Effect of Defatting Method on the Nutritional, Functional, and Bioactive Properties of Black Soldier Fly (Hermetia illucens) Larvae
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Defatting
2.2.2. Physicochemical and Thermal Characterization of FP, DPP, and DPS
Proximal Composition and Caloric Value
Water Activity (Aw)
Color Properties
Differential Scanning Calorimetry (DSC)
2.2.3. Functional and Technological Properties
Water- and Oil-Holding Capacity
Emulsification Capacity and Stability
Water Absorption Index and Swelling Capacity
Hygroscopicity
2.2.4. Bioactive Compounds
Antioxidant Activity
Phytochemical Screening
2.2.5. Statistical Analysis
3. Results
3.1. Physicochemical and Thermal Characterization of FP, DPP, and DPS
3.1.1. Proximal Composition and Caloric Value
3.1.2. Water Activity (Aw)
3.1.3. Color Properties
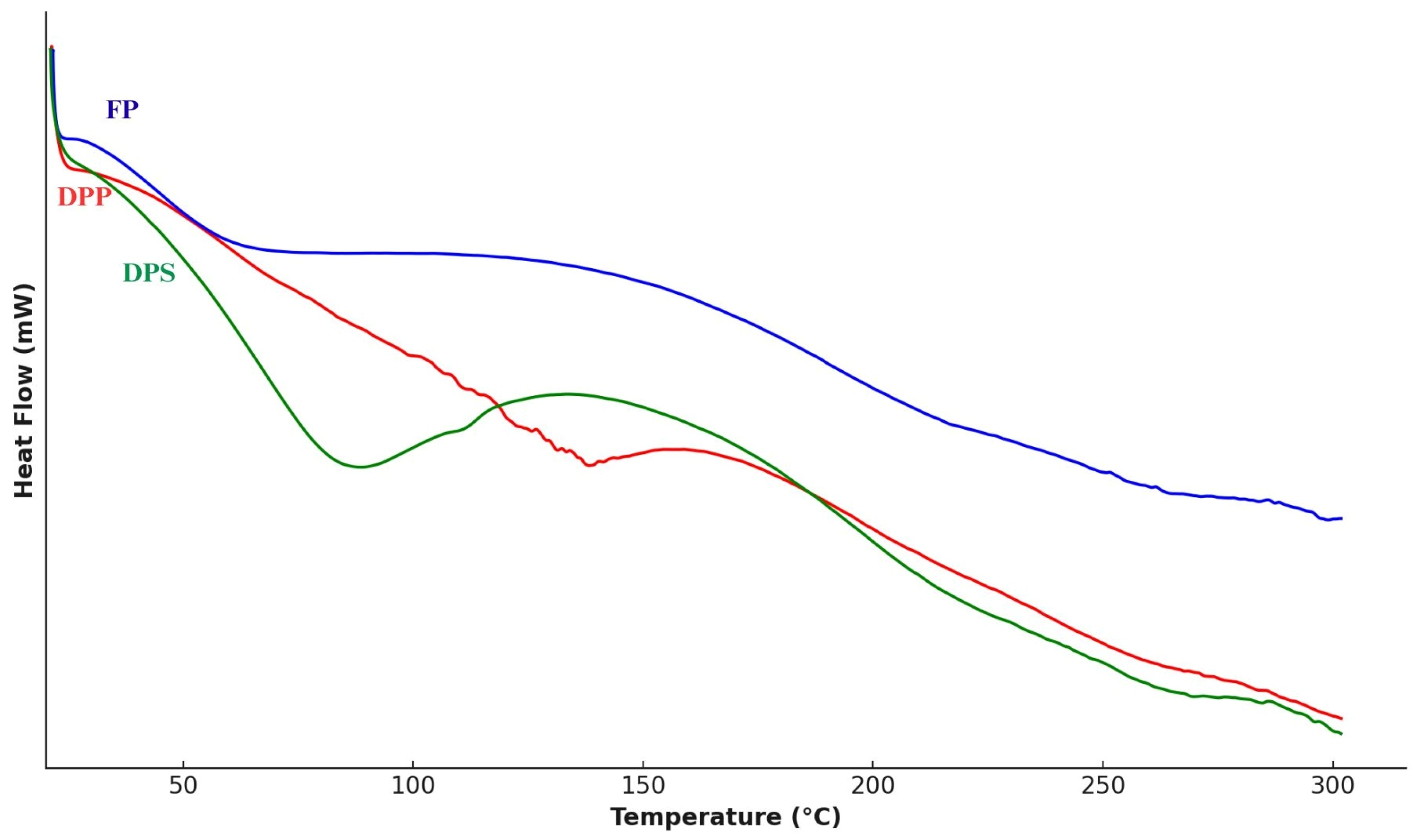
3.1.4. Differential Scanning Calorimetry (DSC)
3.2. Functional and Technological Properties
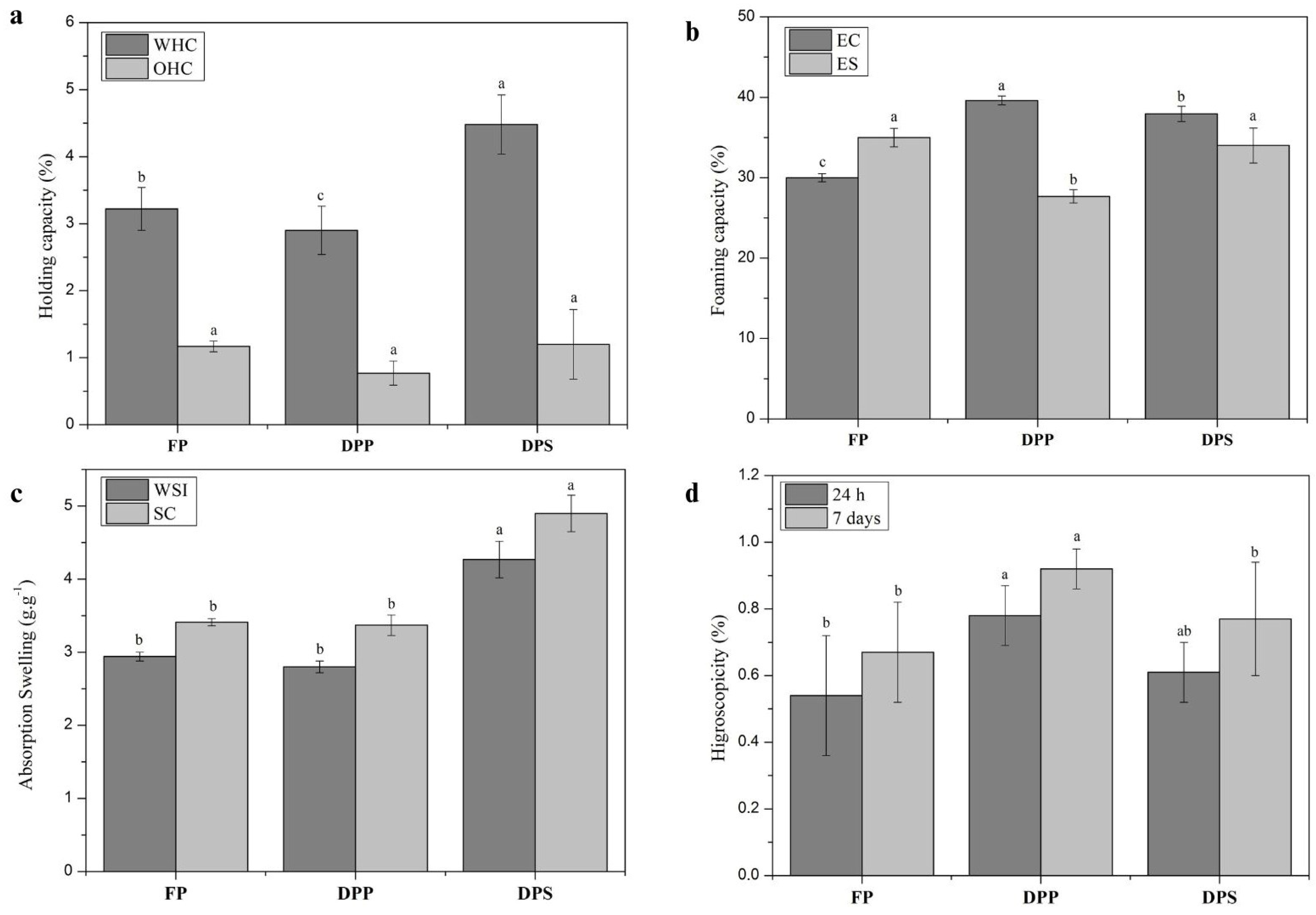
3.2.1. Water- and Oil-Holding Capacity
3.2.2. Emulsification Capacity and Stability
3.2.3. Water Solubility Index and Swelling Capacity
3.2.4. Hygroscopicity
3.3. Bioactive Compounds
3.3.1. Antioxidant Activity
3.3.2. Phytochemical Screening
4. Discussion
4.1. Physicochemical and Thermal Characterization
4.2. Functional and Technological Properties
4.3. Bioactive Compounds
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| FP | Full powder form |
| DPP | Defatted powder by pressing |
| DPS | Defatted powder by solvent |
References
- Lu, H.; Shang, Z.; Ruan, Y.; Jiang, L. Study on Urban Expansion and Population Density Changes Based on the Inverse S-Shaped Function. Sustainability 2023, 15, 10464. [Google Scholar] [CrossRef]
- Gravel, A.; Doyen, A. The Use of Edible Insect Proteins in Food: Challenges and Issues Related to Their Functional Properties. Innov. Food Sci. Emerg. Technol. 2020, 59, 102272. [Google Scholar] [CrossRef]
- Mshayisa, V.V.; Van Wyk, J.; Zozo, B. Nutritional, Techno-Functional and Structural Properties of Black Soldier Fly (Hermetia illucens) Larvae Flours and Protein Concentrates. Foods 2022, 11, 724. [Google Scholar] [CrossRef] [PubMed]
- Rumpold, B.A.; Schlüter, O. Insect-Based Protein Sources and Their Potential for Human Consumption: Nutritional Composition and Processing. Anim. Front. 2015, 5, 20–24. [Google Scholar]
- Vanqa, N.; Mshayisa, V.V.; Basitere, M. Proximate, Physicochemical, Techno-Functional and Antioxidant Properties of Three Edible Insect (Gonimbrasia belina, Hermetia illucens and Macrotermes subhylanus) Flours. Foods 2022, 11, 976. [Google Scholar] [CrossRef]
- Barbi, S.; Macavei, L.I.; Fuso, A.; Luparelli, A.V.; Caligiani, A.; Ferrari, A.M.; Maistrello, L.; Montorsi, M. Valorization of Seasonal Agri-Food Leftovers through Insects. Sci. Total Environ. 2020, 709, 136209. [Google Scholar] [CrossRef]
- Bortolini, S.; Macavei, L.I.; Saadoun, J.H.; Foca, G.; Ulrici, A.; Bernini, F.; Malferrari, D.; Setti, L.; Ronga, D.; Maistrello, L. Hermetia illucens (L.) Larvae as Chicken Manure Management Tool for Circular Economy. J. Clean. Prod. 2020, 262, 121289. [Google Scholar] [CrossRef]
- Shumo, M.; Osuga, I.M.; Khamis, F.M.; Tanga, C.M.; Fiaboe, K.K.M.; Subramanian, S. The Nutritive Value of Black Soldier Fly Larvae Reared on Common Organic Waste Streams in Kenya. Sci. Rep. 2019, 9, 10110. [Google Scholar] [CrossRef]
- Monisha, C.; Loganathan, M. Impact of Drying Methods on the Physicochemical Properties and Nutritional Composition of Defatted Black Soldier Fly (Hermetia illucens) Pre-Pupae Flour. J. Food Process. Preserv. 2022, 46, e16184. [Google Scholar] [CrossRef]
- Bußler, S.; Rumpold, B.A.; Jander, E.; Rawel, H.M.; Schlüter, O.K. Recovery and Techno-Functionality of Flours and Proteins from Two Edible Insect Species: Meal Worm (Tenebrio molitor) and Black Soldier Fly (Hermetia illucens) Larvae. Heliyon 2016, 2, e00218. [Google Scholar] [CrossRef]
- Jongema, Y. List of Edible Insects of the World; Laboratory of Entomology, Wageningen University: Wageningen, The Netherlands, 2017. [Google Scholar]
- Schösler, H.; De Boer, J.; Boersema, J.J. Can We Cut out the Meat of the Dish? Constructing Consumer-Oriented Pathways towards Meat Substitution. Appetite 2012, 58, 39–47. [Google Scholar] [CrossRef]
- Roncolini, A.; Milanović, V.; Cardinali, F.; Osimani, A.; Garofalo, C.; Sabbatini, R.; Clementi, F.; Pasquini, M.; Mozzon, M.; Foligni, R.; et al. Protein Fortification with Mealworm (Tenebrio molitor L.) Powder: Effect on Textural, Microbiological, Nutritional and Sensory Features of Bread. PLoS ONE 2019, 14, e0211747. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-W.; Setyabrata, D.; Lee, Y.J.; Jones, O.G.; Kim, Y.H.B. Pre-Treated Mealworm Larvae and Silkworm Pupae as a Novel Protein Ingredient in Emulsion Sausages. Innov. Food Sci. Emerg. Technol. 2016, 38, 116–123. [Google Scholar] [CrossRef]
- Zhang, F.; Cao, C.; Kong, B.; Sun, F.; Shen, X.; Yao, X.; Liu, Q. Pre-Dried Mealworm Larvae Flour Could Partially Replace Lean Meat in Frankfurters: Effect of Pre-Drying Methods and Replacement Ratios. Meat Sci. 2022, 188, 108802. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.S.G.; Fischer, A.R.H.; Tinchan, P.; Stieger, M.; Steenbekkers, L.P.A.; van Trijp, H.C.M. Insects as Food: Exploring Cultural Exposure and Individual Experience as Determinants of Acceptance. Food Qual. Prefer. 2015, 42, 78–89. [Google Scholar] [CrossRef]
- Wendin, K.M.E.; Nyberg, M.E. Factors Influencing Consumer Perception and Acceptability of Insect-Based Foods. Curr. Opin. Food Sci. 2021, 40, 67–71. [Google Scholar] [CrossRef]
- Toti, E.; Massaro, L.; Kais, A.; Aiello, P.; Palmery, M.; Peluso, I. Entomophagy: A Narrative Review on Nutritional Value, Safety, Cultural Acceptance and a Focus on the Role of Food Neophobia in Italy. Eur. J. Investig. Health Psychol. Educ. 2020, 10, 628–643. [Google Scholar] [CrossRef]
- Kim, T.-K.; Yong, H.I.; Kim, Y.-B.; Kim, H.-W.; Choi, Y.-S. Edible Insects as a Protein Source: A Review of Public Perception, Processing Technology, and Research Trends. Food Sci. Anim. Resour. 2019, 39, 521. [Google Scholar] [CrossRef]
- Imathiu, S. Benefits and Food Safety Concerns Associated with Consumption of Edible Insects. NFS J. 2020, 18, 1–11. [Google Scholar] [CrossRef]
- Zhao, X.; Vázquez-Gutiérrez, J.L.; Johansson, D.P.; Landberg, R.; Langton, M. Yellow Mealworm Protein for Food Purposes—Extraction and Functional Properties. PLoS ONE 2016, 11, e0147791. [Google Scholar] [CrossRef]
- Caparros Megido, R.; Sablon, L.; Geuens, M.; Brostaux, Y.; Alabi, T.; Blecker, C.; Drugmand, D.; Haubruge, É.; Francis, F. Edible Insects Acceptance by B Elgian Consumers: Promising Attitude for Entomophagy Development. J. Sens. Stud. 2014, 29, 14–20. [Google Scholar] [CrossRef]
- Hurtado-Ribeira, R.; Hernández, D.M.; Villanueva-Bermejo, D.; García-Risco, M.R.; Hernández, M.D.; Vázquez, L.; Fornari, T.; Martin, D. Methods Differently Affects Oxidative Quality of the Fat From. Insecta 2023, 14, 14040368. [Google Scholar]
- Yi, L.; Lakemond, C.M.M.; Sagis, L.M.C.; Eisner-Schadler, V.; van Huis, A.; van Boekel, M.A.J.S. Extraction and Characterisation of Protein Fractions from Five Insect Species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef] [PubMed]
- González, C.M.; Garzón, R.; Rosell, C.M. Insects as Ingredients for Bakery Goods. A Comparison Study of H. illucens, A. domestica and T. molitor Flours. Innov. Food Sci. Emerg. Technol. 2019, 51, 205–210. [Google Scholar] [CrossRef]
- Kim, T.-K.; Lee, J.-H.; Yong, H.I.; Kang, M.-C.; Cha, J.Y.; Chun, J.Y.; Choi, Y.-S. Effects of Defatting Methods on the Physicochemical Properties of Proteins Extracted from Hermetia illucens Larvae. Foods 2022, 11, 1400. [Google Scholar] [CrossRef]
- L’hocine, L.; Boye, J.I.; Arcand, Y. Composition and Functional Properties of Soy Protein Isolates Prepared Using Alternative Defatting and Extraction Procedures. J. Food Sci. 2006, 71, C137–C145. [Google Scholar] [CrossRef]
- Kwiatkowski, J.R.; Cheryan, M. Extraction of Oil from Ground Corn Using Ethanol. J. Am. Oil Chem. Soc. 2002, 79, 825–830. [Google Scholar] [CrossRef]
- Ferreira-Dias, S.; Valente, D.G.; Abreu, J.M.F. Comparison Between Ethanol and Hexane for Oil Extraction from Quercus suber L. Fruits. Grasas Y Aceites 2003, 54, 378–383. [Google Scholar] [CrossRef]
- Thanonkaew, A.; Wongyai, S.; McClements, D.J.; Decker, E.A. Effect of Stabilization of Rice Bran by Domestic Heating on Mechanical Extraction Yield, Quality, and Antioxidant Properties of Cold-Pressed Rice Bran Oil (Oryza saltiva L.). LWT-Food Sci. Technol. 2012, 48, 231–236. [Google Scholar] [CrossRef]
- Cruz, V.A.; Vicentini-Polette, C.M.; Magalhaes, D.R.; de Oliveira, A.L. Extraction, Characterization, and Use of Edible Insect Oil—A Review. Food Chem. 2025, 463, 141199. [Google Scholar] [CrossRef]
- Rahman, M.M.; Byanju, B.; Lamsal, B.P. Protein, Lipid, and Chitin Fractions from Insects: Method of Extraction, Functional Properties, and Potential Applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 6415–6431. [Google Scholar] [CrossRef]
- Matthäus, B.; Piofczyk, T.; Katz, H.; Pudel, F. Renewable Resources from Insects: Exploitation, Properties, and Refining of Fat Obtained by Cold-Pressing from Hermetia illucens (Black Soldier Fly) Larvae. Eur. J. Lipid Sci. Technol. 2019, 121, 1800376. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; AOAC: Washington, DC, USA, 2005. [Google Scholar]
- Janssen, R.H.; Vincken, J.-P.; van den Broek, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-Protein Conversion Factors for Three Edible Insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef] [PubMed]
- Instituto Adolfo Lutz (Ed.) Métodos Físico-Químicos Para Análise de Alimentos, 4th ed.; Instituto Adolfo Lutz: São Paulo, Brazil, 2008. [Google Scholar]
- Watt, B.K.; Merrill, A.L. Composition of Foods: Raw, Processed, Prepared; Consumer and Food Economics Institute, Agricultural Research Service: Washington, DC, USA, 1975; ISBN 0486213420. [Google Scholar]
- Jantzen da Silva Lucas, A.; Quadro Oreste, E.; Leão Gouveia Costa, H.; Martín López, H.; Dias Medeiros Saad, C.; Prentice, C. Extraction, Physicochemical Characterization, and Morphological Properties of Chitin and Chitosan from Cuticles of Edible Insects. Food Chem. 2021, 343, 128550. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.G.; Martins, V.G. Functional, Thermal, Bioactive and Antihypertensive Properties of Hot Trub Derived from Brewing Waste as an Alternative Source of Protein. Food Hydrocoll. 2023, 146, 109292. [Google Scholar] [CrossRef]
- Bento, J.A.C.; Morais, D.K.; de Berse, R.S.; Bassinello, P.Z.; Caliari, M.; Soares Júnior, M.S. Functional, Thermal, and Pasting Properties of Cooked Carioca Bean (Phaseolus vulgaris L.) Flours. Appl. Food Res. 2022, 2, 100027. [Google Scholar] [CrossRef]
- Torbica, A.; Belović, M.; Popović, L.; Čakarević, J. Heat and Hydrothermal Treatments of Non-Wheat Flours. Food Chem. 2021, 334, 127523. [Google Scholar] [CrossRef]
- Hong, T.; Ma, Y.; Wu, F.; Jin, Y.; Xu, D.; Xu, X. Understanding the Effects of Dry Heat Treatment on Wheat Flour Pasting: Insights from Protein and Starch Structural Changes. J. Cereal Sci. 2023, 113, 103740. [Google Scholar] [CrossRef]
- Fernandes, S.S.; Greque, L.; Santos, M.D.F.C.; de Novais, L.M.; D’Oca, C.D.; Prentice, C.; Salas-Mellado, M.D.L.M. Effect of the Spray Drying Conditions on the Physicochemical and Structural Characteristics and the Stability of Chia Oil Microparticles. J. Appl. Polym. Sci. 2021, 138, 51015. [Google Scholar] [CrossRef]
- Do Socorro Moura Rufino, M.; Alves, R.E.; de Brito, E.S.; de Morais, S.M.; de Goes Sampaio, C.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Determinação Da Atividade Antioxidante Total Em Frutas Pela Captura Do Radical Livre DPPH. Fortaleza Embrapa Agroindústria Trop. 2007, 127, 1–4. [Google Scholar]
- Herrero, M.; Martín-Álvarez, P.J.; Senorans, F.J.; Cifuentes, A.; Ibáñez, E. Optimization of Accelerated Solvent Extraction of Antioxidants from Spirulina Platensis Microalga. Food Chem. 2005, 93, 417–423. [Google Scholar] [CrossRef]
- Purewal, S.S.; Verma, P.; Kaur, P.; Sandhu, K.S.; Singh, R.S.; Kaur, A.; Salar, R.K. A Comparative Study on Proximate Composition, Mineral Profile, Bioactive Compounds and Antioxidant Properties in Diverse Carrot (Daucus carota L.) Flour. Biocatal. Agric. Biotechnol. 2023, 48, 102640. [Google Scholar] [CrossRef]
- Purewal, S.S.; Kaur, P.; Garg, G.; Sandhu, K.S.; Salar, R.K. Antioxidant, Anti-Cancer, and Debittering Potential of Edible Fungi (Aspergillus oryzae) for Bioactive Ingredient in Personalized Foods. Biocatal. Agric. Biotechnol. 2022, 43, 102406. [Google Scholar] [CrossRef]
- Choi, B.D.; Wong, N.A.K.; Auh, J.-H. Defatting and Sonication Enhances Protein Extraction from Edible Insects. Korean J. Food Sci. Anim. Resour. 2017, 37, 955. [Google Scholar]
- Ribeiro, J.C.; Lima, R.C.; Maia, M.R.G.; Almeida, A.A.; Fonseca, A.J.M.; Cabrita, A.R.J.; Cunha, L.M. Impact of Defatting Freeze-Dried Edible Crickets (Acheta domesticus and Gryllodes sigillatus) on the Nutritive Value, Overall Liking and Sensory Profile of Cereal Bars. LWT 2019, 113, 108335. [Google Scholar] [CrossRef]
- Jeong, M.-S.; Lee, S.-D.; Cho, S.-J. Effect of Three Defatting Solvents on the Techno-Functional Properties of an Edible Insect (Gryllus bimaculatus) Protein Concentrate. Molecules 2021, 26, 5307. [Google Scholar] [CrossRef] [PubMed]
- Lenaerts, S.; Van Der Borght, M.; Callens, A.; Van Campenhout, L. Suitability of Microwave Drying for Mealworms (Tenebrio molitor) as Alternative to Freeze Drying: Impact on Nutritional Quality and Colour. Food Chem. 2018, 254, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Borremans, A.; Bubler, S.; Tchewonpi Sagu, S.; Rawel, H.; Schluter, O.; Leen, V.C. Effect of Blanching Plus Fermentation on Selected. Foods 2020, 9, 917. [Google Scholar] [CrossRef] [PubMed]
- Anusha, S.; Negi, P.S. Characterization and Techno-Functional Properties of Tenebrio molitor Larvae Protein Concentrate. Food Biosci. 2023, 54, 102882. [Google Scholar] [CrossRef]
- Huang, W.; Wang, C.; Chen, Q.; Chen, F.; Hu, H.; Li, J.; He, Q.; Yu, X. Physicochemical, Functional, and Antioxidant Properties of Black Soldier Fly Larvae Protein. J. Food Sci. 2024, 89, 259–275. [Google Scholar] [CrossRef]
- Zozo, B.; Wicht, M.M.; Mshayisa, V.V.; van Wyk, J. The Nutritional Quality and Structural Analysis of Black Soldier Fly Larvae Flour before and After Defatting. Insects 2022, 13, 168. [Google Scholar] [CrossRef]
- D’Antonio, V.; Battista, N.; Sacchetti, G.; Di Mattia, C.; Serafini, M. Functional Properties of Edible Insects: A Systematic Review. Nutr. Res. Rev. 2023, 36, 98–119. [Google Scholar] [CrossRef]
- Wang, J.; Jousse, M.; Jayakumar, J.; Fernández-Arteaga, A.; de Lamo-Castellví, S.; Ferrando, M.; Güell, C. Black Soldier Fly (Hermetia illucens) Protein Concentrates as a Sustainable Source to Stabilize o/w Emulsions Produced by a Low-Energy High-Throughput Emulsification Technology. Foods 2021, 10, 1048. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Decker, E.A.; McClements, D.J. Influence of Protein Concentration and Order of Addition on Thermal Stability of β-Lactoglobulin Stabilized n-Hexadecane Oil-in-Water Emulsions at Neutral PH. Langmuir 2005, 21, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-Y.; Peng, B.; Wang, M.; Zou, X.-G.; Yin, Y.-L.; Deng, Z.-Y. Characteristics and Emulsifying Properties of Two Protein Fractions Derived from the Emulsion Formed during Aqueous Extraction of Camellia Oil. Food Hydrocoll. 2019, 87, 644–652. [Google Scholar] [CrossRef]
- Hall, F.G.; Jones, O.G.; O’Haire, M.E.; Liceaga, A.M. Functional Properties of Tropical Banded Cricket (Gryllodes sigillatus) Protein Hydrolysates. Food Chem. 2017, 224, 414–422. [Google Scholar] [CrossRef]
- Mohd Ali, N.; Yeap, S.K.; Ho, W.Y.; Beh, B.K.; Tan, S.W.; Tan, S.G. The Promising Future of Chia, Salvia hispanica L. Biomed Res. Int. 2012, 2012, 171956. [Google Scholar]
- Fernandes, S.S.; Tonato, D.; Mazutti, M.A.; de Abreu, B.R.; da Costa Cabrera, D.; D’Oca, C.D.R.M.; Prentice-Hernández, C.; de las Mercedes Salas-Mellado, M. Yield and Quality of Chia Oil Extracted via Different Methods. J. Food Eng. 2019, 262, 200–208. [Google Scholar] [CrossRef]
- Navarro del Hierro, J.; Cantero-Bahillo, E.; Fornari, T.; Martin, D. Effect of Defatting and Extraction Solvent on the Antioxidant and Pancreatic Lipase Inhibitory Activities of Extracts from Hermetia illucens and Tenebrio molitor. Insects 2021, 12, 789. [Google Scholar] [CrossRef]
- Scaria, A.; Jayaraj, R.; Sudheesh, P.S. Comparative Phytochemical Profiling of Different Parts of Saraca Asoca. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Gessner, D.K.; Ringseis, R.; Eder, K. Potential of Plant Polyphenols to Combat Oxidative Stress and Inflammatory Processes in Farm Animals. J. Anim. Physiol. Anim. Nutr. 2017, 101, 605–628. [Google Scholar] [CrossRef]
- Tsiplakou, E.; Pitino, R.; Manuelian, C.L.; Simoni, M.; Mitsiopoulou, C.; De Marchi, M.; Righi, F. Plant Feed Additives as Natural Alternatives to the Use of Synthetic Antioxidant Vitamins in Livestock Animal Products Yield, Quality, and Oxidative Status: A Review. Antioxidants 2021, 10, 780. [Google Scholar] [CrossRef]
- Nehme, R.; Andrés, S.; Pereira, R.B.; Ben Jemaa, M.; Bouhallab, S.; Ceciliani, F.; López, S.; Rahali, F.Z.; Ksouri, R.; Pereira, D.M. Essential Oils in Livestock: From Health to Food Quality. Antioxidants 2021, 10, 330. [Google Scholar] [CrossRef]
- Hao, Y.; Huang, S.; Si, J.; Zhang, J.; Gaowa, N.; Sun, X.; Lv, J.; Liu, G.; He, Y.; Wang, W. Effects of Paper Mulberry Silage on the Milk Production, Apparent Digestibility, Antioxidant Capacity, and Fecal Bacteria Composition in Holstein Dairy Cows. Animals 2020, 10, 1152. [Google Scholar] [CrossRef]
- Jaiswal, L.; Ismail, H.; Worku, M. A Review of the Effect of Plant-Derived Bioactive Substances on the Inflammatory Response of Ruminants (Sheep, Cattle, and Goats). Int. J. Vet. Anim. Med. 2020, 3, 130. [Google Scholar]
| Parameter | FP | DPP | DPS | |
|---|---|---|---|---|
| Moisture (g/100 g) | 6.07 ± 0.10 b | 5.20 ± 0.17 c | 7.01 ± 0.32 a | |
| Protein * (g/100 g) | 34.13 ± 2.05 c | 47.16 ± 2.73 b | 54.96 ± 1.26 a | |
| Lipids *(g/100 g) | 32.45 ± 4.53 a | 21.70 ± 1.39 b | 3.18 ± 0.55 c | |
| Crude fiber * (g/100 g) | 8.87 ± 0.62 b | 7.73 ± 0.35 c | 13.90 ± 0.43 a | |
| Ash * (g/100 g) | 8.58 ± 0.08 c | 9.42 ± 0.15 b | 10.70 ± 0.04 a | |
| Carbohydrates * (g/100 g) | 10.61 | 11.37 | 10.45 | |
| Energy value (kcal/100 g) | 471.01 | 407.37 | 288.64 | |
| Aw | 0.571 ± 0.009 a | 0.529 ± 0.006 a | 0.269 ± 0.060 b | |
| L* | 31.88 ± 0.44 b | 33.03 ± 0.73 b | 57.09 ± 0.13 a | |
| a* | 9.54 ± 0.17 a | 9.79 ± 0.20 a | 5.92 ± 0.35 b | |
| b* | 22.53 ± 0.44 b | 23.40 ± 0.26 b | 24.01 ± 0.93 a | |
| h (°) | 67.22 ± 0.10 b | 67.04 ± 0.32 b | 76.15 ± 0.29 a | |
| Thermal properties | Tp (°C) | 138.30 | 54.15 | 88.88 |
| T0 (°C) | 128.34 | 49.46 | 62.11 | |
| Tf (°C) | 144.28 | 80.19 | 118.26 | |
| ΔH (J/g) | 8.77 | 8.37 | 108.66 | |
| Antioxidant Activity (% Inhibition) | ||
|---|---|---|
| ABTS Radical Capture | DPPH Radical Sequestration | |
| FP | 45.43 ± 0.44 b | 52.54 ± 0.27 b |
| DPP | 48.00 ± 1.20 a | 68.30 ± 0.25 a |
| DPS | 46.93 ± 1.31 ab | 54.12 ± 1.53 ab |
| Components | FP | DPP | DPS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Water | Methanol | Ethanol | Water | Methanol | Ethanol | Water | Methanol | Ethanol | |
| Coumarins | (+) | (−) | (−) | (+) | (+) | (+) | (+) | (+) | (+) |
| Flavonoids | (+) | (−) | (−) | (+) | (+) | (+) | (+) | (+) | (+) |
| Saponins | (−) | (+) | (+) | (−) | (−) | (+) | (+) | (−) | (+) |
| Flavanones | (−) | (−) | (−) | (+) | (+) | (+) | (+) | (+) | (+) |
| Steroids | (+) | (−) | (−) | (+) | (−) | (−) | (+) | (−) | (−) |
| Tannins | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
| Quinone | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
| Phenols | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marasca, N.S.; de Sousa Araújo, A.C.; da Silva Noda, K.; de Farias, B.S.; Brizio, A.P.D.R.; Fernandes, S.S.; Martins, V.G. Effect of Defatting Method on the Nutritional, Functional, and Bioactive Properties of Black Soldier Fly (Hermetia illucens) Larvae. Insects 2025, 16, 844. https://doi.org/10.3390/insects16080844
Marasca NS, de Sousa Araújo AC, da Silva Noda K, de Farias BS, Brizio APDR, Fernandes SS, Martins VG. Effect of Defatting Method on the Nutritional, Functional, and Bioactive Properties of Black Soldier Fly (Hermetia illucens) Larvae. Insects. 2025; 16(8):844. https://doi.org/10.3390/insects16080844
Chicago/Turabian StyleMarasca, Natasha Spindola, Alan Carvalho de Sousa Araújo, Karoline da Silva Noda, Bruna Silva de Farias, Ana Paula Dutra Resem Brizio, Sibele Santos Fernandes, and Vilásia Guimarães Martins. 2025. "Effect of Defatting Method on the Nutritional, Functional, and Bioactive Properties of Black Soldier Fly (Hermetia illucens) Larvae" Insects 16, no. 8: 844. https://doi.org/10.3390/insects16080844
APA StyleMarasca, N. S., de Sousa Araújo, A. C., da Silva Noda, K., de Farias, B. S., Brizio, A. P. D. R., Fernandes, S. S., & Martins, V. G. (2025). Effect of Defatting Method on the Nutritional, Functional, and Bioactive Properties of Black Soldier Fly (Hermetia illucens) Larvae. Insects, 16(8), 844. https://doi.org/10.3390/insects16080844







