Biol. Chem. 2012, 287, 40232–40238. [Google Scholar] [CrossRef]
- Wu, S.F.; Mu, X.C.; Dong, Y.X.; Wang, L.X.; Wei, Q.; Gao, C.F. Expression pattern and pharmacological characterisation of two novel alternative splice variants of the glutamate-gated chloride channel in the small brown planthopper Laodelphax striatellus. Pest Manag. Sci. 2017, 73, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; O’Reilly, A.O.; Williamson, M.S.; Puinean, A.M.; Yang, Y.H.; Wu, S.W.; Wu, Y.D. Function and pharmacology of glutamate-gated chloride channel exon 9 splice variants from the diamondback moth Plutella xylostella. Insect Biochem. Mol. Biol. 2019, 104, 58–64. [Google Scholar] [CrossRef]
- Wang, S.; Qiao, S.T.; Guo, F.R.; Xie, Y.; Liu, C.; Liu, J.W.; Zhao, S.Q.; Zhou, L.Q.; Yang, F.X.; Wu, S.F.; et al. Overexpression and alternative splicing of the glutamate-gated chloride channel are associated with emamectin benzoate resistance in the rice stem borer, Chilo suppressalis Walker (Lepidoptera: Crambidae). Pest Manag. Sci. 2025, 81, 2114–2125. [Google Scholar] [CrossRef]
- Wang, X.; Wang, R.; Yang, Y.; Wu, S.; O’Reilly, A.O.; Wu, Y. A point mutation in the glutamate-gated chloride channel of Plutella xylostella is associated with resistance to abamectin. Insect Mol. Biol. 2016, 25, 116–125. [Google Scholar] [CrossRef]
- Wang, X.L.; Puinean, A.M.; O’Reilly, A.O.; Williamson, M.S.; Smelt, C.L.C.; Millar, N.S.; Wu, Y.D. Mutations on M3 helix of Plutella xylostella glutamate-gated chloride channel confer unequal resistance to abamectin by two different mechanisms. Insect Biochem. Mol. Biol. 2017, 86, 50–57. [Google Scholar] [CrossRef]
- Sun, X.; Hua, W.J.; Wang, K.K.; Song, J.J.; Zhu, B.; Gao, X.W.; Liang, P. A novel V263I mutation in the glutamate-gated chloride channel of Plutella xylostella (L.) confers a high level of resistance to abamectin. Int. J. Biol. Macromol. 2023, 230, 123389. [Google Scholar] [CrossRef] [PubMed]
- Wang, S. Resistance Monitoring and Preliminary Study on Resistance Mechanism of Chilo suppressalis to Abamectin. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2021. [Google Scholar]
- Amezian, D.; Nauen, R.; Le Goff, G. Comparative analysis of the detoxification gene inventory of four major Spodoptera pest species in response to xenobiotics. Insect Biochem. Mol. Biol. 2021, 138, 103646. [Google Scholar] [CrossRef] [PubMed]
- Li, X.T.; Huang, Q.C.; Yuan, J.Z.; Tang, Z.H. Fipronil resistance mechanisms in the rice stem borer, Chilo suppressalis Walker. Pestic. Biochem. Physiol. 2007, 89, 169–174. [Google Scholar] [CrossRef]
- He, Y.P.; Zhang, J.F.; Chen, J.M. Effect of Synergists on Susceptibility to Chlorantraniliprole in Field Populations of Chilo suppressalis (Lepidoptera: Pyralidae). J. Econ. Entomol. 2014, 107, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhao, J.; Sun, Y.; Xu, D.J.; Xu, G.C.; Xu, X.L.; Zhang, Y.L.; Huang, S.J.; Han, Z.J.; Gu, Z.Y. Constitutive overexpression of cytochrome P450 monooxygenase genes contributes to chlorantraniliprole resistance in Chilo suppressalis (Walker). Pest Manag. Sci. 2019, 75, 718–725. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, J.; Xu, D.J.; Xu, G.C.; Peng, Y.C.; Zhang, Y.A. New insights into chlorantraniliprole metabolic resistance mechanisms mediated by the striped rice borer cytochrome P450 monooxygenases: A case study of metabolic differences. Sci. Total Environ. 2024, 912, 169229. [Google Scholar] [CrossRef] [PubMed]
- Zibaee, A.; Sendi, J.J.; Ghadamyari, M.; Alinia, F.; Etebari, K. Diazinon Resistance in Different Selected Strains of Chilo suppressalis (Lepidoptera: Crambidae) in Northern Iran. J. Econ. Entomol. 2009, 102, 1189–1196. [Google Scholar] [CrossRef]
- Enayati, A.A.; Ranson, H.; Hemingway, J. Insect glutathione transferases and insecticide resistance. Insect Mol. Biol. 2005, 14, 3–8. [Google Scholar] [CrossRef]
- Meng, X.K.; Wu, Z.L.; Jiang, C.Y.; Guan, D.J.; Zhang, N.; Jiang, H.; Shen, Q.W.; Qian, K.; Wang, J.J. Identification and characterization of glutathione S-transferases and their potential roles in detoxification of abamectin in the rice stem borer, Chilo suppressalis. Pestic. Biochem. Physiol. 2022, 182, 105050. [Google Scholar] [CrossRef]
- Bock, K.W. The UDP-glycosyltransferase (UGT) superfamily expressed in humans, insects and plants: Animal-plant arms-race and co-evolution. Biochem. Pharmacol. 2016, 99, 11–17. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, L.; Sun, Y.; Song, P.P.; Han, Z.J. UDP-Glycosyltransferase Genes in the Striped Rice Stem Borer, Chilo suppressalis (Walker), and Their Contribution to Chlorantraniliprole Resistance. Int. J. Mol. Sci. 2019, 20, 1064. [Google Scholar] [CrossRef]
- Li, X.X.; Zhu, B.; Gao, X.W.; Liang, P. Over-expression of UDP-glycosyltransferase gene UGT2B17 is involved in chlorantraniliprole resistance in Plutella xylostella (L.). Pest Manag. Sci. 2017, 73, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.J.; Yang, X.M.; Jiang, H.; Zhang, N.; Wu, Z.L.; Jiang, C.Y.; Shen, Q.W.; Qian, K.; Wang, J.J.; Meng, X.K. Identification and Validation of ATP-Binding Cassette Transporters Involved in the Detoxification of Abamectin in Rice Stem Borer, Chilo suppressalis. J. Integr. Agric. 2022, 70, 4611–4619. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.C.; Zhao, J.; Sun, Y.; Wan, P.; Hu, Y.Y.; Luo, G.H.; Qin, W.J.; Huang, S.J. Insights into chlorantraniliprole resistance of Chilo suppressalis: Expression profiles of ATP-binding cassette transporter genes in strains ranging from low- to high-level resistance. J. Asia-Pac. Entomol. 2021, 24, 224–231. [Google Scholar] [CrossRef]
- Meng, X.K.; Wu, Z.L.; Yang, X.M.; Guan, D.J.; Wang, J.J. Cloning and Analysis of P-glycoprotein Gene and Its Transcriptional Response to Insecticide in Chilo suppressalis. Sci. Agric. Sin. 2021, 54, 4121–4131. [Google Scholar]
- Xia, X.; Luo, G.H.; Yu, J.L.; Liu, B.Q.; Zhang, R.; Zhang, G.; Shu, Z.L.; Lou, Y.G.; Hoffmann, A.A.; Fang, J.C. Jasmonic acid changes associated with long-term control of lepidopteran rice pests after insecticide seed treatment. Entomol. Gen. 2024, 44, 573–581. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, J.K.; He, C.S.; Gao, C.F. Advancements on the Insecticides Application and Key Technique in the Control of Disaster Pests in Paddy Field. Mod. Agrochem. 2024, 23, 7–12. [Google Scholar]
- Wei, Q.; Zhang, M.; Zhu, X.H.; He, J.C.; Liu, L.M.; Lai, F.X.; Wang, W.X.; Wan, P.J.; Liu, L.S.; Fu, Q. Analysis of Reduced Pesticide Use and Enhanced Effectiveness in Controlling Rice Pests Through Transplanting Insecticide-Pretreated Rice Seedlings. Acta Entomol. Sinica 2024, 67, 477–489. [Google Scholar]
- Huang, X.F.; Chen, H.B.; Li, C.Q.; Xiao, P.F.; Zhu, G.N.; Ye, J.R. Effects of Sex-Pheromone Attractant and Black Light on the Trapping of Rice Stem Borer (Chilo suppressalis) and the Impact Factors. Chin. J. Pestic. Sci. 2020, 22, 602–610. [Google Scholar]
- Liu, B.; Syu, K.J.; Zhang, Y.X.; Gupta, S.; Shen, Y.J.; Chien, W.J.; Tseng, J.C.; Lou, D.W. Practical Synthesis and Field Application of the Synthetic Sex Pheromone of Rice Stem Borer, Chilo suppressalis (Lepidoptera: Pyralidae). J. Chem. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Hou, H.Y.; Dong, X.L.; Zhou, H.; Zhang, M.L.; Wei, L.X.; Gao, S.; Zhu, D.F. The Application of Insect Sex Attractant and Vetiver to Reduce the Damage Caused by Scirpophaga incertulas in Organically Grown Rice. J. Biosaf. 2020, 29, 247–251. [Google Scholar]
- Yao, X.M.; Zhai, J.; Sun, J.; Wang, W.Q.; Zhang, X.M.; Wu, J.X.; Guo, Q.S.; Du, Y.J. Impact of sex pheromone aerosol puff on the behavior of Chilo suppressalis and its control effect in rice fields. China Plant Prot. 2024, 44, 59–63. [Google Scholar]
- Wang, W.Y.; Chen, Y.; Zhao, H.; Guo, Q.S.; Du, Y.J. Evaluation of the Control Efficacy of Mating Disruption by Active High Dose Aerosol Pheromone Dispensers on Rice Pests. Chin. J. Biol. Control 2024, 40, 1293–1301. [Google Scholar]
- Zhang, D.; Qu, L.L.; Song, L.; Li, J.Z.; Zhang, W.M.; Meng, W.; Guo, Q.S.; Du, Y.J. Mating disruption of Chilo suppressalis (Lepidoptera: Crambidae) with novel aerosol dispensers in rice field. Pest Manag. Sci. 2025, 81, 4904–4912. [Google Scholar] [CrossRef]
- Swale, D.R. Perspectives on new strategies for the identification and development of insecticide targets. Pestic. Biochem. Physiol. 2019, 161, 23–32. [Google Scholar] [CrossRef]
- Cheng, S.H.; Li, R.; Chen, Z.B.; Ni, J.P.; Lv, N.N.; Liang, P.Z.; Guo, T.F.; Zhen, C.A.; Liang, P.; Gao, X.W. Comparative susceptibility of Aphis gossypii Glover (Hemiptera: Aphididae) on cotton crops to imidacloprid and a novel insecticide cyproflanilide in China. Ind. Crops Prod. 2023, 192, 116053. [Google Scholar] [CrossRef]
- Ge, W.; Qiao, S.; Liu, C.; Guo, F.; Wang, S.; Sun, H.; Liu, Y.; Yang, F.; Wu, S.; Gao, C.F. Baseline Establishment, Susceptibility Monitoring and Risk Assessment of Cyproflanilide, a Novel Meta-Diamide Insecticide, Against Chilo suppressalis (Lepidoptera: Crambidae) in China. J. Integr. Agric. 2025; in press. [Google Scholar] [CrossRef]
- Tang, T.; Hu, F.; Wang, P.; Fu, W.; Liu, X.Y. Broflanilide effectively controls Helicoverpa armigera and Spodoptera exigua exhibiting diverse susceptibilities to chlorantraniliprole and emamectin benzoate. Pest Manag. Sci. 2020, 77, 1262–1272. [Google Scholar] [CrossRef]
- Wang, X.L.; Shi, T.L.; Tang, P.; Liu, S.N.; Hou, B.F.; Jiang, D.; Lu, J.D.; Yang, Y.H.; Carrière, Y.; Wu, Y.D. Baseline susceptibility of Helicoverpa armigera, Plutella xylostella, and Spodoptera frugiperda to the meta-diamide insecticide broflanilide. Insect Sci. 2022, 30, 1118–1128. [Google Scholar] [CrossRef]
- Wang, H.H.; Zhao, R.; Zhang, S.; Gao, J.; Xiao, X.; Tian, X.Y.; Liang, P.; Gu, S.H. Monitoring broflanilide resistance and its synergism with metaflumizone and tetraniliprole against fall armyworm, Spodoptera frugiperda. Entomol. Gen. 2023, 43, 535–543. [Google Scholar] [CrossRef]
- Shang, J.; Wang, H.S.; Dong, W.Y.; Guo, X.Y.; Zhu, J.H.; Liang, P.; Shi, X.Y. Baseline susceptibility, risk assessment of resistance, changes in insecticides sensitivity and fitness after selection of dimpropyridaz in Aphis gossypii Glover (Hemiptera: Aphididae). Pestic. Biochem. Physiol. 2024, 205, 106138. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.H.; Song, L.W.; Zhang, J.J.; Zang, L.S.; Zhu, L.; Ruan, C.C.; Sun, G.Z. Performance of four Chinese Trichogramma species as biocontrol agents of the rice striped stem borer, Chilo suppressalis, under various temperature and humidity regimes. J. Pest Sci. 2012, 85, 497–504. [Google Scholar] [CrossRef]
- Ko, K.; Liu, Y.D.; Hou, M.L.; Babendreier, D.; Zhang, F.; Song, K. Evaluation for Potential Trichogramma (Hymenoptera: Trichogrammatidae) Strains for Control of the Striped Stem Borer (Lepidoptera: Crambidae) in the Greater Mekong Subregion. J. Econ. Entomol. 2014, 107, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; You, C.M.; Wang, J.Y.; Woodcock, B.A.; Chen, Y.J.; Ji, X.Y.; Wan, N.F. A parasitic wasp-releasing engineering to promote ecosystem services in paddy systems. Agric. Ecosyst. Environ. 2024, 373, 109126. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Wang, C.; Liu, D.F.; Zhang, J.F.; Hu, B.Y. Control Efficacy of Trichogramma chilonis Against Chilo suppressalis in Rice. China Plant Prot. 2024, 44, 58–60. [Google Scholar]
- Peng, G.X.; Xie, J.Q.; Guo, R.; Keyhani, N.O.; Zeng, D.Y.; Yang, P.Y.; Xia, Y.X. Long-term field evaluation and large-scale application of a Metarhizium anisopliae strain for controlling major rice pests. J. Pest Sci. 2021, 94, 969–980. [Google Scholar] [CrossRef]
- Shahriari, M.; Zibaee, A.; Khodaparast, S.A.; Fazeli-Dinan, M. Screening and Virulence of the Entomopathogenic Fungi Associated with Chilo suppressalis Walker. J. Fungi 2021, 7, 34. [Google Scholar] [CrossRef]
- Wang, P.; Li, M.J.; Bai, Q.R.; Ali, A.; Desneux, N.; Dai, H.J.; Zang, L.S. Performance of Trichogramma japonicum as a vector of Beauveria bassiana for parasitizing eggs of rice striped stem borer, Chilo suppressalis. Entomol. Gen. 2021, 41, 147–155. [Google Scholar] [CrossRef]
- Wang, P.; Zheng, M.X.; Li, J.J.; Yu, Y.; Hu, Y.; Monticelli, L.S.; Ruan, C.C.; Desneux, N.; Zhang, J.J. The effect of Beauveria bassiana on the host location of rice striped stem borer, Chilo suppressalis by Trichogramma japonicum. J. Pest Sci. 2023, 97, 1475–1483. [Google Scholar] [CrossRef]
- Lu, Y.H.; Bai, Q.; Zheng, X.S.; Lü, Z.X. Selective tropism of different geographical populations of Chilo suppressalis to the trapping vetiver grass, Vetiveria zizanioiaes. J. Plant Prot. 2017, 44, 968–972. [Google Scholar]
- Lu, Y.H.; Zheng, X.S.; Lü, Z.X. The potential of vetiver grass as a biological control for the rice stem borers Chilo suppressalis and Sesamia inferens. Chin. J. Appl. Entomol. 2018, 55, 1111–1117. [Google Scholar]
- Lu, Y.H.; Zheng, X.S.; Lu, Z.X. Application of vetiver grass Vetiveria zizanioides: Poaceae (L.) as a trap plant for rice stem borer Chilo suppressalis: Crambidae (Walker) in the paddy fields. J. Integr. Agric. 2019, 18, 797–804. [Google Scholar] [CrossRef]
- Lu, Y.H.; Gao, G.C.; Zheng, X.S.; Lü, Z.X. The Lethal Mechanism of Trap Plant Vetiveria zizanioides Against the Larvae of Chilo suppressalis. Sci. Agric. Sin. 2017, 50, 486. [Google Scholar]
- Li, Q.; He, K.; Lu, Y.H.; He, B.B.; Zheng, X.S.; Lu, Z.X.; Li, F.; Xu, H.X. A Vetiver-Specific Terpene Synthase VzTPS9 Contributes to the High Attractiveness of Vetiver to Rice Stem Borer. Proc. Natl. Acad. Sci. USA 2025, 122, e2424863122. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.S.; Lu, Y.H.; Zhong, L.Q.; Huang, X.F.; Xu, F.S.; Yao, X.M.; Xu, H.X.; Lü, Z.X. Optimal planting pattern of trap plant vetiver grass (Vetiveria zizanioides) for controlling rice striped stem borer (Chilo suppressalis). Plant Prot. 2017, 43, 103–108. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, W.; Chen, G.; Wang, M.; Wu, S.; Gao, C. Advances in the Molecular Mechanisms of Resistance in Chilo suppressalis. Insects 2025, 16, 942. https://doi.org/10.3390/insects16090942
Ge W, Chen G, Wang M, Wu S, Gao C. Advances in the Molecular Mechanisms of Resistance in Chilo suppressalis. Insects. 2025; 16(9):942. https://doi.org/10.3390/insects16090942
Chicago/Turabian StyleGe, Wenchao, Guanghang Chen, Mengzhen Wang, Shunfan Wu, and Congfen Gao. 2025. "Advances in the Molecular Mechanisms of Resistance in Chilo suppressalis" Insects 16, no. 9: 942. https://doi.org/10.3390/insects16090942
APA StyleGe, W., Chen, G., Wang, M., Wu, S., & Gao, C. (2025). Advances in the Molecular Mechanisms of Resistance in Chilo suppressalis. Insects, 16(9), 942. https://doi.org/10.3390/insects16090942






