Fenmezoditiaz Inhibited the Acquisition and Transmission of Southern Rice Black-Streaked Dwarf Virus by Sogatella furcifera
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect, Virus, and Antibodies
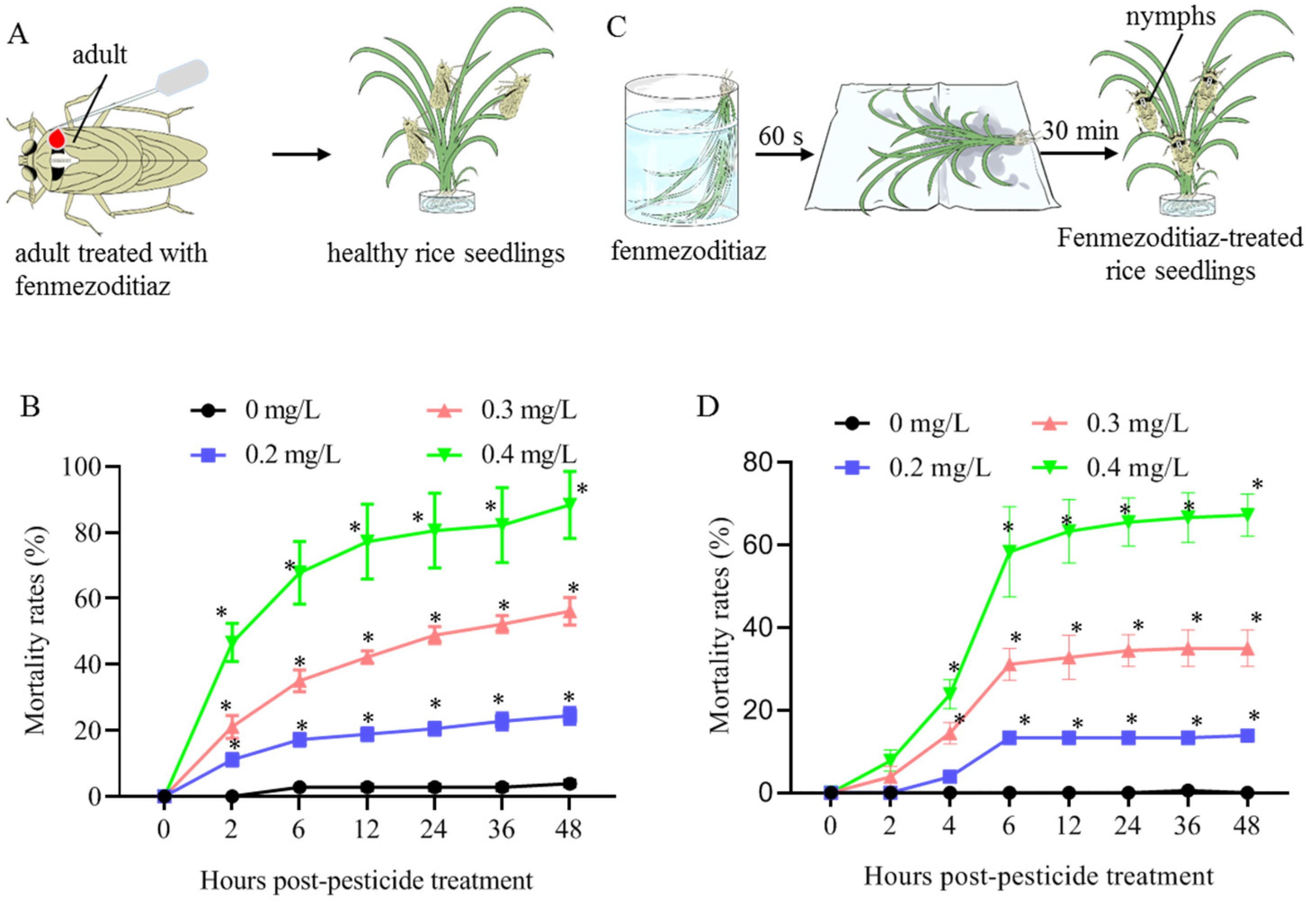
2.2. Determination of Sublethal Concentrations of Fenmezoditiaz
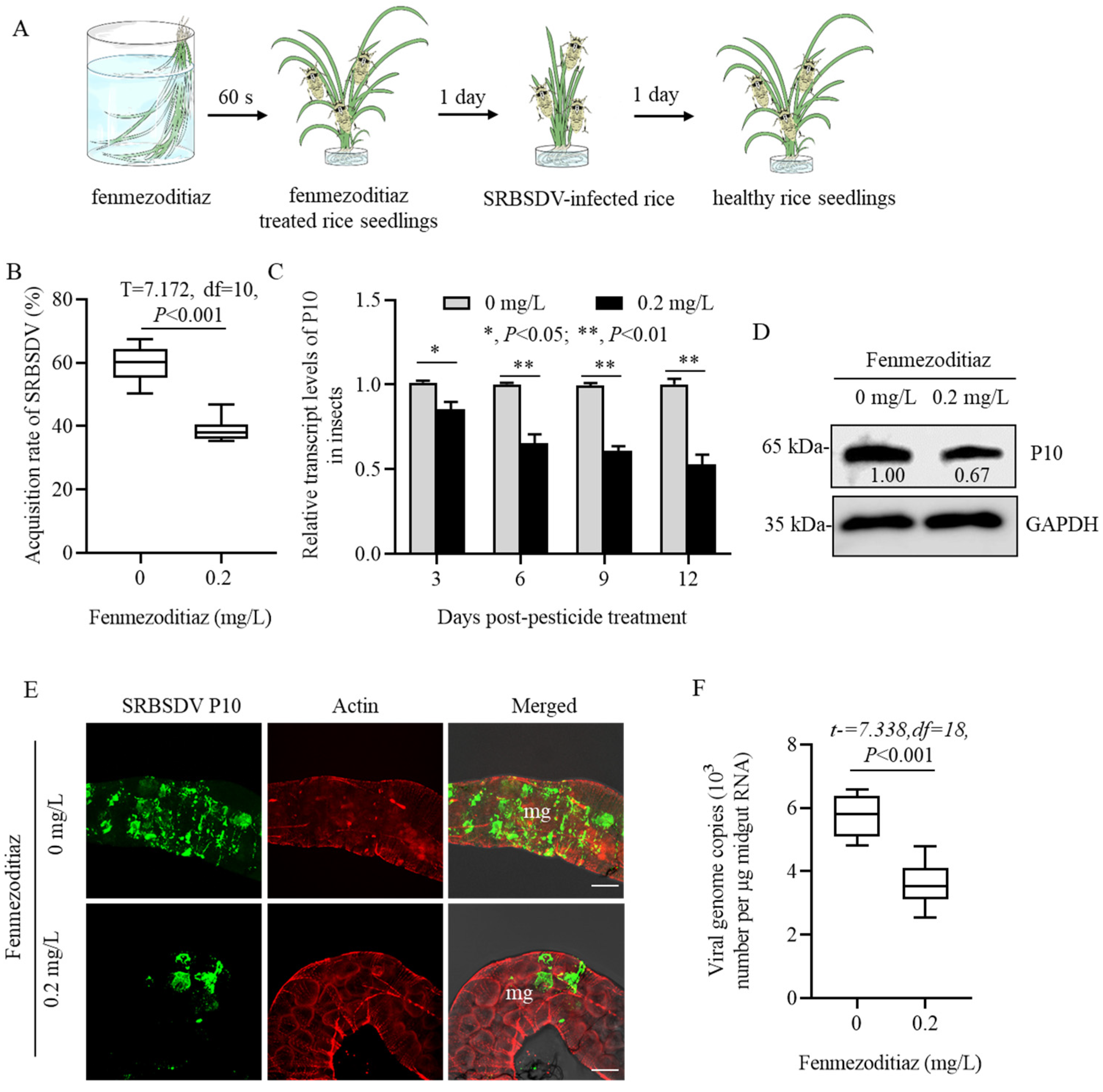
2.3. Influence of Fenmezoditiaz on SRBSDV Acquisition Ability of S. furcifera
2.4. Influence of Fenmezoditiaz on the Propagation of SRBSDV in S. furcifera
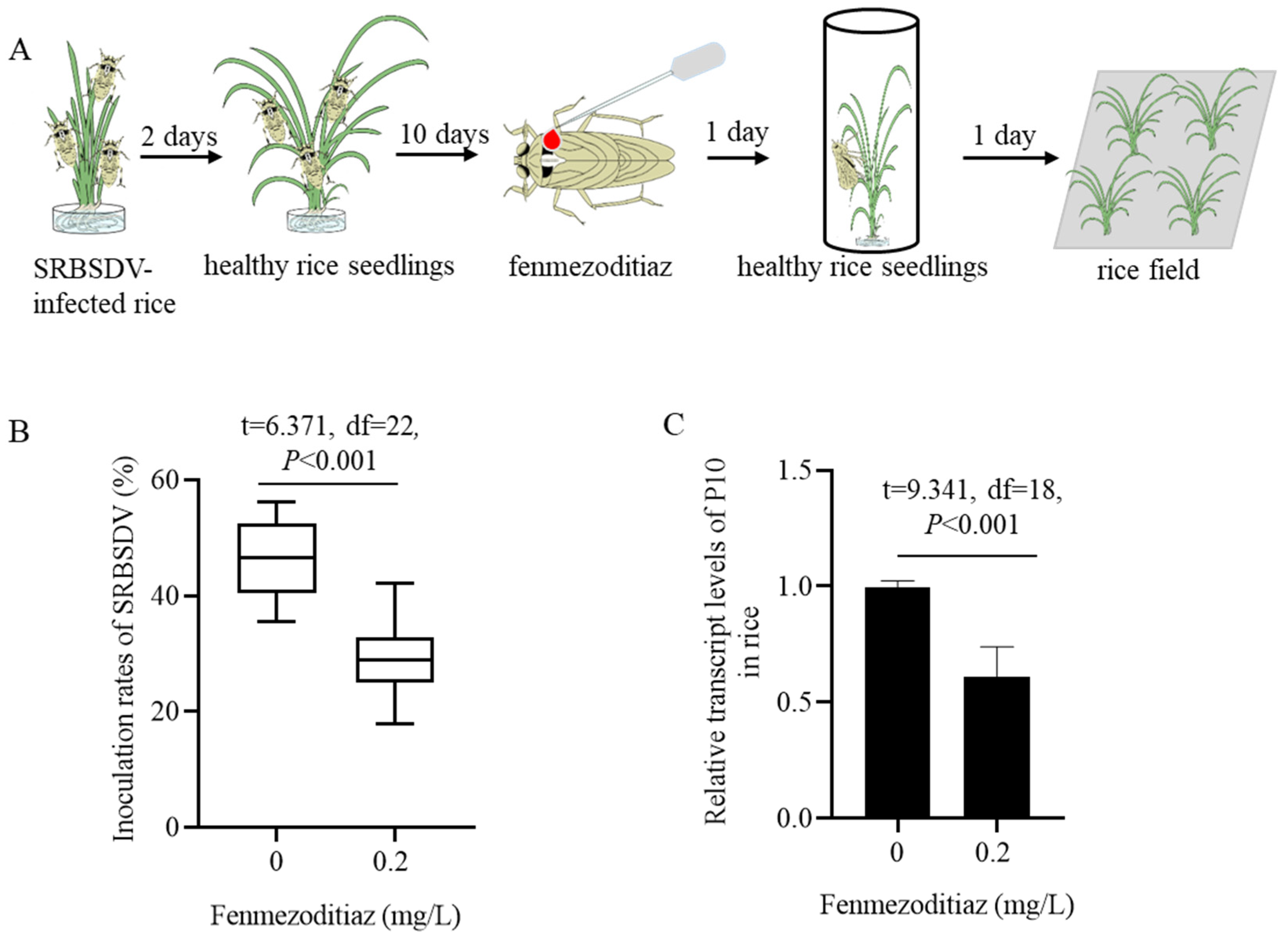
2.5. Influence of Fenmezoditiaz on Inoculation Rate of SRBSDV by S. furcifera
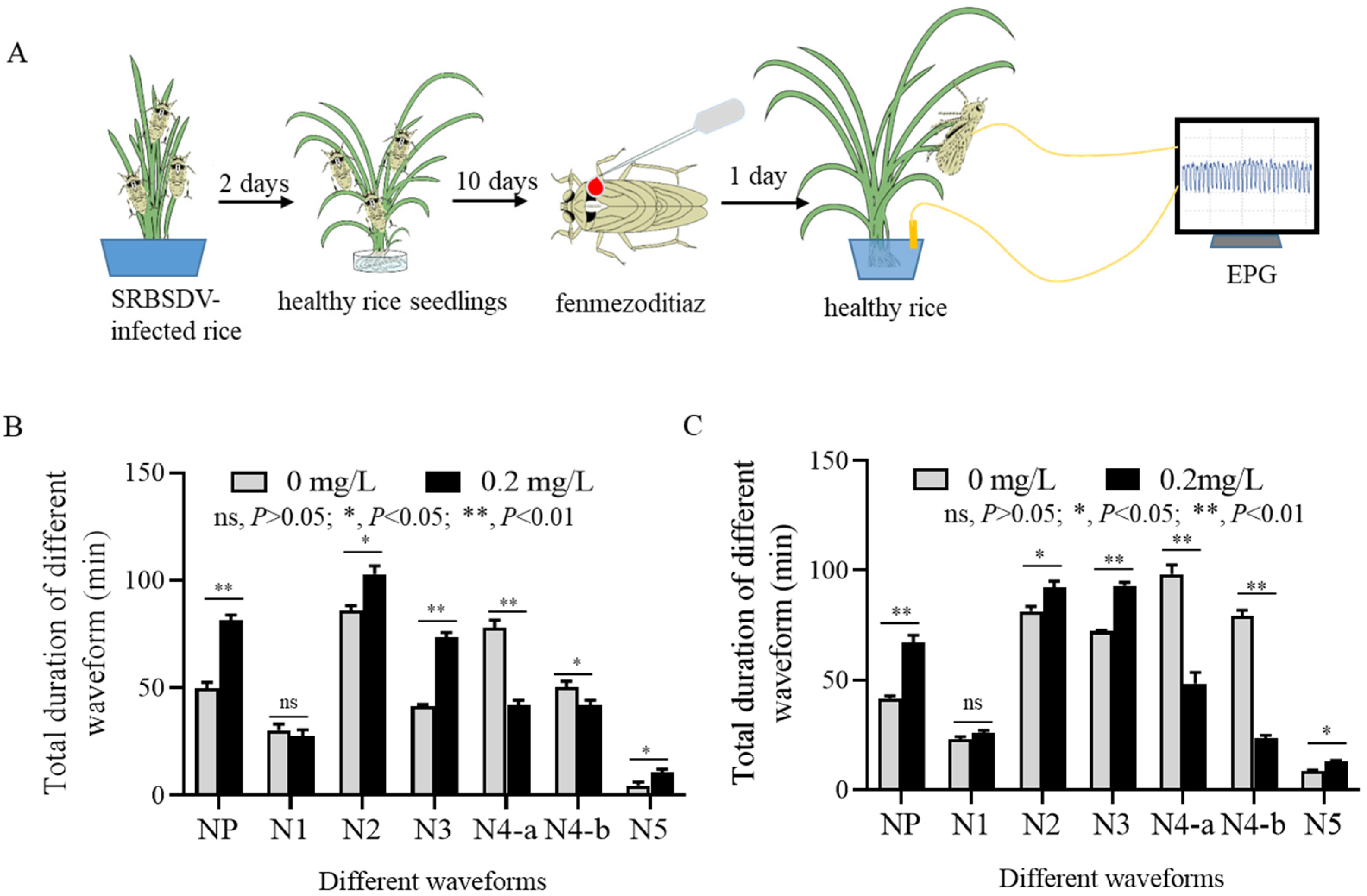
2.6. Influence of Fenmezoditiaz on Feeding Behaviors of S. furcifera Recorded by Electrical Penetration Graphs
2.7. Statistical Analysis
3. Results
3.1. Sublethal Concentrations of Fenmezoditiaz
3.2. Fenmezoditiaz Reduced the Acquisition and Propagation of SRBSDV in S. furcifera
3.3. Fenmezoditiaz Reduced the Inoculation Rate of SRBSDV by S. furcifera
3.4. Fenmezoditiaz Affects the Feeding Behavior of S. furcifera
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| EPG | electrical penetration graph |
| DMSO | Dimethyl sulfoxide |
| SRBSDV | southern rice black-streaked dwarf virus |
| RT-PCR | reverse transcription–polymerase chain reaction |
| padp | post-first access to diseased plants |
References
- Liu, Z.; Zhu, Y.; Shi, H.; Qiu, J.; Ding, X.; Kou, Y. Recent progress in rice broad-spectrum disease resistance. Int. J. Mol. Sci. 2021, 22, 11658. [Google Scholar] [CrossRef]
- Xiong, P.; Zhang, C.; He, L.; Zhan, X.; Han, Y. Deep learning-based rice pest detection research. PLoS ONE 2024, 19, e0313387. [Google Scholar] [CrossRef]
- Di, D.P.; Zhang, Y.L.; Yan, C.; Yan, T.; Zhang, A.H.; Yang, F.; Cao, X.L.; Li, D.W.; Lu, Y.G.; Wang, X.B.; et al. First report of barley yellow striate mosaic virus on wheat in China. Plant Dis. 2014, 98, 1450. [Google Scholar] [CrossRef]
- Wang, P.; Liu, J.; Lyu, Y.; Huang, Z.; Zhang, X.; Sun, B.; Li, P.; Jing, X.; Li, H.; Zhang, C. A review of vector-borne rice viruses. Viruses 2022, 14, 2258. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.G.; Ruan, Y.W.; Gong, C.W.; Xiang, X.; Xu, X.; Zhang, Y.M.; Shen, L.T. Transcriptome analysis of Sogatella furcifera (Homoptera: Delphacidae) in response to Sulfoxaflor and functional verification of resistance-related P450 Genes. Int. J. Mol. Sci. 2019, 20, 4573. [Google Scholar] [CrossRef]
- Liu, D.L.; Li, Z.; Hou, M. Silencing the Autophagy-related genes ATG3 and ATG9 promotes SRBSDV propagation and transmission in Sogatella furcifera. Insects 2022, 13, 394. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Xu, D.; Xu, D.; Zhang, M. Southern rice black-streaked dwarf virus: A white-backed planthopper-transmitted fijivirus threatening rice production in Asia. Front. Microbiol. 2013, 4, 270. [Google Scholar] [CrossRef]
- Guo, Y.; Shao, J.; Wu, Y.; Li, Y. Using Wolbachia to control rice planthopper populations: Progress and challenges. Front. Microbiol. 2023, 14, 1244239. [Google Scholar] [CrossRef]
- Phan, N.T.; Biddinger, D.J.; Rajotte, E.G.; Smagghe, G.; Reddy, G.V.; Ren, Z.X.; Joshi, N.K. Pesticide use in integrated pest and pollinator management framework to protect pollinator health. Pest Manag. Sci. 2025, 81, 1691–1696. [Google Scholar] [CrossRef]
- Datta, J.; Banik, S.C. Insecticide resistance in the brown planthopper, Nilaparvaa lugens (Stål): Mechanisms and status in Asian countries. J. Entomol. Res. Soc. 2021, 23, 225–238. [Google Scholar] [CrossRef]
- Mu, X.C.; Zhang, W.; Wang, L.X.; Zhang, S.; Zhang, K.; Gao, C.F.; Wu, S.F. Resistance monitoring and cross-resistance patterns of three rice planthoppers, Nilaparvata lugens, Sogatella furcifera and Laodelphax striatellus to dinotefuran in China. Pestic. Biochem. Physiol. 2016, 134, 8–13. [Google Scholar] [CrossRef]
- Ruan, Y.; Wang, X.; Xiang, X.; Xu, X.; Guo, Y.; Liu, Y.; Yin, Y.; Wu, Y.; Cheng, Q.; Gong, C.; et al. Status of insecticide resistance and biochemical characterization of chlorpyrifos resistance in Sogatella furcifera (Hemiptera: Delphacidae) in Sichuan Province, China. Pestic. Biochem. Physiol. 2021, 171, 104723. [Google Scholar] [CrossRef]
- Huang, H.; Dickhaut, J.; Weisel, M.; Mao, L.; Rankl, N.; Takeda, H.; Stam, L.F.; Peacock, Q.M.; Höffken, H.W. Discovery and biological characterization of a novel mesoionic insecticide fenmezoditiaz. Pest Manag. Sci. 2025, 81, 2535–2552. [Google Scholar] [CrossRef]
- Jung, M.; Kim, S.; Kim, H.G.; Lee, D.H. Lethal and sublethal effects of synthetic insecticides on the locomotory and feeding behavior of Riptortus pedestris (Hemiptera: Alydidae) under laboratory conditions. J. Asia-Pacific Entomol. 2018, 21, 179–185. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Liu, W.; Li, Q. Single-cell transcriptomics dissecting the development and evolution of nervous system in insects. Curr. Opin. Insect Sci. 2024, 63, 101201. [Google Scholar] [CrossRef] [PubMed]
- Rajarushi, C.N.; Nebapure, S.M.; Biswas, A.; Rajna, S.; Subramanian, S. Contact toxicity of insecticides against rice weevil, Sitophilus oryzae L. and its effect on progeny production. Sci. Rep. 2024, 14, 28404. [Google Scholar] [CrossRef] [PubMed]
- Raisch, T.; Raunser, S. The modes of action of ion-channel-targeting neurotoxic insecticides: Lessons from structural biology. Nat. Struct. Mol. Biol. 2023, 30, 1411–1427. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.E.; Oliveira, R.S.; de Faria Barbosa, R.; Hyslop, S.; Dal Belo, C.A. Recent advances on the influence of fipronil on insect behavior. Curr. Opin. Insect Sci. 2024, 65, 101251. [Google Scholar] [CrossRef]
- Civolani, S.; Cassanelli, S.; Chicca, M.; Rison, J.L.; Bassi, A.; Alvarez, J.M.; Annan, I.B.; Parrella, G.; Giorgini, M.; Fano, E.A. An EPG study of the probing behavior of adult Bemisia tabaci biotype Q (Hemiptera: Aleyrodidae) following exposure to cyantraniliprole. J. Econ. Entomol. 2014, 107, 910–919. [Google Scholar] [CrossRef]
- Garzo, E.; Moreno, A.; Plaza, M.; Fereres, A. Feeding behavior and virus-transmission ability of insect vectors exposed to systemic insecticides. Plants 2020, 9, 895. [Google Scholar] [CrossRef]
- Maluta, N.K.P.; Lopes, J.R.S.; Fiallo-Olivé, E.; Navas-Castillo, J.; Loureno, A.L. Foliar application of systemic insecticides disrupts feeding behavior of the whitefly Bemisia tabaci MEAM1 and the transmission of tomato chlorosis virus in potato plants. J. Pest Sci. 2021, 94, 1265–1276. [Google Scholar] [CrossRef]
- Jia, D.; Luo, G.; Guan, H.; Yu, T.; Sun, X.; Du, Y.; Wang, Y.; Chen, H.; Wei, T. Arboviruses antagonize insect Toll antiviral immune signaling to facilitate the coexistence of viruses with their vectors. PLoS Pathog. 2024, 20, e1012318. [Google Scholar] [CrossRef]
- Li, Z.; Qin, Y.; Jin, R.; Li, J.; Li, J.; Zhang, X. Insecticide resistance monitoring in field populations of the white backed planthopper Sogatella furcifera (Horvath) in China, 2019–2020. Insects 2021, 12, 1078. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.; Chen, H.; Liu, Y.; Jiang, C.; Mao, Q.; Jia, D.; Chen, Q.; Wei, T. Small interfering RNA pathway modulates initial viral infection in midgut epithelium of insect after ingestion of virus. J. Virol. 2015, 90, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Jia, D.; Mao, Q.; Chen, H.; Lu, C.; Wu, W.; Chen, H.; Chen, Q.; Jia, D.; Wei, T. Virus-induced tubule: A vehicle for rapid spread of virions through basal lamina from midgut epithelium in the insect vector. J. Virol. 2014, 88, 10488–10500. [Google Scholar] [CrossRef]
- Kang, Y.; Koo, H.N.; Kim, H.K.; Kim, G.H. Analysis of the feeding behavior and life table of Nilaparvata lugens and Sogatella furcifera (Hemiptera: Delphacidae) under sublethal concentrations of Imidacloprid and Sulfoxaflor. Insects 2022, 13, 1130. [Google Scholar] [CrossRef]
- Lei, W.; Li, P.; Han, Y.; Gong, S.; Yang, L.; Hou, M. EPG recordings reveal differential feeding behaviors in Sogatella furcifera in response to plant virus infection and transmission success. Sci. Rep. 2016, 6, 30240. [Google Scholar] [CrossRef]
- Yu, J.; Zhong, Y.; Dai, C.; Hou, M. Sublethal concentrations of pymetrozine reduce Sogatella furcifera transmission of Southern rice black-streaked dwarf virus. Pest Manag. Sci. 2024, 80, 797–804. [Google Scholar] [CrossRef]
- Cameron, R.; Lang, E.B.; Alvarez, J.M. Use of honeydew production to determine reduction in feeding by Bemisia tabaci (Hemiptera: Aleyrodidae) adults when exposed to cyantraniliprole and imidacloprid treatments. J. Econ. Entomol. 2014, 107, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, G.; Jiang, Y.; Ma, C.; Yang, G. Sublethal effects of imidacloprid on fecundity, apoptosis and virus transmission in the small brown planthopper Laodelphax striatellus. Insects 2021, 12, 1131. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zan, N.; He, H.; Hu, D.; Song, B. Piperazine derivatives containing the alpha-ketoamide moiety discovered as potential anti-tomato spotted wilt virus agents. J. Agric. Food Chem. 2023, 71, 6301–6313. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Mao, L.; Ding, X.; Liu, H.; Vyas, D.J.; Jia, D. Fenmezoditiaz Inhibited the Acquisition and Transmission of Southern Rice Black-Streaked Dwarf Virus by Sogatella furcifera. Insects 2025, 16, 875. https://doi.org/10.3390/insects16090875
Chen Y, Mao L, Ding X, Liu H, Vyas DJ, Jia D. Fenmezoditiaz Inhibited the Acquisition and Transmission of Southern Rice Black-Streaked Dwarf Virus by Sogatella furcifera. Insects. 2025; 16(9):875. https://doi.org/10.3390/insects16090875
Chicago/Turabian StyleChen, Yuting, Lixin Mao, Xiulan Ding, Hengchien Liu, Devendra J. Vyas, and Dongsheng Jia. 2025. "Fenmezoditiaz Inhibited the Acquisition and Transmission of Southern Rice Black-Streaked Dwarf Virus by Sogatella furcifera" Insects 16, no. 9: 875. https://doi.org/10.3390/insects16090875
APA StyleChen, Y., Mao, L., Ding, X., Liu, H., Vyas, D. J., & Jia, D. (2025). Fenmezoditiaz Inhibited the Acquisition and Transmission of Southern Rice Black-Streaked Dwarf Virus by Sogatella furcifera. Insects, 16(9), 875. https://doi.org/10.3390/insects16090875



