The Threshold and Lag Effects of Temperature on Pine Wilt Disease Show Significant Spatial Heterogeneity
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
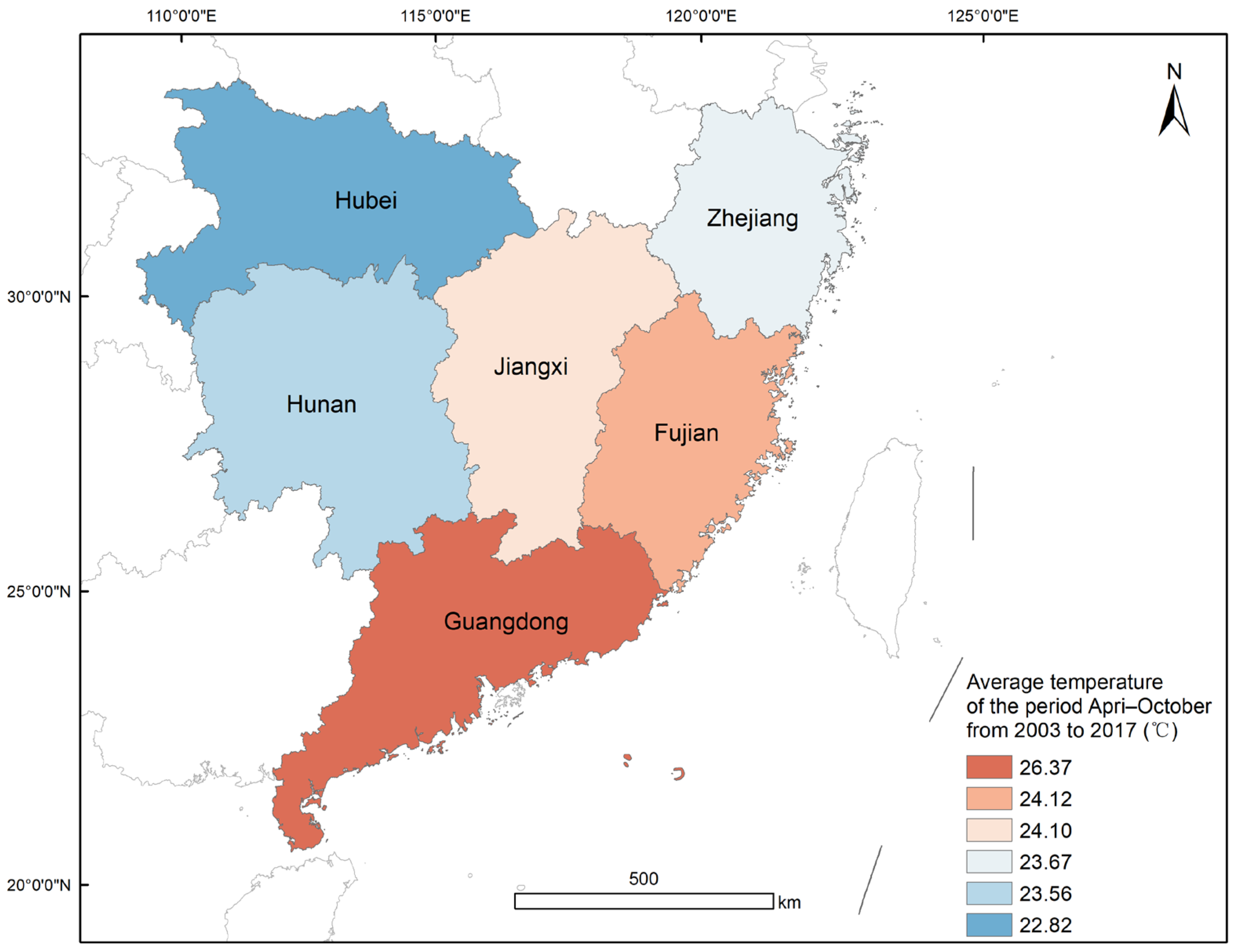
2.1. Study Area
2.2. Data
2.3. Distributed Lag Non-Linear Model
3. Results
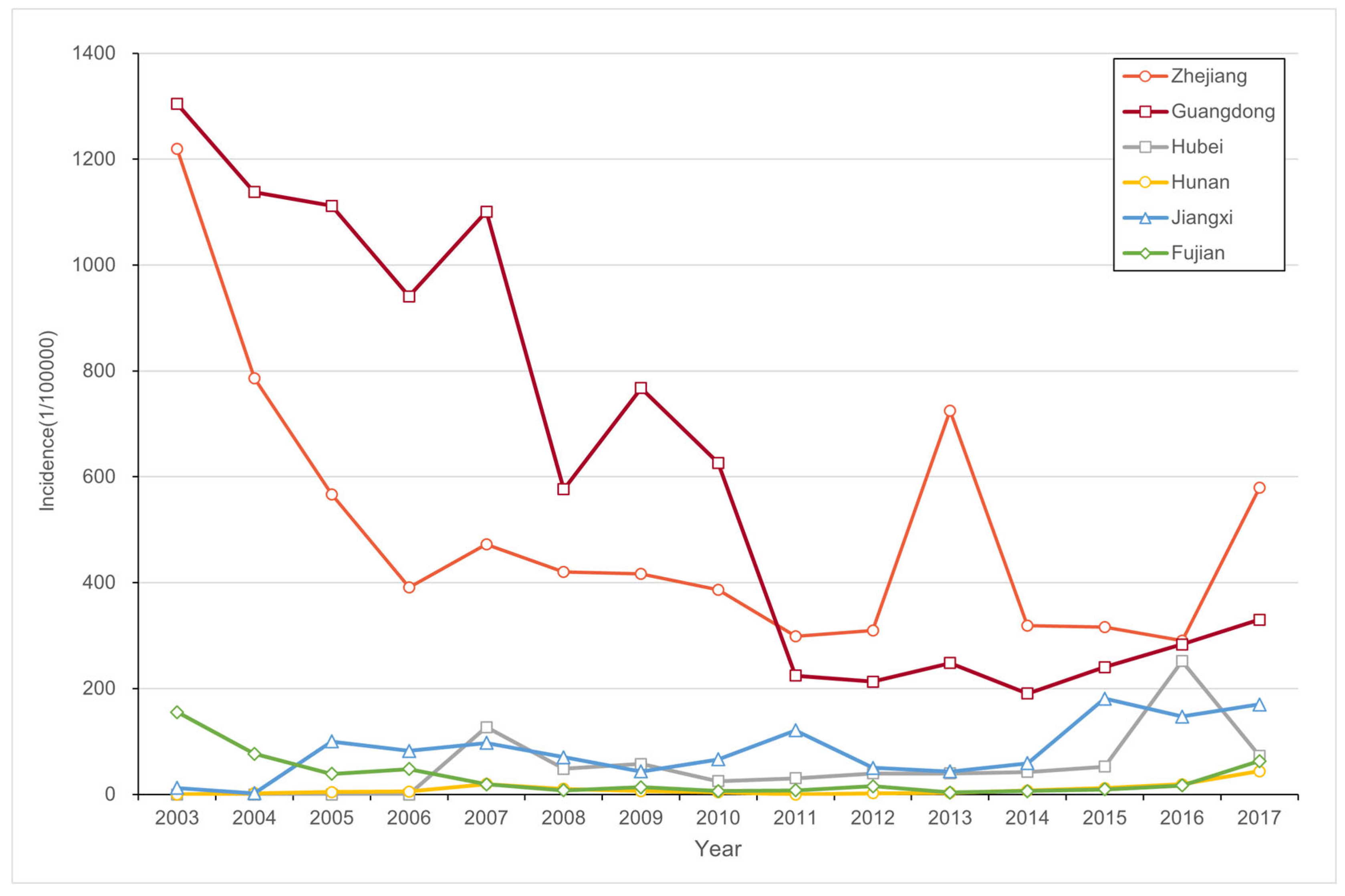
3.1. PWD Spatiotemporal Pattern Evolution
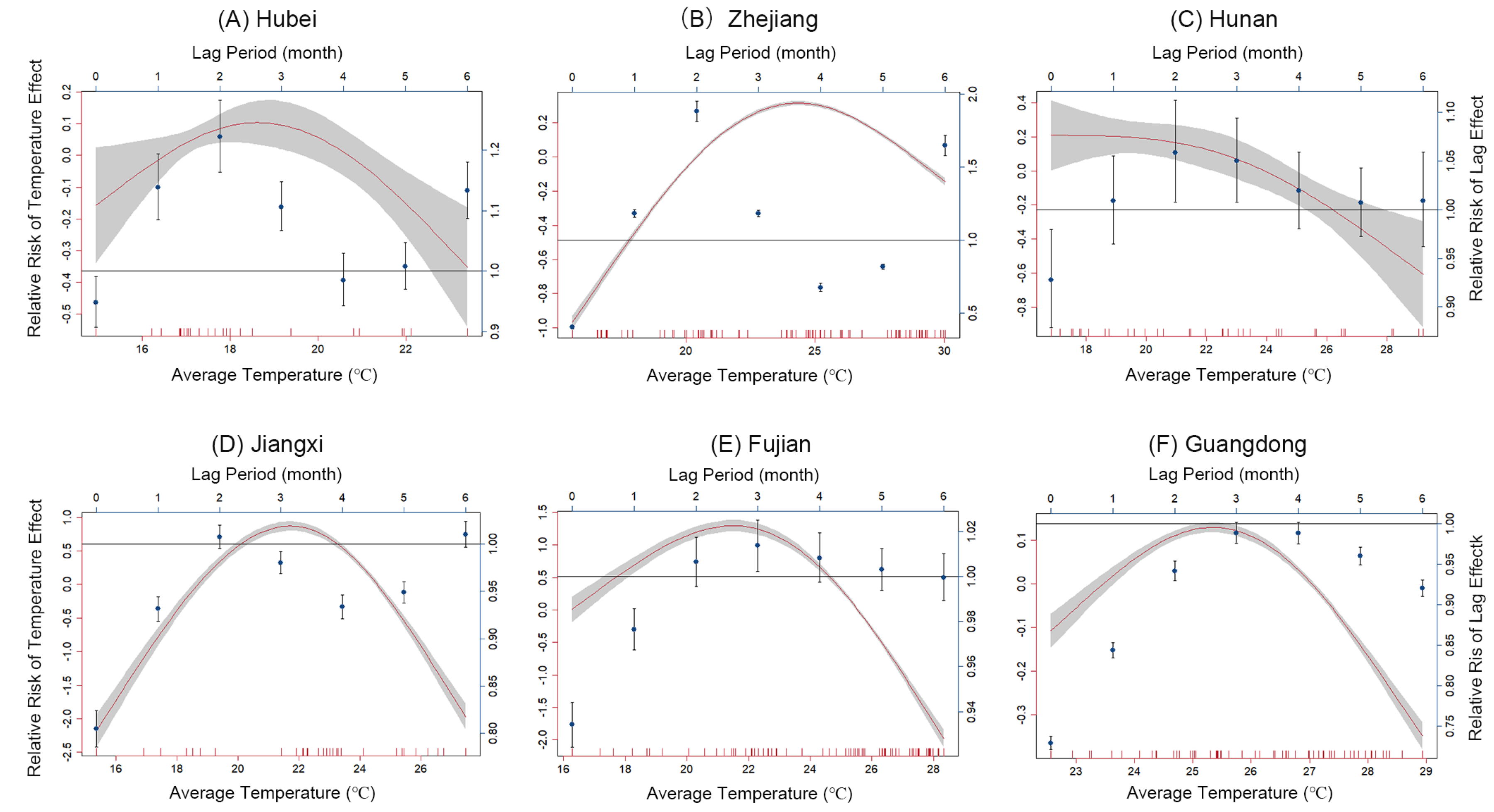
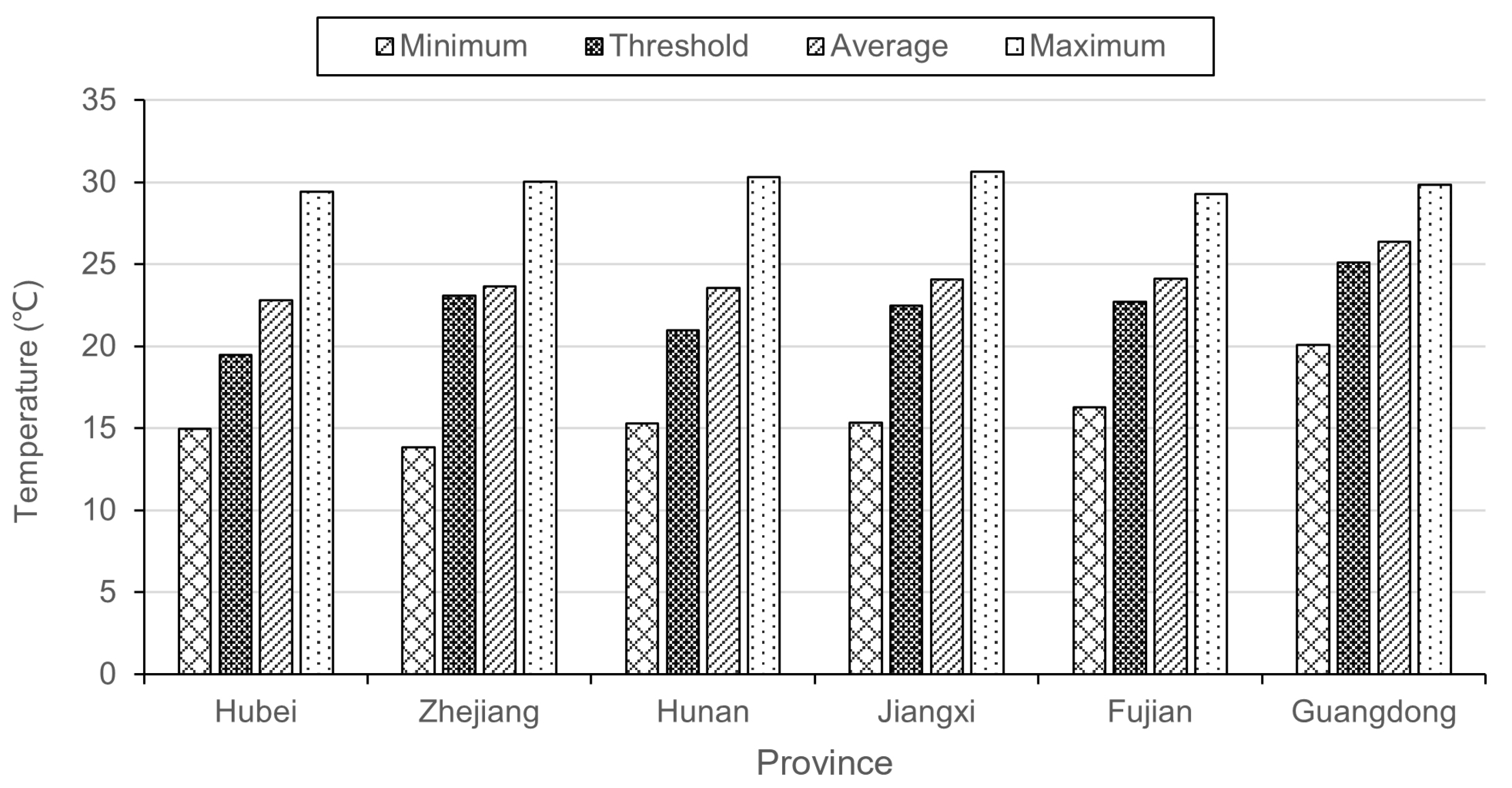
3.2. The Threshold Effects of Temperature on PWD
3.3. The Lag Effects of Temperature on the PWD
3.4. Correlation of Threshold Effect, Lag Effects, and Temperature
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PWD | Pine wilt disease |
| PWN | Pine wilt nematode, Bursaphelenchus xylophilus |
| DLNM | Distributed lag non-linear model |
| GAM | Linear dichroism Generalized Additive Model |
References
- Jung, C.S.; Koh, S.-H.; Nam, Y.; Ahn, J.J.; Lee, C.Y.; Choi, W.I. A Model for Predicting Spring Emergence of Monochamus saltuarius (Coleoptera: Cerambycidae) from Korean White Pine, Pinus koraiensis. J. Econ. Entomol. 2015, 108, 1830–1836. [Google Scholar] [CrossRef]
- Futai, K. Pine Wood Nematode, Bursaphelenchus xylophilus. Annu. Rev. Phytopathol. 2013, 51, 61–83. [Google Scholar] [CrossRef]
- Wang, X.; Cao, Y.; Wang, L.; Piao, C.; Li, C. Current Status of Pine Wilt Disease and Its Control Status. J. Environ. Entomol. 2018, 2, 256–267. [Google Scholar]
- Hao, Z.; Huang, J.; Zhou, Y.; Fang, G. Spatiotemporal Pattern of Pine Wilt Disease in the Yangtze River Basin. Forests 2021, 12, 731. [Google Scholar] [CrossRef]
- Wu, H.Y.; Tan, Q.Q.; Jiang, S.X. First Report of Pine Wilt Disease Caused by Bursaphelenchus xylophilus on Pinus thunbergii in the Inland City of Zibo, Shandong, China. Plant Dis. 2013, 97, 1126. [Google Scholar] [CrossRef]
- An, H.; Lee, S.; Cho, S. The Effects of Climate Change on Pine Wilt Disease in South Korea: Challenges and Prospects. Forests 2019, 10, 486. [Google Scholar] [CrossRef]
- Vicente, C.; Espada, M.; Vieira, P.; Mota, M. Pine Wilt Disease: A Threat to European Forestry. Eur. J. Plant Pathol. 2012, 133, 89–99. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.; Wei, Y.; Zhou, J.; Zhang, W.; Qin, P.; Chinta, S.; Kong, X.; Liu, Y.; Yu, H.; et al. Ascarosides Coordinate the Dispersal of a Plant-Parasitic Nematode with the Metamorphosis of Its Vector Beetle. Nat. Commun. 2016, 7, 12341. [Google Scholar] [CrossRef]
- Tao, J.; Xiao, W.; Yang, W.; Dong, J.; Liu, F. Effects of Prescribed Burning on Bursaphelenchus xylophilus. J. Cent. South Univ. For. Technol. 2021, 11, 65–72. [Google Scholar]
- Li, Y.; Zhang, X. High Risk of Invasion and Expansion of Pine Wood Nematode in Middle Temperate Zone of China. J. Temp. For. Res. 2018, 1, 3–6. [Google Scholar]
- Menéndez-Gutiérrez, M.; Alonso, M.; Toval, G.; Díaz, R. Variation in Pinewood Nematode Susceptibility among Pinus Pinaster Ait. Provenances from the Iberian Peninsula and France. Ann. For. Sci. 2017, 74, 76. [Google Scholar] [CrossRef]
- Zhou, H.; Yuan, X.; Zhou, H.; Shen, H.; Ma, L.; Sun, L.; Fang, G.; Sun, H. Surveillance of Pine Wilt Disease by High Resolution Satellite. J. For. Res. 2022, 33, 1401–1408. [Google Scholar] [CrossRef]
- Gao, R.; Luo, Y.; Wang, Z.; Yu, H.; Shi, J. Patterns of Biomass, Carbon, and Nitrogen Storage Distribution Dynamics after the Invasion of Pine Forests by Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae) in the Three Gorges Reservoir Region. J. For. Res. 2018, 29, 459–470. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Synthesis Report; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Hao, Z.; Fang, G.; Huang, W.; Ye, H.; Zhang, B.; Li, X. Risk Prediction and Variable Analysis of Pine Wilt Disease by a Maximum Entropy Model. Forests 2022, 13, 342. [Google Scholar] [CrossRef]
- Ikegami, M.; Jenkins, T.A.R. Estimate Global Risks of a Forest Disease under Current and Future Climates Using Species Distribution Model and Simple Thermal Model—Pine Wilt Disease as a Model Case. For. Ecol. Manag. 2018, 409, 343–352. [Google Scholar] [CrossRef]
- Xiao, Y.; Guo, Q.; Xie, N.; Yuan, G.; Liao, M.; Gui, Q.; Ding, G. Predicting the Global Potential Distribution of Bursaphelenchus xylophilus Using an Ecological Niche Model: Expansion Trend and the Main Driving Factors. BMC Ecol. Evo. 2024, 24, 48. [Google Scholar] [CrossRef]
- Cheng, G.; Lv, Q.; Feng, Y.; Li, Y.; Wang, Y.; Zhang, X. Temporal and Spatial Dynamic Pattern of Pine Wilt Disease Distribution in China Predicted under Climate Change Scenario. Sci. Silvae Sin. 2015, 45, 119–126. [Google Scholar]
- Tang, X.; Yuan, Y.; Li, X.; Zhang, J. Maximum Entropy Modeling to Predict the Impact of Climate Change on Pine Wilt Disease in China. Front. Plant Sci. 2021, 12, 652500. [Google Scholar] [CrossRef]
- Shi, P.; Sandhu, H.S.; Ge, F. Could the Intrinsic Rate of Increase Represent the Fitness in Terrestrial Ectotherms? J. Therm. Biol. 2013, 38, 148–151. [Google Scholar] [CrossRef]
- Matsuhashi, S.; Hirata, A.; Akiba, M.; Nakamura, K.; Oguro, M.; Takano, K.T.; Nakao, K.; Hijioka, Y.; Matsui, T. Developing a Point Process Model for Ecological Risk Assessment of Pine Wilt Disease at Multiple Scales. For. Ecol. Manag. 2020, 463, 118010. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Matsunaga, K.; Watanabe, A. Influence of Temperature on Pine Wilt Disease Progression in Pinus thunbergii Seedlings. Eur. J. Plant Pathol. 2020, 156, 581–590. [Google Scholar] [CrossRef]
- Gao, R.; Wang, Z.; Wang, H.; Hao, Y.; Shi, J. Relationship between Pine Wilt Disease Outbreaks and Climatic Variables in the Three Gorges Reservoir Region. Forests 2019, 10, 816. [Google Scholar] [CrossRef]
- Pimentel, C.S.; Ayres, M.P. Latitudinal Patterns in Temperature-Dependent Growth Rates of a Forest Pathogen. J. Therm. Biol. 2018, 72, 39–43. [Google Scholar] [CrossRef]
- Sun, H.; Liu, S. Research on the Invasion Rules and Prevention Measures of Pine Wilt Disease in Qianxinan Prefecture. Plant Quar. 2019, 3, 13–20. [Google Scholar]
- Boyd, I.L.; Freer-Smith, P.H.; Gilligan, C.A.; Godfray, H.C.J. The Consequence of Tree Pests and Diseases for Ecosystem Services. Science 2013, 342, 1235773. [Google Scholar] [CrossRef]
- Gasparrini, A. Distributed Lag Linear and Non-Linear Models in R: The Package dlnm. J. Stat. Soft. 2011, 43, 1–20. [Google Scholar] [CrossRef]
- Sritong-aon, C.; Thomya, J.; Kertpromphan, C.; Phosri, A. Estimated Effects of Meteorological Factors and Fire Hotspots on Ambient Particulate Matter in the Northern Region of Thailand. Air Qual. Atmos. Health 2021, 14, 1857–1868. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y.; Tang, H.; Wang, Y.; Gu, A.; Zhai, X.; Zheng, M. Cumulative Effects of Temperature on Blood Pressure during Pregnancy: A Cohort Study of Differing Effects in Three Trimesters. Sci. Total Environ. 2023, 859, 160143. [Google Scholar] [CrossRef]
- Davis, R.E.; Roney, P.C.; Pane, M.M.; Johnson, M.C.; Leigh, H.V.; Basener, W.; Curran, A.L.; DeMarcy, B.; Jang, J.; Schroeder, C.; et al. Climate and Human Mortality in Virginia, 2005–2020. Sci. Total Environ. 2023, 894, 164825. [Google Scholar] [CrossRef]
- Luo, X.; Hong, H.; Lu, Y.; Deng, S.; Wu, N.; Zhou, Q.; Chen, Z.; Feng, P.; Zhou, Y.; Tao, J.; et al. Impact of Air Pollution and Meteorological Factors on Incidence of Allergic Rhinitis: A Low-latitude Multi-city Study in China. Allergy 2023, 78, 1656–1659. [Google Scholar] [CrossRef]
- Sofwan, N.M.; Mahiyuddin, W.R.W.; Latif, M.T.; Ayub, N.A.; Yatim, A.N.M.; Mohtar, A.A.A.; Othman, M.; Aizuddin, A.N.; Sahani, M. Risks of Exposure to Ambient Air Pollutants on the Admission of Respiratory and Cardiovascular Diseases in Kuala Lumpur. Sustain. Cities Soc. 2021, 75, 103390. [Google Scholar] [CrossRef]
- Yang, L.; Yang, J.; Liu, M.; Sun, X.; Li, T.; Guo, Y.; Hu, K.; Bell, M.L.; Cheng, Q.; Kan, H.; et al. Nonlinear Effect of Air Pollution on Adult Pneumonia Hospital Visits in the Coastal City of Qingdao, China: A Time-Series Analysis. Environ. Res. 2022, 209, 112754. [Google Scholar] [CrossRef]
- Bigler, C.; Vitasse, Y. Daily Maximum Temperatures Induce Lagged Effects on Leaf Unfolding in Temperate Woody Species Across Large Elevational Gradients. Front. Plant Sci. 2019, 10, 398. [Google Scholar] [CrossRef]
- Hastie, T.J.; Tibshirani, R.J. Generalized Additive Models, 1st ed.; Routledge: London, UK, 2017; ISBN 978-0-203-75378-1. [Google Scholar]
- Qiao, Y.; Liu, F.; Tan, L.; Chen, T.; Mao, H. Information Extraction and Classification of Forest Land Based on the Object-Oriented Methods. J. Temp. For. Res. 2019, 2, 25–33. [Google Scholar] [CrossRef]
- Yan, W.; Wang, H.; Jiang, C.; Jin, S.; Ai, J.; Sun, O.J. Satellite View of Vegetation Dynamics and Drivers over Southwestern China. Ecol. Indic. 2021, 130, 108074. [Google Scholar] [CrossRef]
- Piao, S.; Fang, J.; Zhou, L.; Ciais, P.; Zhu, B. Variations in Satellite-derived Phenology in China’s Temperate Vegetation. Glob. Change Biol. 2006, 12, 672–685. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Chen, X.; Gao, Y.; Xie, S.; Mi, J. GLC_FCS30: Global Land-Cover Product with Fine Classification System at 30 m Using Time-Series Landsat Imagery. Earth Syst. Sci. Data 2021, 13, 2753–2776. [Google Scholar] [CrossRef]
- Agrawal, S.; Chakraborty, A. Evaluation of ESACCI Satellite Soil Moisture Product Using In-Situ CTCZ Observations over India. J. Earth Syst. Sci. 2020, 129, 129. [Google Scholar] [CrossRef]
- Pimentel, C.S.; Gonçalves, E.V.; Firmino, P.N.; Calvão, T.; Fonseca, L.; Abrantes, I.; Correia, O.; Máguas, C. Differences in Constitutive and Inducible Defences in Pine Species Determining Susceptibility to Pinewood Nematode. Plant Pathol. 2017, 66, 131–139. [Google Scholar] [CrossRef]
- Gao, R.; Liu, L.; Fan, S.; Zheng, W.; Liu, R.; Zhang, Z.; Huang, R.; Zhao, L.; Shi, J. Occurrence and Potential Diffusion of Pine Wilt Disease Mediated by Insect Vectors in China under Climate Change. Pest Manag. Sci. 2024, 80, 6068–6081. [Google Scholar] [CrossRef]
- Ohsawa, M.; Akiba, M. Possible Altitude and Temperature Limits on Pine Wilt Disease: The Reproduction of Vector Sawyer Beetles (Monochamus alternatus), Survival of Causal Nematode (Bursaphelenchus xylophilus), and Occurrence of Damage Caused by the Disease. Eur. J. For. Res. 2014, 133, 225–233. [Google Scholar] [CrossRef]
- Lu, Y. Influence of Temperature on the Development of Bursaphelenchus xylophilus. Ph.D. Thesis, Shandong Agricultural University, Tai’an, China, 2015. [Google Scholar]
- Naves, P.; De Sousa, E. Threshold Temperatures and Degree-Day Estimates for Development of Post-Dormancy Larvae of Monochamus galloprovincialis (Coleoptera: Cerambycidae). J. Pest Sci. 2009, 82, 1–6. [Google Scholar] [CrossRef]
- Roques, A.; Zhao, L.; Sun, J.; Robinet, C. Pine Wood Nematode, Pine Wilt Disease, Vector Beetle and Pine Tree: How a Multiplayer System Could Reply to Climate Change. In Climate Change and Insect Pests; CABI: Wallingford, UK, 2015; pp. 220–234. [Google Scholar]
- Rutherford, T.A.; Webster, J.M. Distribution of Pine Wilt Disease with Respect to Temperature in North America, Japan, and Europe. Can. J. For. Res. 1987, 17, 1050–1059. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Matsunaga, K.; Hirao, T.; Tamura, M.; Watanabe, A. Spatiotemporal Analysis of Pine Wilt Disease: Relationship between Pinewood Nematode Distribution and Defence Response in Pinus thunbergii Seedlings. For. Pathol. 2019, 49, e12518. [Google Scholar] [CrossRef]
- Petrella, L.N. Natural Variants of C. Elegans Demonstrate Defects in Both Sperm Function and Oogenesis at Elevated Temperatures. PLoS ONE 2014, 9, e112377. [Google Scholar] [CrossRef]
- Li, Z. Cold Tolerance Research of Pine Wood Nematode Bursaphelenchus xylophilus strains from Different Area in China; Beijing Forestry University: Beijing, China, 2022. [Google Scholar]
- Klosin, A.; Casas, E.; Hidalgo-Carcedo, C.; Vavouri, T.; Lehner, B. Transgenerational Transmission of Environmental Information in C. elegans. Science 2017, 356, 320–323. [Google Scholar] [CrossRef]
- Marré, J.; Traver, E.C.; Jose, A.M. Extracellular RNA Is Transported from One Generation to the next in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2016, 113, 12496–12501. [Google Scholar] [CrossRef]
- Houri-Ze’evi, L.; Korem, Y.; Sheftel, H.; Faigenbloom, L.; Toker, I.A.; Dagan, Y.; Awad, L.; Degani, L.; Alon, U.; Rechavi, O. A Tunable Mechanism Determines the Duration of the Transgenerational Small RNA Inheritance in C. elegans. Cell 2016, 165, 88–99. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Huang, J.; Zhao, X.; Liu, Y.; Fang, G.; Zhou, Y.; Hu, M. The Threshold and Lag Effects of Temperature on Pine Wilt Disease Show Significant Spatial Heterogeneity. Insects 2025, 16, 834. https://doi.org/10.3390/insects16080834
Zhang R, Huang J, Zhao X, Liu Y, Fang G, Zhou Y, Hu M. The Threshold and Lag Effects of Temperature on Pine Wilt Disease Show Significant Spatial Heterogeneity. Insects. 2025; 16(8):834. https://doi.org/10.3390/insects16080834
Chicago/Turabian StyleZhang, Ruicong, Jixia Huang, Xiaoting Zhao, Yanqing Liu, Guofei Fang, Yantao Zhou, and Maogui Hu. 2025. "The Threshold and Lag Effects of Temperature on Pine Wilt Disease Show Significant Spatial Heterogeneity" Insects 16, no. 8: 834. https://doi.org/10.3390/insects16080834
APA StyleZhang, R., Huang, J., Zhao, X., Liu, Y., Fang, G., Zhou, Y., & Hu, M. (2025). The Threshold and Lag Effects of Temperature on Pine Wilt Disease Show Significant Spatial Heterogeneity. Insects, 16(8), 834. https://doi.org/10.3390/insects16080834



