Heortia vitessoides Infests Aquilaria sinensis: A Systematic Review of Climate Drivers, Management Strategies, and Molecular Mechanisms
Simple Summary
Abstract
1. Introduction
2. Biological Characteristics, Occurrence Patterns, and Climate Response of Heortia vitessoides
2.1. Growth and Development Characteristics
2.2. Occurrence Patterns of Heortia vitessoides in Different Regions
2.3. Climate Response of Heortia vitessoides
3. Management Strategies for Heortia vitessoides
3.1. Physical Trapping
3.2. Chemical Control
3.3. Prevention and Control of Natural Enemies
3.4. Modeling of the Population Dynamics of Heortia vitessoides
4. Molecular Mechanism of Heortia vitessoides: From Gene to Environmental Adaptation
4.1. Toxin-Related Genes
4.2. Genes Related to Temperature Stress
4.3. Genes for Metamorphosis of Heortia vitessoides
4.4. Genes Related to the Chemoreceptor of Heortia vitessoides
4.5. RNAi Technology: Bridging Genomic Research and Pest Control in Heortia vitessoides
| Gene Name | Gene Coding Protein | Subcellular Localization | Developmental Stage Expression Pattern | Organizational Expression Pattern | Regulatory Factor | RNAi | Gene Function | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| Detoxification-related genes | HvGSTs1 | Glutathione S-transferases | Cytoplasm (52.2%) | Expressed at all developmental stages, highest expression at pupal stage | In fourth-instar larvae, fat body expression significantly exceeded midgut, head, and cuticle expression; in 2-day-old adults, abdominal and thoracic expression significantly surpassed head, leg, and wing expression | Protects against toxic substances in the body | [66] | ||
| Temperature stress-related genes | Tpx | Thioredoxin peroxidase | Cytoplasm (69.6%) | HvTpx expression was induced by 0 °C, 10 °C, and 35 °C temperatures | Coping with temperature stress | [75] | |||
| CAT | Catalase | Expressed at all developmental stages, highest expression in fifth-instar larvae | Expression in the fat body of fifth-instar larvae was significantly higher than in the head, cuticle, midgut, and Malpighian tubule; expression in the adult abdomen was significantly higher than in the head, thorax, legs, and wings | Expression of this gene was induced under high-temperature stress conditions (35, 37, and 39 °C) | Participation in resistance to heat | [76] | |||
| HvGP | Glycogen phosphorylase | Cytoplasm | Expressed at all developmental stages, highest expression at egg stage | Expression in the fat body of fourth to fifth-instar larvae was significantly higher than in the head, legs, cuticle, midgut, and hindgut; expression in the adult wing was significantly higher than in the thorax, abdomen, head, legs, and antennae | A cold stress interval of 5–20 °C induced the expression of this gene; a heat stress temperature interval of 30–40 °C repressed the expression of this gene | Participating in the fight against hypothermia | [80] | ||
| HvAK | Arginine kinase | Cytoplasm | Expressed at all developmental stages, highest expression in fifth-instar larvae | Expression in the head of fourth-instar larvae was significantly higher than in the fat body, midgut, Malpighian tubule, and legs | Both high- (35 °C) and low-temperature (4 °C) stresses caused the upregulation of expression | Responding to adverse environments | [82] | ||
| Metamorphic development-related genes | HvTPS | Trehalose-6-phosphate synthase | Expressed at all developmental stages, with the highest expression after pupation and before emergence | Expression in the fat body of fifth-instar larvae was significantly higher than in the head, cuticle, midgut, and Malpighian tubule | Both pupal and adult stages showed deformities, and survival significantly reduced compared to the control | Involved in biosynthesis of chitin and lipids | [88] | ||
| HvCDA1, HvCDA2 | Chitin deacetylase | Heortia vitessoides showed abnormalities or even death in the molting, pupating, and emergence stages, and all adults fortunate enough to have life characteristics showed wing folds | Requirements for the growth and development of Heortia vitessoides | [89] | |||||
| HvEcR | Ecdysone receptor | Mitochondria | Expressed in all developmental stages, with significantly higher expression in fifth-instar larvae and adults | Significantly higher expression in the fat body of fourth to fifth-instar larvae than in cuticle, head, midgut, and Malpighian tubule | Expression levels may be regulated by 20-hydroxyecdysone | [90] | |||
| HvFABP | Fatty acid binding protein | Significantly higher expression from prepupal to adult stage than larval stage | Expression in the midgut of fifth-instar larvae was significantly higher than in the head, cuticle, fat body, foregut, and hindgut; expression in the wings of adults was significantly higher than in the head, thorax, abdomen, and legs | Starvation induces HvFABP expression, and 20-E induces upregulation of its expression | Heortia vitessoides dies because of molting failure | Participating in Heortia vitessoides molting process | [91] | ||
| Chemosensitive genes | HvitOR42, HvitOR43 | Pheromone receptor | Highly expressed in the antennae of adult males (more than 10-fold greater expression than females) | May be associated with the recognition of female sex pheromones | [94] | ||||
| HvitOR20 | Odorant receptor | Highly expressed in the antennae of adult females (more than 10-fold greater expression than males) | May play a role in female oviposition-related behaviors | [94] | |||||
| HvitGOBP2, Hvit-PBP1, HvitPBP2, HvitOBP2, HvitOBP10, HvitOBP11, HvitOBP13, HvitOBP15 | Odorant binding protein | Highly expressed in the mouthparts of adult males (expression more than 10-fold greater than females) | May be associated with the recognition of sex pheromones | [94] | |||||
| HvitCSP8, HvitCSP15, HvitCSP17 | Chemosensory protein | Preferentially expressed in males (more than 3-fold greater expression than females) | May be associated with the recognition of sex pheromones | [94] |
5. Interaction Between Heortia vitessoides and Aquilaria sinensis
6. Conclusions and Outlook
- Gene Editing and Functional Validation: Utilize CRISPR/Cas9 technology to target and knockout key genes in H. vitessoides, elucidating the regulatory networks governing its development and behavior.
- Field Application of RNAi Technology: Design double-stranded RNA (dsRNA) formulations targeting multiple genes (e.g., detoxification enzymes, chemosensory genes) based on current research for environmentally friendly field deployment.
- Constructing Cross-Species Molecular Interaction Networks: Employ multi-omics integration to decipher the molecular mechanisms underlying natural enemy responses to host signals. Systematically analyze the induced expression patterns of H. vitessoides genes in response to host defense compounds, while identifying the temporal regulation of JA pathway genes in A. sinensis triggered by herbivory. This will reveal the dynamic molecular interaction network of “host defense—H. vitessoides response”.
- Multi-Omics-Driven Integrated Modeling: Combine genomic, transcriptomic, and metabolomic data to construct multi-scale models of H. vitessoides environmental adaptability and population dynamics. Develop time-delay differential equation models to simulate the nonlinear regulatory effects of different control measures (e.g., RNAi spray frequency, natural enemy release intervals) on population density, optimizing control strategies.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Syazwan, S.A.; Lee, S.Y.; Ong, S.P.; Mohamed, R. Damaging insect pests and diseases and their threats to agarwood tree plantations. Sains Malays. 2019, 48, 497–507. [Google Scholar]
- Su, Y.P. Biological characteristics of Aquilaria sinensis Heortia vitessoides. J. Chin. Med. Mater. 1994, 12, 7–9. [Google Scholar]
- Chen, Z.Y.; Li, D.W.; Wang, L.; Li, Y.Z.; Huang, X.R.; Qin, C.S. Studies on biological characteristics of Heortia vitessoides Moore on Aquilaris sinensis. China Plant Prot. 2011, 31, 10–14. [Google Scholar]
- Zhou, Y.K.; Zhan, Q.Q.; Zhao, X.S.; Lu, L.L. Brief introduction to the control of Aquilaria sinensis Heortia vitessoides and prospect of transitivity pheromone. China Agric. Inf. 2017, 03, 49–52. [Google Scholar]
- Qiao, H.L.; Lu, P.F.; Chen, J.; Xu, C.Q.; Ma, W.S.; Qin, R.M.; Li, X.M.; Cheng, H.Z. Biological characteristics and occurrence patterns of Heortia vitessoides. Chin. J. Appl. Entomol. 2013, 50, 1244–1252. [Google Scholar]
- Qiao, H.L.; Xu, C.Q.; Zhou, Y.K.; Xu, R.; Guo, K.; Wei, J.H.; Li, X.M.; Chen, J. Study on the application of insecticidal lamp to control the Heortia vitessoides of Aquilaria sinensis. J. Chin. Med. Mater. 2017, 40, 2026–2029. [Google Scholar]
- Chen, Z.Y.; Dong, X.; Wang, L.; Li, D.W.; Mo, X.; Kong, D.Q. Study on the monitoring effect of solar electric lamp on Aquilaria sinensis in Zhongshan City. Prot. For. Sci. Technol. 2023, 03, 34–36. [Google Scholar]
- Huang, Z.J.; Yuan, P.Y.; Xie, W.L.; Ma, T.; Wen, X.J. Effect of group size on the survival and growth of Heortia vitessoides Moore (Lepidoptera: Pyralidae). J. Environ. Entomol. 2022, 44, 1037–1042. [Google Scholar]
- Yang, X.; Li, G.; Wang, C. Evidence of Cuticle Chemicals of Heortia vitessoides (Lepi-doptera: Crambidae) Larvae Influencing the Aggregation Behavior of Conspecific Larvae. Insects 2024, 15, 746. [Google Scholar] [CrossRef]
- Jin, X.F.; Zhang, M.; Liu, Z.T.; Wen, X.J. Selection of insect-resistant varieties of Aquilaria sinensis and preliminary identification of its resistance to Heortia vitessoides. In Proceedings of the 2014 Annual Meeting of China Plant Protection Society, Shenyang, China, 26 July–4 August 2014. [Google Scholar]
- Peng, S.Y.; Zhao, P.F.; Chang, M.S.; Yang, J.; Yang, Z.D. Behavior characteristics of Heortia vitessoides larva and its control. Guangxi For. Sci. 2022, 51, 280–284. [Google Scholar]
- Kuntadi, K.; Irianto, R.S.B.; Andadari, L. Dinamika Serangan Ulat Heortia Vitessoides Moore (Lepidoptera: Crambidae) pada Tanaman Gaharu di Hutan Penelitian Carita, Propinsi Banten. J. Penelit. Hutan Tanam. 2016, 13, 83–93. [Google Scholar] [CrossRef]
- Prathapan, K.D.; Santhoshkumar, T. First report of infestation of the agar defoliator Heortia vitessoides (Moore) on the agar wood tree Aquilaria malaccensis Benth. in South India. Indian J. Entomol. 2023, 85, 654–656. [Google Scholar] [CrossRef]
- Li, T.; Zhao, G.X.; Zhao, D.X.; Chen, L.Y.; Zhou, J.S.; Wang, S.M. Investigation of Anthracnose and Heortia vitessoides on Aquilaria sinensis in Hekou of Yunnan. Guangdong Agric. Sci. 2015, 42, 63–67. [Google Scholar]
- Xu, D.; Li, X.Y.; Jin, Y.W.; Zhuo, Z.H.; Yang, H.J.; Hu, J.M.; Wang, R.Y. Influence of climatic factors on the potential distribution of pest Heortia vitessoides Moore in China. Glob. Ecol. Conserv. 2020, 23, e01107. [Google Scholar] [CrossRef]
- Liang, S.P.; Cai, J.C.; Chen, X.; Jin, Z.Y.; Zhang, J.K.; Huang, Z.J.; Tang, L.P.; Sun, Z.H.; Wen, X.J.; Wang, C. Larval aggregation of Heortia vitessoides Moore (Lepidoptera: Crambidae) and evidence of horizontal transfer of avermectin. Forests 2019, 10, 331. [Google Scholar] [CrossRef]
- Singh, S.; Barthakur, N.D.; Gurung, D. Bioecology of Heortia vitessoides Moore (Lepidoptera: Pyralidae: Odentiinae), a major defoliator of Aquilaria malaccensis Lam. (Indian eagle wood). Ann. For. Sci. 2000, 8, 109–115. [Google Scholar]
- Rahman, I. Biology of Heortia vitessoides More, a major insect pest of Aquilaria Malaccensis Lamk in northeast India. Indian J. Entomol. 2018, 80, 1725–1728. [Google Scholar] [CrossRef]
- Wen, Y.Z.; Jin, X.F.; Zhu, C.Q.; Chen, X.; Ma, T.; Zhang, S.N.; Zhang, Y.; Zeng, S.C.; Chen, X.Y.; Sun, Z.H.; et al. Effect of substrate type and moisture on pupation and emergence of Heortia vitessoides (Lepidoptera: Crambidae): Choice and no-choice studies. J. Insect Behav. 2016, 29, 473–489. [Google Scholar] [CrossRef]
- Wen, Y.Z.; Qin, W.Q.; Chen, X.; Wen, X.J.; Ma, T.; Dong, X.; Lin, S.C.; Sun, Z.H.; Zeng, S.C.; Wang, C. Soil moisture effects on pupation behavior, physiology, and morphology of Heortia vitessoides (Lepidoptera: Crambidae). J. Entomol. Sci. 2017, 52, 229–238. [Google Scholar] [CrossRef]
- Sha, L.H.; Chen, L.; Luo, X.Y.; Xu, J.H.; Sun, X.D. Research progress on biological characteristics of Heortia vitessoides Moore. World J. 2018, 7, 16–18. [Google Scholar]
- Wu, Y.J. Heortia vitessoides and Cryptophlebia ombrodelta: The 15th in a series of popular science articles on pest control. For. GuangXi 2016, 8, 46. [Google Scholar] [CrossRef]
- Yan, Z.; Yue, J.J. Effects of temperature and supplementary foods on the development and fecundity of Heortia vitessoides. Chin. J. Trop. Crops 2019, 40, 1789–1795. [Google Scholar]
- Rishi, R.R.; Pandey, S.; Kumar, R. Management of Heortia vitessoides Moore. A major insect pest of Aquilaria malaccensis Lamk, in North East India. J. Entomol. Zool. Stud. 2016, 4, 335–338. [Google Scholar]
- Zhou, Y.K.; Zhan, Q.Q.; Zhao, X.S.; Lu, L.L.; Gan, B.C. Developmental threshold temperature and effective accumulative temperature of Heortia vitessoides in Hainan. China Plant Prot. 2017, 37, 46–48. [Google Scholar]
- Sasaki, F.; Shiba, T.; Matsukura, K. Novel method of determining parameters for the effective accumulated temperature model by using seasonal pest occurrence data. Ecol. Model. 2024, 490, 110651. [Google Scholar] [CrossRef]
- Royer, L.; McNeil, J.N. Effect of relative humidity conditions on responsiveness of European corn borer (Ostrinia nubilalis) males to female sex pheromone in a wind tunnel. J. Chem. Ecol. 1993, 19, 61–69. [Google Scholar] [CrossRef]
- Zhang, Q.H. A review on the factors affecting emission and reception of sex pheromones by lepidopt erous moths. Chin. J. Biol. Control 1993, 9, 80–86. [Google Scholar]
- Ren, P.; Néron, V.; Rossi, S.; Liang, E.; Bouchard, M.; Deslauriers, A. Warming counteracts defoliation-induced mismatch by increasing herbivore-plant phenological synchrony. Glob. Change Biol. 2020, 26, 2072–2080. [Google Scholar] [CrossRef]
- Maharjan, R.; Ahn, J.; Yi, H. Interactive effects of temperature and plant host on the development parameters of Spodoptera exigua (Hübner)(Lepidoptera: Noctuidae). Insects 2022, 13, 747. [Google Scholar] [CrossRef]
- Abarca, M.; Spahn, R. Direct and indirect effects of altered temperature regimes and phenological mismatches on insect populations. Curr. Opin. Insect Sci. 2021, 47, 67–74. [Google Scholar] [CrossRef]
- Kollberg, I.; Bylund, H.; Jonsson, T.; Schmidt, A.; Gershenzon, J.; Bjorkman, C. Temperature affects insect outbreak risk through tritrophic interactions mediated by plant secondary compounds. Ecosphere 2015, 6, 1–17. [Google Scholar] [CrossRef]
- Laws, A.N. Climate change effects on predator–prey interactions. Curr. Opin. Insect Sci. 2017, 23, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.L.; Xu, C.Q.; Xu, R.; Guo, K.; Wei, J.H.; Li, X.M.; Chen, J. Evaluation of efficiency of traps baited with frequency trembler grid lamps and trap plants for control Heortia vitessoides infectwed in Aquilaria sinensis. China J. Chin. Mater. Med. 2016, 41, 2025–2029. [Google Scholar]
- Grunsven, R.H.A.; Deijk, J.R.; Donners, M.; Berendse, F.; Visser, M.E.; Veenendaal, E.; Spoelstra, K. Experimental light at night has a negative long-term impact on macro-moth populations. Curr. Biol. 2020, 30, R694–R695. [Google Scholar] [CrossRef]
- Zhang, B.J.; Li, Y.R.; Wei, X.H.; Li, X.W.; Zhao, L.L.; Ma, R.Y.; Xiang, H.M. Improvement of attractant and trap of Grapholita molesta (Busck) in peach. Chin. J. Biol. Control. 2025, 41, 71–79. [Google Scholar]
- Chen, Z.Y.; Wang, L.; Li, D.W.; Li, Y.Z.; Huang, X.R.; Qin, C.S. A screening experiment on insecticides against Heortia vitessoides Moore. J. For. Eng. 2012, 26, 117–119. [Google Scholar]
- Lu, J.; Liu, Y.; Li, L.L.; Zhang, M.; Wen, X.J.; Li, Y.Z. A experiment on 7 kinds of insecticides against Heortia vitessoides Moore. Shandong For. Sci. Technol. 2014, 44, 37–39. [Google Scholar]
- Zhou, Y.K.; Zhan, Q.Q.; Lu, L.L.; Yang, Y.; Liu, Y.Y.; Gan, B.C. Toxicity and efficacy evaluation of six biopesticides against Heortia vitessoides larvae. For. Pest Dis. 2016, 35, 31–33. [Google Scholar]
- Chen, R.H.; Zheng, W.; Tian, L.Y.; Huang, H.; Wei, J.X. Indoor toxicity test of six insecticides against larvae of different instars of Heortia vitessoides. Trop. For. 2023, 51, 92–96. [Google Scholar]
- Qian, C.Y.; Ma, T.; Qiu, H.L.; Lyu, H.L.; Liang, S.P.; Shao, Y.H.; Yuan, P.Y.; Shen, L.M.; Wen, X.J.; Wang, C. Lethal, transmission, behavioral, and physiological effects of Metarhizium anisopliae against gregarious larvae of Heortia vitessoides and synergistic effects between Metarhizium anisopliae and insecticides. Pest Manag. Sci. 2023, 79, 2191–2205. [Google Scholar] [CrossRef]
- Zhao, P.F.; Chang, M.S.; Luo, J.; Wu, Y.J.; Yang, J.; Yang, Z.D. Infection effect of a strain of Aspergillus sp. Q527 on Heortiavites soides and its biological characteristics. Guangdong Agric. Sci. 2019, 46, 106–112. [Google Scholar]
- Lovett, B.; Bilgo, E.; Millogo, S.A.; Ouattarra, A.K.; Sareet, I.; Gnambani, E.J.; Dabire, R.K.; Diabate, A.; Leger, R.J.S. Transgenic Metarhizium rapidly kills mosquitoes in a malaria-endemic region of Burkina Faso. Science 2019, 364, 894–897. [Google Scholar] [CrossRef] [PubMed]
- Usta, C. Microorganisms in biological pest control—A review (bacterial toxin application and effect of environmental factors). Curr. Prog. Biol. Res. 2013, 13, 287–317. [Google Scholar]
- Land, M.; Bundschuh, M.; Hopkins, R.J.; Poulin, B.; McKie, B.G. Effects of mosquito control using the microbial agent Bacillus thuringiensis israelensis (Bti) on aquatic and terrestrial ecosystems: A systematic review. Environ. Evid. 2023, 12, 26. [Google Scholar] [CrossRef]
- Yan, Z.; Yue, J.J.; Yang, C.Y. Potential use of Trichogramma pintoi as a biocontrol agent against Heortia vitessoides (Lepidoptera: Pyralidae). J. Econ. Entomol. 2020, 113, 654–659. [Google Scholar] [CrossRef]
- Luo, L.F.; Wen, W.Z.; Luo, J.; Li, M.F. Control effect of Trichogramma on agarwood Heortia vitessoides. Agric. Technol. Serv. 2021, 38, 24–26. [Google Scholar]
- Yan, Z.; Yue, J.J.; Zhang, Y.Y. Biotic and abiotic factors that affect parasitism in Trichogramma pintoi (Hymenoptera: Trichogrammatidae) as a biocontrol agent against Heortia vitessoides (Lepidoptera: Pyralidae). Environ. Entomol. 2023, 52, 301–308. [Google Scholar] [CrossRef]
- Xian, S.Q.; Wang, X.; Lin, S.C.; Chen, L.S.; Li, K.Y.; Xian, Z.Y. A new parasitic natural enemy against Heortia vitessoides: Trichogramma evanescens Westwood. For. Pest Dis. 2021, 40, 24–28. [Google Scholar]
- Wang, X.; Lin, S.C.; Xian, S.Q.; Bu, M.Y.; Chen, Q.W.; Chen, L.S.; Huang, Y.H. Study on parasitism habit of Trichogramma evanesceus in Heortia vitessoides eggs. For. Environ. Sci. 2022, 38, 94–100. [Google Scholar]
- Xian, S.Q.; Wang, X.; Lin, S.C.; Xian, Z.Y.; Bu, M.Y.; Chen, Q.W. A preliminary report on the experiment of controlling Heortia vitessoides by Trichogramma evanescens. Hunan For. Sci. Technol. 2023, 50, 107–112. [Google Scholar]
- Li, W.H.; Jia, C.J.; Chen, H.P.; Fu, L.; Wen, J.; Chen, K.W. Functional response of Eocanthecona furcellate (Wolff) to the larvae of Heortia vitessoides (Moore). J. Environ. Entomol. 2015, 37, 843–848. [Google Scholar]
- Xiao, N.; Wang, X.; Lin, S.C.; Bu, M.Y.; Chen, Q.W.; Liu, W.H. Study on selecting of natural enemy mantis of Heortia vitessoides. Anhui Agric. Sci. 2021, 49, 165–166, 189. [Google Scholar]
- Wang, X.; Xiao, N.; Lin, S.C.; Liu, W.H.; Chen, Q.W.; Bu, M.Y.; Li, M.L. Investigation on the effect of controlling Heortia vitessoides with Hierodula patellifera. Hunan For. Sci. Technol. 2022, 49, 44–48. [Google Scholar]
- Crowder, D.W.; Jabbour, R. Relationships between biodiversity and biological control in agroecosystems: Current status and future challenges. Biol. Control. 2014, 75, 8–17. [Google Scholar] [CrossRef]
- Loomans, A.J.M. Every generalist biological control agent requires a special risk assessment. BioControl 2021, 66, 23–35. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Huang, X.; Li, S.G.; Zhang, Z.X.; Zhan, A. Incorporating adaptive genomic variation into predictive models for invasion risk assessment. Environ. Sci. Ecotechnol. 2024, 18, 100299. [Google Scholar] [CrossRef]
- Anguelov, R.; Dufourd, C.; Dumont, Y. Mathematical model for pest–insect control using mating disruption and trapping. Appl. Math. Model. 2017, 52, 437–457. [Google Scholar] [CrossRef]
- Xiang, Z.; Tang, S.; Xiang, C.; Wu, J. On impulsive pest control using integrated intervention strategies. Appl. Math. Comput. 2015, 269, 930–946. [Google Scholar] [CrossRef]
- Law, S.T.S.; Nong, W.; So, W.L.; Baril, T.; Swale, T.; Chan, C.B.; Tobe, S.S.; Kai, Z.P.; Bendena, W.G.; Hayward, A.; et al. Chromosomal-level reference genome of the moth Heortia vitessoides (Lepidoptera: Crambidae), a major pest of agarwood-producing trees. Genomics 2022, 114, 110440. [Google Scholar] [CrossRef]
- Ranson, H.; Claudianos, C.; Ortelli, F.; Abgrall, C.; Hemingway, J.; Sharakhova, M.V.; Unger, M.F.; Collins, F.H.; Feyereisen, R. Evolution of supergene families associated with insecticide resistance. Science 2002, 298, 179–181. [Google Scholar] [CrossRef]
- Ruan, C.L.; Mi, Z.; Zhu, Y. Research progress on mechanism of insect resistance to insecticides. Sci. Seric. 2012, 38, 322–328. [Google Scholar]
- Liao, C.Y.; Zhang, K.; Niu, J.Z.; Ding, T.B.; Zhong, R.; Xia, W.K.; Dou, W.; Wang, J.J. Identification and characterization of seven glutathione S-transferase genes from citrus red mite, Panonychus citri (McGregor). Int. J. Mol. Sci. 2013, 14, 24255–24270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Wang, J.X.; Zhang, M.; Qin, G.H.; Li, D.Q.; Zhu, K.Y.; Ma, E.B.; Zhang, J.Z. Molecular cloning, characterization and positively selected sites of the glutathione S-transferase family from Locusta migratoria. PLoS ONE 2014, 9, e114776. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R. Genomic organization of the glutathione S-transferase family in insects. Mol. Phylogenet. Evol. 2011, 61, 924–932. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, C.Y.; Lin, T. Characterization and expression pattern of glutathione S-transferase Sigma 1 gene from Heortia vitessoides. Acta Agric. Boreali Sin. 2018, 33, 99–105. [Google Scholar]
- Zhu, C.Q.; Wang, Z.; Xie, W.Z.; Zhang, M.; Lu, X.L.; Li, Y.Z.; Wen, X.J. Effects of Aquilaris sinensis leaves on development and protective enzymes activity of Heortia vitessoides Moore larval. China Plant Prot. 2017, 37, 19–23. [Google Scholar]
- Horn, D.J. Temperature synergism in integrated pest management. In Temperature Sensitivity in Insects and Application in Integrated Pest Management; Westview Press: Boulder, CO, USA, 2019; pp. 125–140. [Google Scholar]
- Ghazanfar, M.U.; Hagenbucher, S.; Romeis, J.; Grabenweger, G.; Meissle, M. Fluctuating temperatures influence the susceptibility of pest insects to biological control agents. J. Pest Sci. 2020, 93, 1007–1018. [Google Scholar] [CrossRef]
- Shi, F.; Xing, Y.; Niu, Y.; Cheng, L.; Xu, Y.; Li, X.; Ren, L.; Zong, S.; Tao, J. Unveiling winter survival strategies: Physiological and metabolic responses to cold stress of Monochamus saltuarius larvae during overwintering. Pest Manag. Sci. 2024, 80, 5656–5671. [Google Scholar] [CrossRef]
- Yao, P.B.; Chen, X.B.; Yan, Y.; Liu, F.; Zhang, Y.Y.; Guo, X.Q.; Xu, B.H. Glutaredoxin 1, glutaredoxin 2, thioredoxin 1, and thioredoxin peroxidase 3 play important roles in antioxidant defense in Apis cerana cerana. Free Radic. Biol. Med. 2014, 68, 335–346. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.B.; Liu, T.Y.; Ma, X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Li, T.P.; Guo, J.; Hu, G.L.; Cao, F.; Su, H.Y.; Shen, M.D.; Wang, H.M.; You, M.S.; Liu, Y.Y.; Gurr, G.M.; et al. Zinc finger proteins facilitate adaptation of a global insect pest to climate change. BMC Biol. 2024, 22, 303. [Google Scholar] [CrossRef]
- Cheng, J.; Lv, Z.H.; Lin, T. Identification and expression analysis of thioredoxin peroxidase Tpx gene in Heortia vitessoides Moore. J. Huazhong Agric. Univ. 2018, 37, 56–63. [Google Scholar]
- Cheng, J.; Wang, C.Y.; Lyu, Z.H.; Chen, J.X.; Lin, T. Identification and characterization of the catalase gene involved in resistance to thermal stress in Heortia vitessoides using RNA interference. J. Therm. Biol. 2018, 78, 114–121. [Google Scholar] [CrossRef]
- Chen, C.P.; Denlinger, D.L. Activation of phosphorylase in response to cold and heat stress in the flesh fly, Sarcophaga crassipalpis. J. Insect Physiol. 1990, 36, 549–553. [Google Scholar] [CrossRef]
- Bahjou, A.; Gourdoux, L.; Moreau, R.; Dutrieu, J. In vitro regulation of glycogen phosphorylase of the larval fat body of Tenebrio molitor. Gen. Comp. Endocrinol. 1988, 71, 205–211. [Google Scholar] [CrossRef]
- Liu, L.; He, L.F.; Liu, H.; Yan, R.; Wan, Q.H. The change of carbohydrate of Musca domestica larva in the diapause. Chin. J. Pest Control 2008, 24, 803–804. [Google Scholar]
- Lv, Z.H.; Wang, C.Y.; Lin, T. Temporal and spatial expression dynamics of glycogen phosphorylase gene and its response to temperature stress in Heortia vitessoides. J. Nanjing Agric. Univ. 2019, 42, 276–283. [Google Scholar]
- Rosenthal, G.A.; Dahlman, D.L.; Robinson, G.W. L-Arginine kinase from tobacco hornworm, Manduca sexta (L.). Purification, properties, and interaction with L-canavanine. J. Biol. Chem. 1977, 252, 3679–3683. [Google Scholar] [CrossRef]
- Wang, C.Y.; Lv, Z.H.; Lin, T. Identification and expression analysis of arginine kinase gene in Heortia vitessoides. J. Huazhong Agric. Univ. 2019, 38, 39–46. [Google Scholar]
- Zhang, J.; Lu, A.; Kong, L.; Zhang, Q.; Ling, E. Functional analysis of insect molting fluid proteins on the protection and regulation of ecdysis. J. Biol. Chem. 2014, 289, 35891–35906. [Google Scholar] [CrossRef] [PubMed]
- Si, W.; Wang, Q.; Li, Y.; Dong, D.J. Label-free quantitative proteomic analysis of insect larval and metamorphic molts. BMC Dev. Biol. 2020, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Wang, S.; Wang, S.G.; Wang, H.J.; Zhang, J.Y.; Cui, S.Y. Invertebrate trehalose-6-phosphate synthase gene: Genetic architecture, biochemistry, physiological function, and potential applications. Front. Physiol. 2018, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.P.; Liu, J.P.; An, G.L.; Li, W.H.; Si, W.J.; Sun, D.X.; Zhu, Y. Genome-wide identification and characterization of the trehalose-6-phosphate synthetase (TPS) gene family in watermelon (Citrullus lanatus) and their transcriptional responses to salt stress. Int. J. Mol. Sci. 2021, 23, 276. [Google Scholar] [CrossRef]
- Yu, H.Z.; Huang, Y.L.; Lu, Z.J.; Zhang, Q.; Su, H.N.; Du, Y.M.; Yi, L.; Zhong, B.L.; Chen, C.X. Inhibition of trehalase affects the trehalose and chitin metabolism pathways in Diaphorina citri (Hemiptera: Psyllidae). Insect Sci. 2021, 28, 718–734. [Google Scholar] [CrossRef]
- Chen, J.X.; Lyu, Z.H.; Wang, C.Y.; Cheng, J.; Lin, T. RNA interference of a trehalose-6-phosphate synthase gene reveals its roles in the biosynthesis of chitin and lipids in Heortia vitessoides (Lepidoptera: Crambidae). Insect Sci. 2020, 27, 212–223. [Google Scholar] [CrossRef]
- Wang, C.Y.; Cheng, J.; Lyu, Z.H.; Li, Z.X.; Chen, J.X.; Lin, T. Chitin deacetylase 1 and 2 are indispensable for larval–pupal and pupal–adult molts in Heortia vitessoides (Lepidoptera: Crambidae). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2019, 237, 110325. [Google Scholar] [CrossRef]
- Lv, Z.H.; Li, Z.X.; Lin, T. Identification and expression analysis of ecdysone receptor gene HvEcR of Heortia vitessoides. Acta Agric. Boreali-Occident. Sin. 2019, 28, 1187–1194. [Google Scholar]
- Ye, Q.Y.; Li, Z.X.; Chen, Q.L.; Sun, M.X.; Yin, M.L.; Lin, T. Fatty acid-binding protein gene is indispensable for molting process in Heortia vitessoides (Lepidoptera: Crambidae). J. Integr. Agric. 2023, 22, 495–504. [Google Scholar] [CrossRef]
- Kou, G.X.; Shi, M.F.; Bai, L.C.; Lai, Y.P.; Liu, Z.L.; Ding, R.; Zhou, Y.T. Ultramorphological structure observation of Drgyia antiqua. Pratacult. Sci. 2024, 16, 157. Available online: https://link.cnki.net/urlid/62.1069.S.20240731.1616.002 (accessed on 7 March 2025).
- Dahanukar, A.; Hallem, E.A.; Carlson, J.R. Insect chemoreception. Curr. Opin. Neurobiol. 2005, 15, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.C.; Liu, L.; Yang, B.; Yan, S.C.; Wang, G.R. Identification and analysis of chemosensory genes in Heortia vitessoides. Plant Prot. 2021, 47, 34–48. [Google Scholar]
- Alphey, L.S.; Crisanti, A.; Randazzo, F.; Akbari, O.S. Standardizing the definition of gene drive. Proc. Natl. Acad. Sci. USA 2020, 117, 30864–30867. [Google Scholar] [CrossRef] [PubMed]
- Bellés, X. Beyond Drosophila: RNAi in vivo and functional genomics in insects. Annu. Rev. Entomol. 2010, 55, 111–128. [Google Scholar] [CrossRef]
- Barrangou, R.; Doudna, J.A. Applications of CRISPR technologies in research and beyond. Nat. Biotechnol. 2016, 34, 933–941. [Google Scholar] [CrossRef]
- Bourtzis, K.; Vreysen, M.J.B. Sterile insect technique (SIT) and its applications. Insects 2021, 12, 638. [Google Scholar] [CrossRef]
- Bi, J.; Wang, Y.F. The effect of the endosymbiont Wolbachia on the behavior of insect hosts. Insect Sci. 2020, 27, 846–858. [Google Scholar] [CrossRef]
- He, L.; Huang, Y.; Tang, X. RNAi-based pest control: Production, application and the fate of dsRNA. Front. Bioeng. Biotechnol. 2022, 10, 1080576. [Google Scholar] [CrossRef]
- Head, G.P.; Carroll, M.W.; Evans, S.P.; Ruleet, D.M.; Willse, A.R.; Clarkal, T.L.; Storer, N.P.; Flannagan, R.D.; Samuel, L.W.; Meinke, L.G. Evaluation of SmartStax and SmartStax PRO maize against western corn rootworm and northern corn rootworm: Efficacy and resistance management. Pest Manag. Sci. 2017, 73, 1883–1899. [Google Scholar] [CrossRef]
- Hunter, W.B.; Glick, E.; Paldi, N.; Bextine, B.R. Advances in RNA interference: dsRNA treatment in trees and grapevines for insect pest suppression. Southwest. Entomol. 2012, 37, 85–87. [Google Scholar] [CrossRef]
- Székács, A.; Ammour, A.S.; Mendelsohn, M.L. RNAi based pesticides. Front. Plant Sci. 2021, 12, 714116. [Google Scholar] [CrossRef]
- Dubelman, S.; Fischer, J.; Zapata, F.; Huizinga, K.; Jiang, C.; Uffmanet, J.; Levineal, S.; Carson, D. Environmental fate of double-stranded RNA in agricultural soils. PLoS ONE 2014, 9, e93155. [Google Scholar] [CrossRef] [PubMed]
- Futuyma, D.J.; Agrawal, A.A. Macroevolution and the biological diversity of plants and herbivores. Proc. Natl. Acad. Sci. USA 2009, 106, 18054–18061. [Google Scholar] [CrossRef] [PubMed]
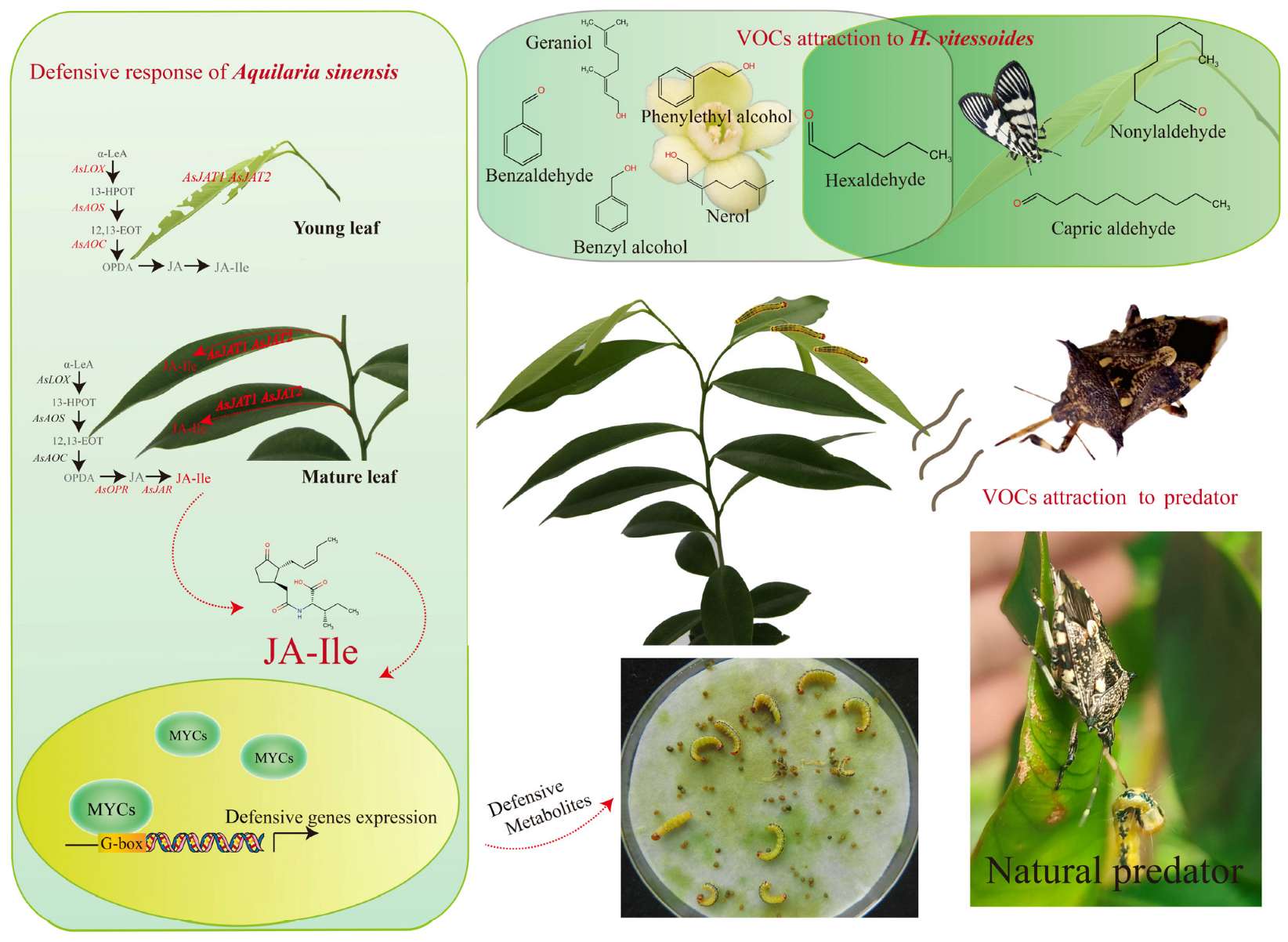
- Qiao, H.L.; Lu, P.F.; Chen, J.; Ma, W.S.; Qin, R.M.; Li, X.M. Antennal and behavioural responses of Heortia vitessoides females to host plant volatiles of Aquilaria sinensis. Entomol. Exp. Appl. 2012, 143, 269–279. [Google Scholar] [CrossRef]
- Qian, C.Y.; Xie, W.Q.; Su, Z.Q.; Wen, X.J.; Ma, T. Quantitative analysis and characterization of floral volatiles, and the role of active compounds on the behavior of Heortia vitessoides. Front. Plant Sci. 2024, 15, 1439087. [Google Scholar] [CrossRef]
- Qiao, H.L.; Lu, P.F.; Liu, S.; Xu, C.Q.; Guo, K.; Xu, R.; Chen, J. Volatiles from Aquilaria sinensis damaged by Heortia vitessoides larvae deter the conspecific gravid adults and attract its predator Cantheconidea concinna. Sci. Rep. 2018, 8, 15067. [Google Scholar] [CrossRef]
- Mao, Y.T.; Zhang, M.; Jin, X.F.; Ma, T.; Wang, C.; Sun, C.H.; Chen, X.Y.; Li, Y.Z.; Wen, X.J. Study on resistance of Aquilaria sinensis against Heortia vitessoides. J. South China Agric. Univ. 2017, 38, 89–96. [Google Scholar]
- Zhu, C.Q.; Zhang, M.; Ma, T.; Sun, C.H.; Li, Y.Z.; Wen, X.J. Resistance machenism of Aquilaria sinensis against Heortia vitessoides Moore. For. Pest Dis. 2017, 36, 5–8. [Google Scholar]
- Chen, Y.; Zhou, G.Y.; Chen, G.D.; Rao, D.D.; Dong, X.N.; Han, Y. Comparative analysis of metabolites in different resistant plants of Aquilaria sinensis. J. Environ. Entomol. 2024, 46, 988–997. [Google Scholar]
- Chen, Y.Y.; Liang, S.H.; Wang, S.Y.; Li, B.C.; Wang, K.; Zhu, Y.J.; Yang, R.S.; Hao, X.; Yang, Z.Y.; Shen, Y.B.; et al. Repeated mechanical damage enhanced Aquilaria sinensis resistance to Heortia vitessoides through jasmonic acid. Front. Plant Sci. 2023, 14, 1183002. [Google Scholar] [CrossRef]
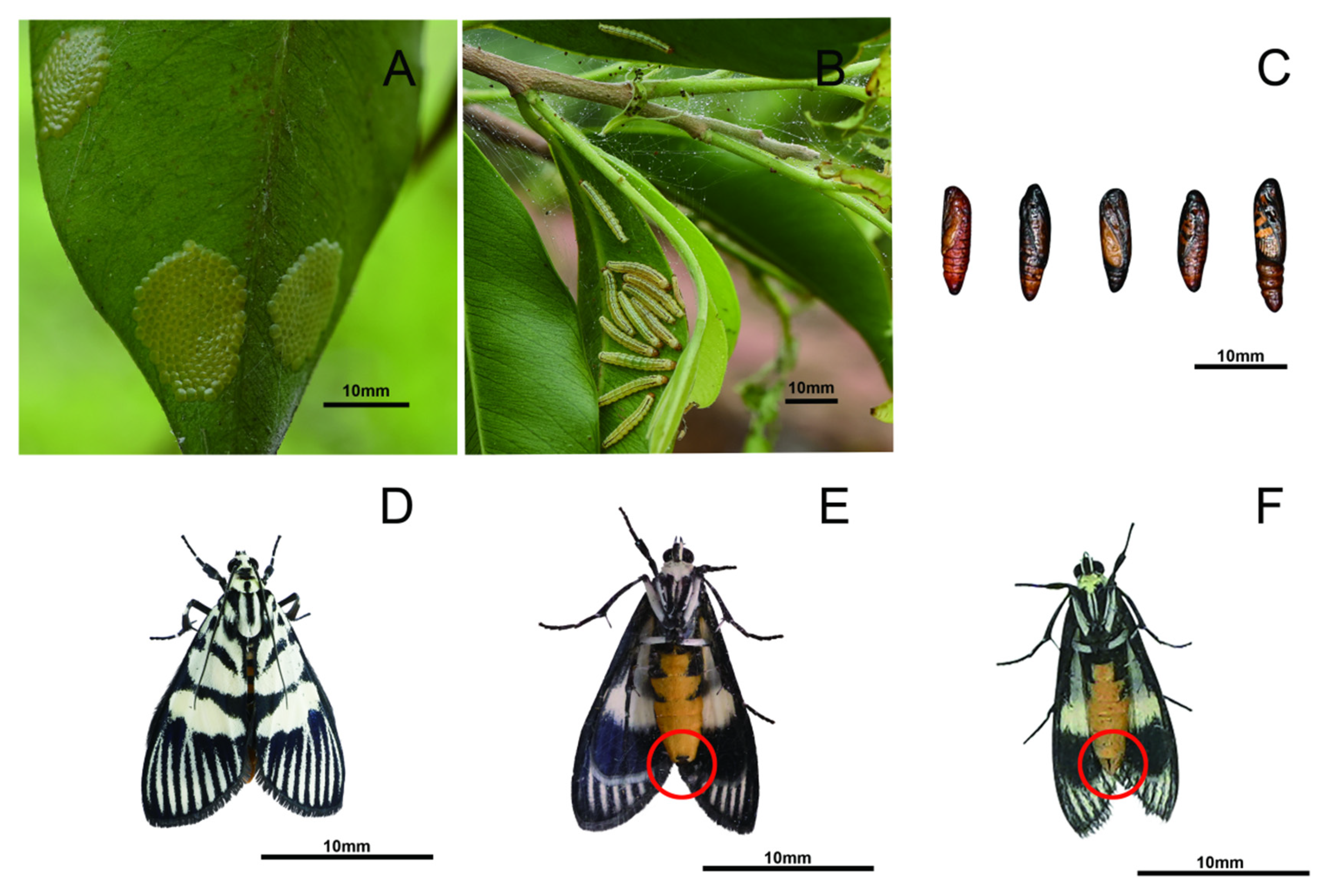
| Region | Annual Generation Algebra | Damage Period (Month) | Damage Peak Period | Monthly Average Temperature in Peak Period (°C) | Monthly Average Humidity During the Peak Period (%) | Average Monthly Rainfall During the Peak Period (mm) | Annual Average Temperature (°C) | Annual Average Humidity (%) | Mean Annual Precipitation (mm) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Hainan, China | 8~10 | 2~11 | April | 24.9~29.1 | 73~85 | 30.3~124.7 | 22.5~25.6 | 81~83 | 1500~2500 | [4,21] |
| Guangdong, China | 6~8 | 4~12 | - | - | - | - | 22~22.6 | 74~82 | 2040~2888.1 | [5] |
| Guangxi, China | 5~6 | 3~12 | Late April to early May; October | 17.7~24.6; 18.1~22.3 | 76~83; 66~83 | 80.8~351.9; 53.6~102.7 | 20.7~22.27 | 75~83 | 1086.8~1569.3 | [22] |
| Yunnan, China | 6 | - | April to May | 5.5~22.5 | 56~80 | 18.5~194.4 | 22.6 | 74~77 | 1136.6 | [23] |
| India | 4~5 | 2~9 | - | - | - | - | 24~27 | 72.9 | 1200 | [24] |
| Indonesia | - | All year around | July to September | - | - | - | 23~32 | 77~85 | 3950 | [12] |
| Drug Type | Drug Name | Insecticidal Efficiency (%) | Concentration | Insecticidal Principle | References |
|---|---|---|---|---|---|
| Antibiotics | Emamectin benzoate | 100.00 | 5.0 × 106 dilution of 0.5% | Contact killing and stomach toxicity | [36] |
| Avermectins | 100.00 | 5.0 × 106 dilution of 1.8% | Contact killing and stomach toxicity | [36] | |
| Mixed class | Sendebao | 98.90 | 30 times of synergistic powder | [38] | |
| Insect growth regulators | Fenoxycarb | 98.90 | 8000 dilution of 3% | Contact killing, stomach toxicity, and exhibiting strong juvenile hormone activity | [38] |
| Spinetoram·methoxyfenozide | 100.00 | Diluted 1000-fold | [11] | ||
| Plant source | Eucalyptol SL | 90.88 | 1000 times of 5% | Mainly contact killing | [39] |
| Matrine | 0.30% | Paralysis | [40] | ||
| Microbial source | Spinetoram | 100.00 | Diluted 1000-fold | Contact killing, stomach toxicity, and interfering with nerve activity | [11] |
| Beauveria bassiana | 84.00~90.00 | 2.4 × 108 spores/mL | Infection | [24] | |
| Metarhizium anisopliae | 40.00~52.00 | 2.4 × 1010 spores/mL | Infection | [24,41] | |
| 100.00 | 1 × 109 spores/mL | ||||
| Aspergillus nomius Q527 | 83.30 | Infection | [42] | ||
| Helicoverpa. armigera NPV | 2 billion PIB/mL | Infection | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Z.; Chen, Y.; Xue, H.; Li, X.; Li, B.; Liang, J.; Zhu, Y.; Long, K.; Yang, J.; Pang, J.; et al. Heortia vitessoides Infests Aquilaria sinensis: A Systematic Review of Climate Drivers, Management Strategies, and Molecular Mechanisms. Insects 2025, 16, 690. https://doi.org/10.3390/insects16070690
Yin Z, Chen Y, Xue H, Li X, Li B, Liang J, Zhu Y, Long K, Yang J, Pang J, et al. Heortia vitessoides Infests Aquilaria sinensis: A Systematic Review of Climate Drivers, Management Strategies, and Molecular Mechanisms. Insects. 2025; 16(7):690. https://doi.org/10.3390/insects16070690
Chicago/Turabian StyleYin, Zongyu, Yingying Chen, Huanrong Xue, Xiaofei Li, Baocai Li, Jiaming Liang, Yongjin Zhu, Keyu Long, Jinming Yang, Jiao Pang, and et al. 2025. "Heortia vitessoides Infests Aquilaria sinensis: A Systematic Review of Climate Drivers, Management Strategies, and Molecular Mechanisms" Insects 16, no. 7: 690. https://doi.org/10.3390/insects16070690
APA StyleYin, Z., Chen, Y., Xue, H., Li, X., Li, B., Liang, J., Zhu, Y., Long, K., Yang, J., Pang, J., Li, K., & Ye, S. (2025). Heortia vitessoides Infests Aquilaria sinensis: A Systematic Review of Climate Drivers, Management Strategies, and Molecular Mechanisms. Insects, 16(7), 690. https://doi.org/10.3390/insects16070690





