Temporal Dynamics of Host Plant Use and Parasitism of Three Stink Bug Species: A Multi-Trophic Perspective
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Surveyed Sites
2.2. Field Sampling of Stink Bugs
2.3. Field Collection of Eggs and Identification of Emerged Parasitoids
2.4. Data Analysis
3. Results
3.1. Seasonal Abundance
3.2. Host Plant Utilization Patterns
3.3. Parasitism Patterns
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schaefer, C.W.; Panizzi, A.R. Stink Bugs (Pentatomidae). In Heteroptera of Economic Importance; CRC Press: Boca Raton, FL, USA, 2000; Volume 13, p. 856. ISBN 978-0-429-11838-8. [Google Scholar]
- Sosa-Gómez, D.R.; Corrêa-Ferreira, B.S.; Kraemer, B.; Pasini, A.; Husch, P.E.; Delfino Vieira, C.E.; Reis Martinez, C.B.; Negrão Lopes, I.O. Prevalence, Damage, Management and Insecticide Resistance of Stink Bug Populations (Hemiptera: Pentatomidae) in Commodity Crops. Agric. For. Entomol. 2020, 22, 99–118. [Google Scholar] [CrossRef]
- Leskey, T.C.; Nielsen, A.L. Impact of the Invasive Brown Marmorated Stink Bug in North America and Europe: History, Biology, Ecology, and Management. Annu. Rev. Entomol. 2018, 63, 599–618. [Google Scholar] [CrossRef]
- Hamilton, G.C.; Ahn, J.J.; Bu, W.; Leskey, T.C.; Nielsen, A.L.; Park, Y.-L.; Rabitsch, W.; Hoelmer, K.A. Halyomorpha Halys (Stål). In Invasive Stink Bugs and Related Species (Pentatomoidea); CRC Press: Boca Raton, FL, USA, 2018; Volume 4, p. 840. ISBN 978-1-315-37122-1. [Google Scholar]
- Grabarczyk, E.E.; Cottrell, T.E.; Tillman, G. Characterizing the Spatiotemporal Distribution of Three Native Stink Bugs (Hemiptera: Pentatomidae) across an Agricultural Landscape. Insects 2021, 12, 854. [Google Scholar] [CrossRef]
- Panizzi, A.R.; Lucini, T.L. The Overlooked Role of Weed Plants Affecting Pest Stink Bug (Hemiptera: Heteroptera: Pentatomidae) Bioecology in the Neotropics. Arthropod-Plant Interact. 2022, 16, 1–14. [Google Scholar] [CrossRef]
- Berteloot, O.H.; Peusens, G.; Beliën, T.; De Clercq, P.; Van Leeuwen, T. Unveiling the Diet of Two Generalist Stink Bugs, Halyomorpha halys and Pentatoma rufipes (Hemiptera: Pentatomidae), through Metabarcoding of the ITS2 Region from Gut Content. Pest Manag. Sci. 2024, 80, 5694–5705. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, R.; Calvy, M.; Valentie, E.; Driss, L.; Guignet, J.; Thomas, M.; Tavella, L. Symptoms Resulting from the Feeding of True Bugs on Growing Hazelnuts. Entomol. Exp. Appl. 2022, 170, 477–487. [Google Scholar] [CrossRef]
- Powell, G. The Biology and Control of an Emerging Shield Bug Pest, Pentatoma rufipes (L.) (Hemiptera: Pentatomidae). Agric. For. Entomol. 2020, 22, 298–308. [Google Scholar] [CrossRef]
- Falagiarda, M.; Tortorici, F.; Bortolini, S.; Melchiori, M.; Wolf, M.; Tavella, L. Ecological Dynamics of True Bugs (Hemiptera: Pentatomidae, Acanthosomatidae and Coreidae) and Associated Egg Parasitoids (Hymenoptera) in an Alpine Region of Italy. Bull. Entomol. Res. 2025. under review. [Google Scholar]
- Wermelinger, B.; Wyniger, D.; Forster, B. First Records of an Invasive Bug in Europe: Halyomorpha halys Stål (Heteroptera: Pentatomidae), a New Pest on Woody Ornamentals and Fruit Trees? Mitteilungen Schweiz. Entomol. Ges. 2008, 81, 1–8. [Google Scholar]
- Rolando, G.; Moraglio, S.T.; Caratti, A.; Cordero, C.; Borreani, G.; Tavella, L. Quantitative and Qualitative Damage Caused by Halyomorpha halys (Hemiptera: Pentatomidae) on Soybean Crop at Different Growth Stages. Crop Prot. 2025, 187, 106987. [Google Scholar] [CrossRef]
- Fischnaller, S.; Frasconi-Wendt, C.; Rottensteiner, A.; Gruber, A.; Schmidt, S. A Local Exploration of Herbivorous Stink Bugs’ Host Plant Colonization in Apple Orchard Ecotones. Heteropteron 2024, 72, 30. [Google Scholar]
- Driss, L.; Hamidi, R.; Andalo, C.; Magro, A. Study of the Overwintering Ecology of the Hazelnut Pest, Palomena prasina (L.) (Hemiptera: Pentatomidae) in a Perspective of Integrated Pest Management. J. Appl. Entomol. 2024, 148, 34–48. [Google Scholar] [CrossRef]
- Bergmann, E.J.; Venugopal, P.D.; Martinson, H.M.; Raupp, M.J.; Shrewsbury, P.M. Host Plant Use by the Invasive Halyomorpha halys (Stål) on Woody Ornamental Trees and Shrubs. PLoS ONE 2016, 11, e0149975. [Google Scholar] [CrossRef] [PubMed]
- Panizzi, A.R. Wild Hosts of Pentatomids: Ecological Significance and Role in Their Pest Status on Crops. Annu. Rev. Entomol. 1997, 42, 99–122. [Google Scholar] [CrossRef] [PubMed]
- Moura, R.R.; Ribeiro, P.V.A.; Pereira, B.G.; Quero, A.; Carvalho, R.L.; Oliveira, D.C. Food, Shelter or Competitors? Overlapping of Life Stages and Host Plant Selection in a Neotropical Stink Bug Species. J. Plant Interact. 2017, 12, 560–566. [Google Scholar] [CrossRef]
- Zobel, E.S.; Hooks, C.R.R.; Dively, G.P. Seasonal Abundance, Host Suitability, and Feeding Injury of the Brown Marmorated Stink Bug, Halyomorpha halys (Heteroptera: Penatomidae), in Selected Vegetables. J. Econ. Entomol. 2016, 109, 1289–1302. [Google Scholar] [CrossRef]
- Tillman, P.G.; Grabarczyk, E.E.; Kesheimer, K.A.; Balusu, R. Seasonal Density and Natural Mortality of Halyomorpha halys (Stål) and Indigenous Stink Bugs (Hemiptera: Pentatomidae) in a Field Crop Agroecosystem. J. Econ. Entomol. 2023, 116, 1636–1648. [Google Scholar] [CrossRef]
- Falagiarda, M.; Carnio, V.; Chiesa, S.G.; Pignalosa, A.; Anfora, G.; Angeli, G.; Ioriatti, C.; Mazzoni, V.; Schmidt, S.; Zapponi, L. Factors Influencing Short-Term Parasitoid Establishment and Efficacy for the Biological Control of Halyomorpha halys with the Samurai Wasp Trissolcus japonicus. Pest Manag. Sci. 2023, 79, 2397–2414. [Google Scholar] [CrossRef]
- Costi, E.; Di Bella, E.; Iotti, D.; Maistrello, L. Biocontrol Implications of Multiparasitism by Trissolcus mitsukurii and Trissolcus japonicus on the Invasive Brown Marmorated Stink Bug. Entomol. Exp. Appl. 2022, 170, 772–781. [Google Scholar] [CrossRef]
- Mele, A.; Scaccini, D.; Pozzebon, A. Hyperparasitism of Acroclisoides sinicus (Huang and Liao) (Hymenoptera: Pteromalidae) on Two Biological Control Agents of Halyomorpha halys. Insects 2021, 12, 617. [Google Scholar] [CrossRef]
- MIPAAF. Decreto 42967 del 9 Giugno 2020: Immissione in Natura Della Specie non Autoctona Trissolcus japonicus Quale Agente di Controllo Biologico del Fitofago Halyomorpha halys ai Sensi del Decreto del Presidente della Repubblica 8 Settembre 1997, n. 357, art. 12; Ministero delle Politiche Agricole, Alimentari e Forestali: Rome, Italy, 2020. [Google Scholar]
- Conti, E.; Avila, G.; Barratt, B.; Cingolani, F.; Colazza, S.; Guarino, S.; Hoelmer, K.; Laumann, R.A.; Maistrello, L.; Martel, G.; et al. Biological Control of Invasive Stink Bugs: Review of Global State and Future Prospects. Entomol. Exp. Appl. 2021, 169, 28–51. [Google Scholar] [CrossRef]
- Conti, E.; Colazza, S. Chemical Ecology of Egg Parasitoids Associated with True Bugs. Psyche A J. Entomol. 2012, 2012, 651015. [Google Scholar] [CrossRef]
- Blassioli Moraes, M.C.; Laumann, R.; Sujii, E.R.; Pires, C.; Borges, M. Induced Volatiles in Soybean and Pigeon Pea Plants Artificially Infested with the Neotropical Brown Stink Bug, Euschistus heros, and Their Effect on the Egg Parasitoid, Telenomus podisi. Entomol. Exp. Appl. 2005, 115, 227–237. [Google Scholar] [CrossRef]
- Martorana, L.; Foti, M.C.; Rondoni, G.; Conti, E.; Colazza, S.; Peri, E. An Invasive Insect Herbivore Disrupts Plant Volatile-Mediated Tritrophic Signalling. J. Pest Sci. 2017, 90, 1079–1085. [Google Scholar] [CrossRef]
- Fatouros, N.E.; Dicke, M.; Mumm, R.; Meiners, T.; Hilker, M. Foraging Behavior of Egg Parasitoids Exploiting Chemical Information. Behav. Ecol. 2008, 19, 677–689. [Google Scholar] [CrossRef]
- Tillman, G. Ecosystem-Based Incorporation of Nectar-Producing Plants for Stink Bug Parasitoids. Insects 2017, 8, 65. [Google Scholar] [CrossRef]
- Foti, M.C.; Rostás, M.; Peri, E.; Park, K.C.; Slimani, T.; Wratten, S.D.; Colazza, S. Chemical Ecology Meets Conservation Biological Control: Identifying Plant Volatiles as Predictors of Floral Resource Suitability for an Egg Parasitoid of Stink Bugs. J. Pest Sci. 2017, 90, 299–310. [Google Scholar] [CrossRef]
- Lee, J.C.; Heimpel, G.E.; Leibee, G.L. Comparing Floral Nectar and Aphid Honeydew Diets on the Longevity and Nutrient Levels of a Parasitoid Wasp. Entomol. Exp. Appl. 2004, 111, 189–199. [Google Scholar] [CrossRef]
- Foti, M.C.; Peri, E.; Wajnberg, E.; Colazza, S.; Rostás, M. Contrasting Olfactory Responses of Two Egg Parasitoids to Buckwheat Floral Scent Are Reflected in Field Parasitism Rates. J. Pest Sci. 2019, 92, 747–756. [Google Scholar] [CrossRef]
- Herlihy, M.V.; Talamas, E.J.; Weber, D.C. Attack and Success of Native and Exotic Parasitoids on Eggs of Halyomorpha halys in Three Maryland Habitats. PLoS ONE 2016, 11, e0150275. [Google Scholar] [CrossRef]
- Cottrell, T.E.; Tillman, G.; Grabarczyk, E.E.; Toews, M.; Sial, A.; Lahiri, S. Habitat and Vertical Stratification Affect Capture of Stink Bugs (Hemiptera: Pentatomidae) and Biological Control of the Invasive Brown Marmorated Stink Bug. Environ. Entomol. 2023, 52, 593–605. [Google Scholar] [CrossRef]
- Südtiroler Beratungsring. Leitfaden Apfelanbau 2022; Pötzelberger Druck GmbH: Meran, Italy, 2022. [Google Scholar]
- Ribes, J.; Pagola-Carte, S. Faune de France 96: Hèmiptères Pentatomoidea Euro-Méditerranéens; Faune de France; Fèdération Francaise des Sociétés de Sciences Naturelles: Paris, France, 2013; Volume 2, ISBN 978-2-903052-35-5. [Google Scholar]
- Sabbatini Peverieri, G.; Giovannini, L.; Benvenuti, C.; Madonni, L.; Hoelmer, K.; Roversi, P.F. Characteristics of the Meconia of European Egg Parasitoids of Halyomorpha halys. J. Hymenopt. Res. 2020, 77, 187–201. [Google Scholar] [CrossRef]
- Askew, R.R.; Nieves-Aldrey, J.L. Nuevas observaciones sobre Eupelminae (Hymenoptera, Chalcidoidea, Eupelmidae) de la Península Ibérica e Islas Canarias incluyendo descripciones de nuevas especies. Graellsia 2004, 60, 27–39. [Google Scholar] [CrossRef]
- Peng, L.; Gibson, G.a.P.; Tang, L.; Xiang, J. Review of the Species of Anastatus (Hymenoptera: Eupelmidae) Known from China, with Description of Two New Species with Brachypterous Females. Zootaxa 2020, 4767, 351–401. [Google Scholar] [CrossRef] [PubMed]
- Talamas, E.J.; Johnson, N.F.; Buffington, M. Key to Nearctic Species of Trissolcus Ashmead (Hymenoptera, Scelionidae), Natural Enemies of Native and Invasive Stink Bugs (Hemiptera, Pentatomidae). J. Hymenopt. Res. 2015, 43, 45–110. [Google Scholar] [CrossRef]
- Talamas, E.J.; Buffington, M.L.; Hoelmer, K. Revision of Palearctic Trissolcus Ashmead (Hymenoptera, Scelionidae). J. Hymenopt. Res. 2017, 56, 3–185. [Google Scholar] [CrossRef]
- Tortorici, F.; Talamas, E.J.; Moraglio, S.T.; Pansa, M.G.; Asadi-Farfar, M.; Tavella, L.; Caleca, V. A Morphological, Biological and Molecular Approach Reveals Four Cryptic Species of Trissolcus Ashmead (Hymenoptera, Scelionidae), Egg Parasitoids of Pentatomidae (Hemiptera). J. Hymenopt. Res. 2019, 93, 153–200. [Google Scholar] [CrossRef]
- Moraglio, S.T.; Tortorici, F.; Visentin, S.; Pansa, M.G.; Tavella, L. Trissolcus kozlovi in North Italy: Host Specificity and Augmentative Releases against Halyomorpha halys in Hazelnut Orchards. Insects 2021, 12, 464. [Google Scholar] [CrossRef]
- Tortorici, F.; Orrù, B.; Timokhov, A.V.; Bout, A.; Bon, M.-C.; Tavella, L.; Talamas, E.J. Telenomus Haliday (Hymenoptera, Scelionidae) Parasitizing Pentatomidae (Hemiptera) in the Palearctic Region. J. Hymenopt. Res. 2024, 97, 591–620. [Google Scholar] [CrossRef]
- Sabbatini Peverieri, G.; Mitroiu, M.-D.; Bon, M.-C.; Balusu, R.; Benvenuto, L.; Bernardinelli, I.; Fadamiro, H.; Falagiarda, M.; Fusu, L.; Grove, E.; et al. Surveys of Stink Bug Egg Parasitism in Asia, Europe and North America, Morphological Taxonomy, and Molecular Analysis Reveal the Holarctic Distribution of Acroclisoides sinicus (Huang & Liao) (Hymenoptera, Pteromalidae). J. Hymenopt. Res. 2019, 74, 123–151. [Google Scholar] [CrossRef]
- Francati, S.; Masetti, A.; Martinelli, R.; Mirandola, D.; Anteghini, G.; Busi, R.; Dalmonte, F.; Spinelli, F.; Burgio, G.; Dindo, M.L. Halyomorpha halys (Hemiptera: Pentatomidae) on Kiwifruit in Northern Italy: Phenology, Infestation, and Natural Enemies Assessment. J. Econ. Entomol. 2021, 114, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Haye, T.; Abdallah, S.; Gariepy, T.; Wyniger, D. Phenology, Life Table Analysis and Temperature Requirements of the Invasive Brown Marmorated Stink Bug, Halyomorpha halys, in Europe. J. Pest Sci. 2014, 87, 407–418. [Google Scholar] [CrossRef]
- Rot, M.; Maistrello, L.; Costi, E.; Trdan, S. Biological Parameters, Phenology and Temperature Requirements of Halyomorpha halys (Hemiptera: Pentatomidae) in the Sub-Mediterranean Climate of Western Slovenia. Insects 2022, 13, 956. [Google Scholar] [CrossRef] [PubMed]
- Moraglio, S.T.; Tortorici, F.; Pansa, M.G.; Castelli, G.; Pontini, M.; Scovero, S.; Visentin, S.; Tavella, L. A 3-Year Survey on Parasitism of Halyomorpha halys by Egg Parasitoids in Northern Italy. J. Pest Sci. 2020, 93, 183–194. [Google Scholar] [CrossRef]
- Zapponi, L.; Tortorici, F.; Anfora, G.; Bardella, S.; Bariselli, M.; Benvenuto, L.; Bernardinelli, I.; Butturini, A.; Caruso, S.; Colla, R.; et al. Assessing the Distribution of Exotic Egg Parasitoids of Halyomorpha halys in Europe with a Large-Scale Monitoring Program. Insects 2021, 12, 316. [Google Scholar] [CrossRef]
- Abram, P.K.; Mills, N.J.; Beers, E.H. Classical Biological Control of Invasive Stink Bugs with Egg Parasitoid—What Does Success Look Like? Pest Manag. Sci. 2020, 76, 1980–1992. [Google Scholar] [CrossRef]
- Moraglio, S.T.; Tortorici, F.; Giromini, D.; Pansa, M.G.; Visentin, S.; Tavella, L. Field Collection of Egg Parasitoids of Pentatomidae and Scutelleridae in Northwest Italy and Their Efficacy in Parasitizing Halyomorpha halys under Laboratory Conditions. Entomol. Exp. Appl. 2021, 169, 52–63. [Google Scholar] [CrossRef]
- Saulich, A.K.; Musolin, D.L. Diapause in the Seasonal Cycle of Stink Bugs (Heteroptera, Pentatomidae) from the Temperate Zone. Entmol. Rev. 2012, 92, 1–26. [Google Scholar] [CrossRef]
- Saulich, A.K.; Musolin, D.L. Seasonal Cycles in Stink Bugs (Heteroptera, Pentatomidae) from the Temperate Zone: Diversity and Control. Entmol. Rev. 2014, 94, 785–814. [Google Scholar] [CrossRef]
- Kehrli, P.; Pasquier, D.; Höhn, H. Die Rotbeinige Baumwanze, Ein Sporadisch Auftretender Schädling imObstbau. Schweiz. Z. Obs. Weinbau 2011, 147, 10–13. [Google Scholar]
- Zapponi, L.; Bon, M.C.; Fouani, J.M.; Anfora, G.; Schmidt, S.; Falagiarda, M. Assemblage of the Egg Parasitoids of the Invasive Stink Bug Halyomorpha halys: Insights on Plant Host Associations. Insects 2020, 11, 588. [Google Scholar] [CrossRef]
- Bakken, A.J.; Schoof, S.C.; Bickerton, M.; Kamminga, K.L.; Jenrette, J.C.; Malone, S.; Abney, M.A.; Herbert, D.A.; Reisig, D.; Kuhar, T.P.; et al. Occurrence of Brown Marmorated Stink Bug (Hemiptera: Pentatomidae) on Wild Hosts in Nonmanaged Woodlands and Soybean Fields in North Carolina and Virginia. Environ. Entomol. 2015, 44, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Formella, A.; McIntyre, K.; Kuhar, T.P. Effect of Vegetable Host Plant Type on Halyomorpha halys (Hemiptera: Pentatomidae) Nymphal Development. J. Econ. Entomol. 2022, 115, 2105–2109. [Google Scholar] [CrossRef] [PubMed]
- Floren, A.; Gogala, A. Heteroptera from Beech (Fagus sylvatica) and Silver Fir (Abies alba) Trees of the Primary Forest Reserve Rajhenavski Rog, Slovenia. Acta Entomol. Slov. 2002, 10, 25–33. [Google Scholar]
- Hawkins, Roger Shieldbugs of Surrey; Surrey Wildlife Atlas Series; Surrey Wildlife Trust: Woking, UK, 2003; ISBN 978-0-9526065-7-4.
- Kehrli, P.; Pasquier, D. Biology and Impact of the Forest Bug Pentatoma rufipes L. (Heteroptera, Pentatomidae) in Pear and Apricot Orchards. IOBC-WRPS Bull. 2012, 74, 33–37. [Google Scholar]
- Gossner, M. Heteroptera (Insecta: Hemiptera) Communities in Tree Crowns of Beech, Oak and Spruce in Managed Forests: Diversity, Seasonality, Guild Structure, and Tree Specificity. In Canopy Arthropod Research in Central Europe-Basic and Applied Studies from the High Frontier; Bioform: Nürnberg, Germany, 2008; pp. 119–143. [Google Scholar]
- Holthouse, M.C.; Spears, L.R.; Alston, D.G. Urban Host Plant Utilisation by the Invasive Halyomorpha halys (Stål) (Hemiptera, Pentatomidae) in Northern Utah. NeoBiota 2021, 64, 87–101. [Google Scholar] [CrossRef]
- Formella, A.; Dorman, S.J.; Taylor, S.V.; Kuhar, T.P. Effects of Aggregation Lure and Tree Species on Halyomorpha HALYS (Hemiptera: Pentatomidae) Seasonal Oviposition. J. Econ. Entomol. 2020, 113, 203–210. [Google Scholar] [CrossRef]
- Laterza, I.; Dioli, P.; Tamburini, G. Semi-Natural Habitats Support Populations of Stink Bug Pests in Agricultural Landscapes. Agric. Ecosyst. Environ. 2023, 342, 108223. [Google Scholar] [CrossRef]
- Körner, C. The Use of ‘Altitude’ in Ecological Research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef]
- Kenzhegaliev, A.M.; Esenbekova, P.A.; Myrkasymova, A.C.; Zhaksybaev, M.B. Arboreal Hemiptera (Heteroptera) of mountain ecosystems of South-Eastern Kazakhstan. Exp. Biol. 2023, 94, 134–142. [Google Scholar] [CrossRef]
- Fent, M.; Dursun, A. An Up-to-Date Checklist of Turkish Pentatomidae (Hemiptera: Heteroptera) with Additional Records. Trak. Univ. J. Nat. Sci. 2022, 23, 65–111. [Google Scholar] [CrossRef]
- Alma, A.; Bocca, M.; Čermak, V.; Chen, P.P.; D’Urso, V.; Exnerová, A.; Goula, M.; Guglielmino, A.; Kunz, G.; Lauterer, P.; et al. Insecta Hemiptera Collected in the Mont Avic Natural Park (Aosta Valley, Northwest Italy). Rev. Valdôtaine D’histoire Nat. 2009, 63, 109–124. [Google Scholar]
- Zhang, J.; Zhang, F.; Gariepy, T.; Mason, P.; Gillespie, D.; Talamas, E.; Haye, T. Seasonal Parasitism and Host Specificity of Trissolcus japonicus in Northern China. J. Pest Sci. 2017, 90, 1127–1141. [Google Scholar] [CrossRef]
- Lowenstein, D.M.; Andrews, H.; Hilton, R.J.; Kaiser, C.; Wiman, N.G. Establishment in an Introduced Range: Dispersal Capacity and Winter Survival of Trissolcus japonicus, an Adventive Egg Parasitoid. Insects 2019, 10, 443. [Google Scholar] [CrossRef] [PubMed]
- Lara, J.R.; Pickett, C.H.; Kamiyama, M.T.; Figueroa, S.; Romo, M.; Cabanas, C.; Bazurto, V.; Strode, V.; Briseno, K.; Lewis, M.; et al. Physiological Host Range of Trissolcus japonicus in Relation to Halyomorpha halys and Other Pentatomids from California. BioControl 2019, 64, 513–528. [Google Scholar] [CrossRef]
- Haye, T.; Fischer, S.; Zhang, J.; Gariepy, T. Can Native Egg Parasitoids Adopt the Invasive Brown Marmorated Stink Bug, Halyomorpha halys (Heteroptera: Pentatomidae), in Europe? J. Pest Sci. 2015, 88, 693–705. [Google Scholar] [CrossRef]
- Giovannini, L.; Sabbatini Peverieri, G.; Tillman, P.G.; Hoelmer, K.A.; Roversi, P.F. Reproductive and Developmental Biology of Acroclisoides sinicus, a Hyperparasitoid of Scelionid Parasitoids. Biology 2021, 10, 229. [Google Scholar] [CrossRef]
- Sanchez, C.; Gamez, M.; Burguillo, F.J.; Garay, J.; Cabello, T. Comparison of Predator-Parasitoid-Prey Interaction Models for Different Host Plant Qualities. Community Ecol. 2018, 19, 125–132. [Google Scholar] [CrossRef]
- Gingras, D.; Dutilleul, P.; Boivin, G. Modeling the Impact of Plant Structure on Host-Finding Behavior of Parasitoids. Oecologia 2002, 130, 396–402. [Google Scholar] [CrossRef]
- Scaccini, D.; Falagiarda, M.; Tortorici, F.; Martinez-Sañudo, I.; Tirello, P.; Reyes-Domínguez, Y.; Gallmetzer, A.; Tavella, L.; Zandigiacomo, P.; Duso, C.; et al. An Insight into the Role of Trissolcus mitsukurii as Biological Control Agent of Halyomorpha halys in Northeastern Italy. Insects 2020, 11, 306. [Google Scholar] [CrossRef]
- Reznik, S.Y.; Karpun, N.N.; Zakharchenko, V.Y.; Shoshina, Y.I.; Dolgovskaya, M.Y.; Saulich, A.K.; Musolin, D.L. To Every Thing There Is a Season: Phenology and Photoperiodic Control of Seasonal Development in the Invasive Caucasian Population of the Brown Marmorated Stink Bug, Halyomorpha halys (Hemiptera: Heteroptera: Pentatomidae). Insects 2022, 13, 580. [Google Scholar] [CrossRef]
- Benelli, G.; Giunti, G.; Tena, A.; Desneux, N.; Caselli, A.; Canale, A. The Impact of Adult Diet on Parasitoid Reproductive Performance. J. Pest Sci. 2017, 90, 807–823. [Google Scholar] [CrossRef]
- Feng, Y.; Kravchuk, O.; Sandhu, H.; Wratten, S.D.; Keller, M.A. The Activities of Generalist Parasitoids Can Be Segregated between Crop and Adjacent Non-Crop Habitats. J. Pest Sci. 2017, 90, 275–286. [Google Scholar] [CrossRef]
- Gillespie, M.A.K.; Gurr, G.M.; Wratten, S.D. Beyond Nectar Provision: The Other Resource Requirements of Parasitoid Biological Control Agents. Entomol. Exp. Appl. 2016, 159, 207–221. [Google Scholar] [CrossRef]
- Lundgren, J.G.; Fausti, S.W. Trading Biodiversity for Pest Problems. Sci. Adv. 2015, 1, e1500558. [Google Scholar] [CrossRef] [PubMed]
- Philpott, S.M.; Lucatero, A.; Bichier, P.; Egerer, M.H.; Jha, S.; Lin, B.; Liere, H. Natural Enemy-Herbivore Networks along Local Management and Landscape Gradients in Urban Agroecosystems. Ecol. Appl. 2020, 30, e02201. [Google Scholar] [CrossRef] [PubMed]
- Staton, T.; Walters, R.J.; Smith, J.; Breeze, T.D.; Girling, R.D. Evaluating a Trait-Based Approach to Compare Natural Enemy and Pest Communities in Agroforestry vs. Arable Systems. Ecol. Appl. 2021, 31, e02294. [Google Scholar] [CrossRef]
- Cingolani, M.F. Conservation Biological Control as an Alternative to Reduce Stink Bugs Outbreaks. In Stink Bugs (Hemiptera: Pentatomidae) Research and Management: Recent Advances and Case Studies from Brazil, Europe, and USA; Bueno, A.F., Panizzi, A.R., Eds.; Springer Nature: Cham, Switzerland, 2024; Volume 9, pp. 81–94. ISBN 978-3-031-69742-5. [Google Scholar]

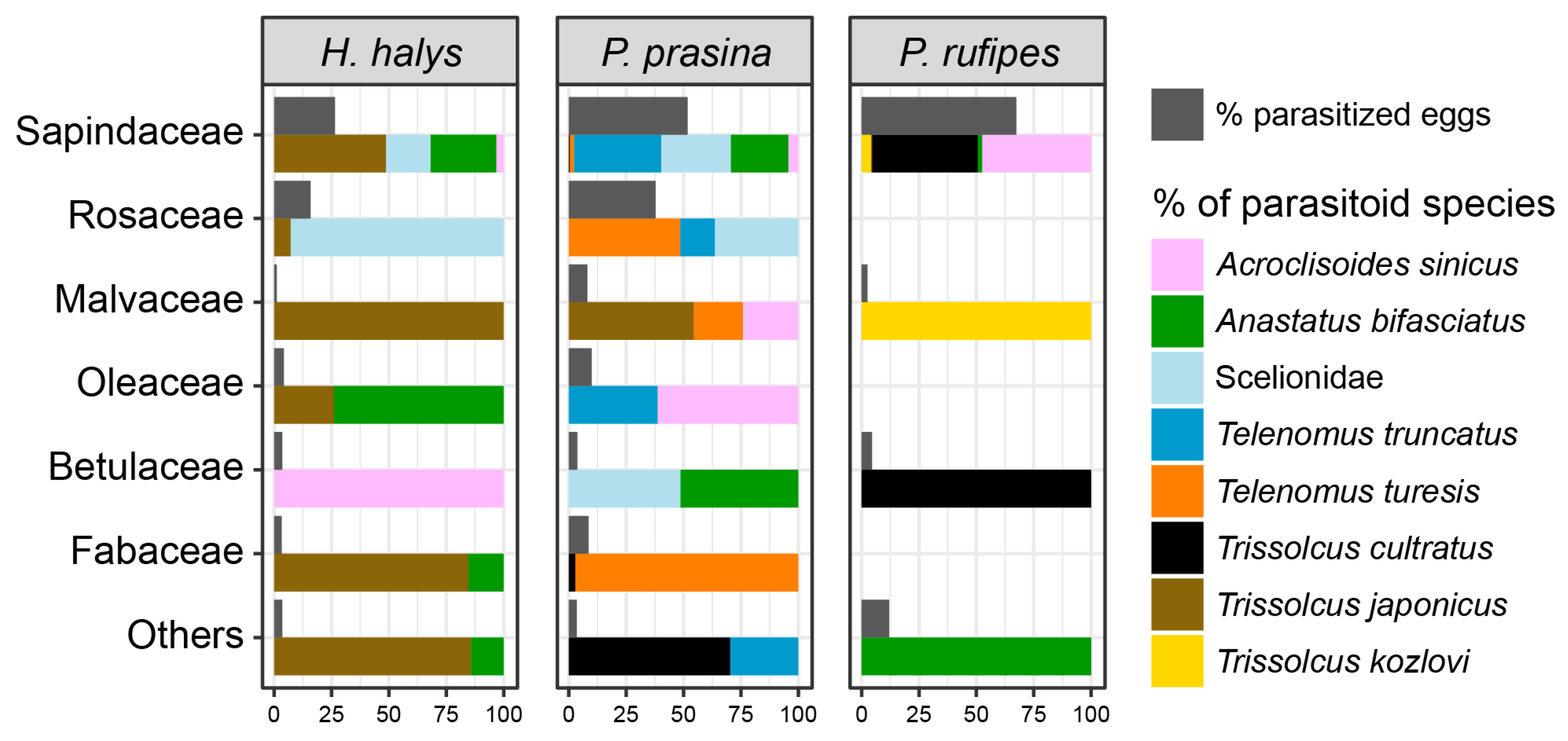

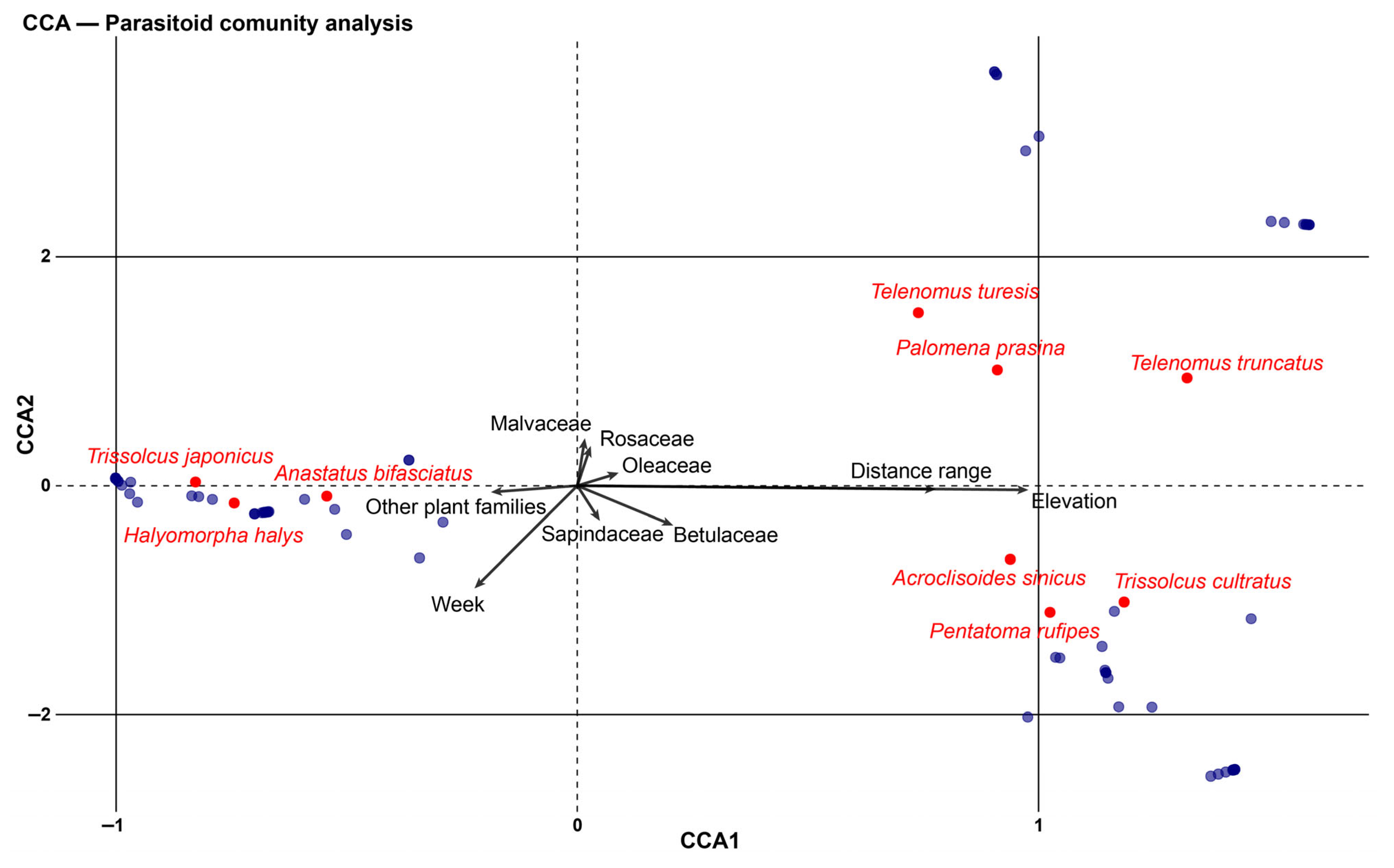
| April | May | June | July | August | September | ||
|---|---|---|---|---|---|---|---|
| H. halys | Eggs (% parasitized) | 0 | 0 | 249 (22.1) | 654 (22.3) | 962 (14.3) | 1226 (44.2) |
| Nymphs | 0 | 0 | 72 | 69 | 65 | 82 | |
| Adults | 2 | 6 | 18 | 11 | 41 | 42 | |
| P. prasina | Eggs (% parasitized) | 0 | 28 (0) | 634 (62.9) | 358 (44.1) | 137 (0) | 18 (0) |
| Nymphs | 0 | 0 | 13 | 47 | 26 | 7 | |
| Adults | 11 | 20 | 4 | 8 | 23 | 14 | |
| P. rufipes | Eggs (% parasitized) | 0 | 0 | 56 (0) | 16 (0) | 14 (100) | 556 (52) |
| Nymphs | 4 | 21 | 50 | 6 | 0 | 1 | |
| Adults | 0 | 0 | 15 | 91 | 97 | 28 |
| Plant Family | H. halys | P. prasina | P. rufipes |
|---|---|---|---|
| Adoxaceae | −0.74 | 0.34 | 0.65 |
| Betulaceae | −1.20 | 0.72 | 0.86 |
| Cornaceae | 1.11 | 0.51 | −2.06 * |
| Fabaceae | 0.95 | 0.19 | −1.50 |
| Fagaceae | −2.15 * | 0.08 | 2.85 ** |
| Lamiaceae | 2.49 * | −1.69 | −1.58 |
| Malvaceae | 0.57 | −0.72 | 0.00 |
| Oleaceae | 0.03 | 0.86 | −0.97 |
| Rosaceae | −0.20 | 0.64 | −0.41 |
| Sapindaceae | −0.58 | −0.51 | 1.34 |
| Simaroubaceae | 2.23 * | −1.51 | −1.41 |
| Ulmaceae | −0.74 | −0.63 | 1.68 |
| Comparison | Chi-Square p-Value | Fisher’s Exact p-Value | Significant After Bonferroni Correction |
|---|---|---|---|
| P. rufipes vs. P. prasina | 0.0675 | 0.0650 | No |
| P. rufipes vs. H. halys | 2.77 × 10−5 | 0.0001 | Yes |
| P. prasina vs. H. halys | 0.0412 | 0.0272 | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falagiarda, M.; Tortorici, F.; Bortolini, S.; Melchiori, M.; Wolf, M.; Tavella, L. Temporal Dynamics of Host Plant Use and Parasitism of Three Stink Bug Species: A Multi-Trophic Perspective. Insects 2025, 16, 731. https://doi.org/10.3390/insects16070731
Falagiarda M, Tortorici F, Bortolini S, Melchiori M, Wolf M, Tavella L. Temporal Dynamics of Host Plant Use and Parasitism of Three Stink Bug Species: A Multi-Trophic Perspective. Insects. 2025; 16(7):731. https://doi.org/10.3390/insects16070731
Chicago/Turabian StyleFalagiarda, Martina, Francesco Tortorici, Sara Bortolini, Martina Melchiori, Manfred Wolf, and Luciana Tavella. 2025. "Temporal Dynamics of Host Plant Use and Parasitism of Three Stink Bug Species: A Multi-Trophic Perspective" Insects 16, no. 7: 731. https://doi.org/10.3390/insects16070731
APA StyleFalagiarda, M., Tortorici, F., Bortolini, S., Melchiori, M., Wolf, M., & Tavella, L. (2025). Temporal Dynamics of Host Plant Use and Parasitism of Three Stink Bug Species: A Multi-Trophic Perspective. Insects, 16(7), 731. https://doi.org/10.3390/insects16070731






