Extract of Tangerine Peel as a Botanical Insecticide Candidate for Smallholder Potato Cultivation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
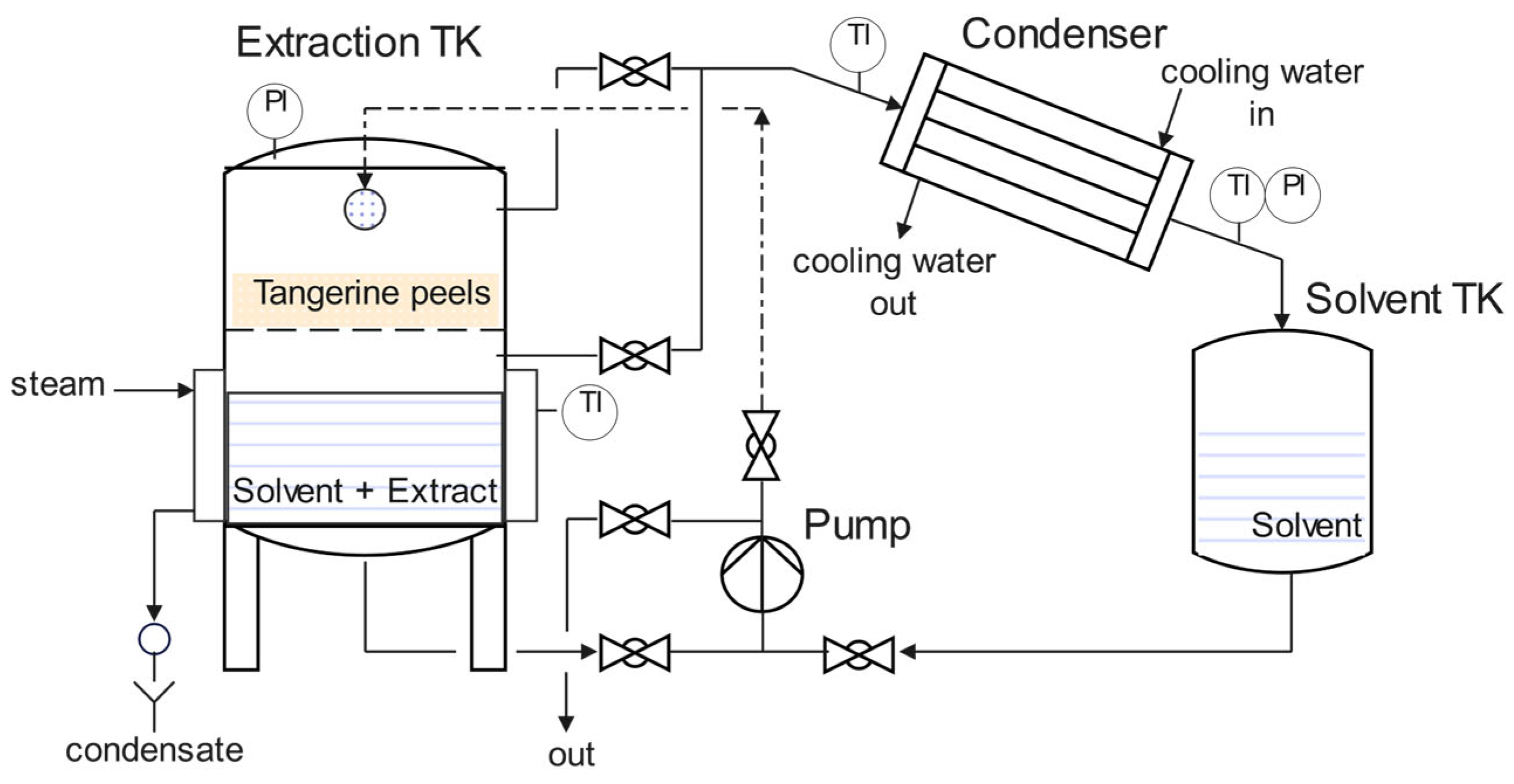
2.2. Pilot-Scale Extraction–Concentration of Ethanolic Extract of Tangerine Peels
2.3. Characterisation of the Ethanolic Extract of Tangerine Peels
2.3.1. Phytochemical Screening of Ethanolic Tangerine Peel Extract
Protein and Amino Acids Determinations (Ninhydrin Assay)
Detection for Phenols and Tannins
Test for Carbohydrates
Flavonoid Assays (Alkaline Reagent and Shinoda Tests)
Test for Steroids (Libermann Test)
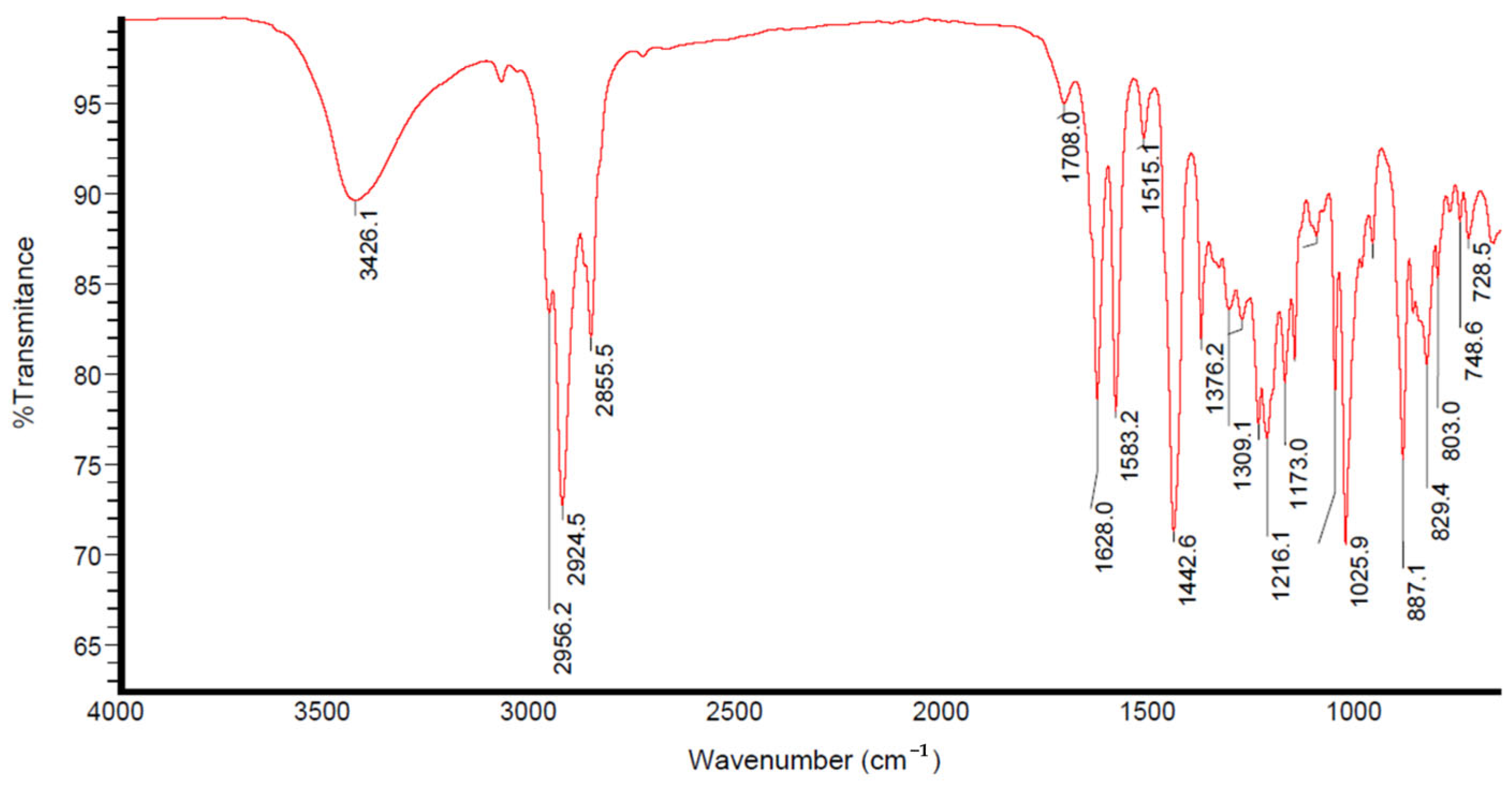
2.3.2. FTIR Study of Ethanolic Tangerine Peel Extract
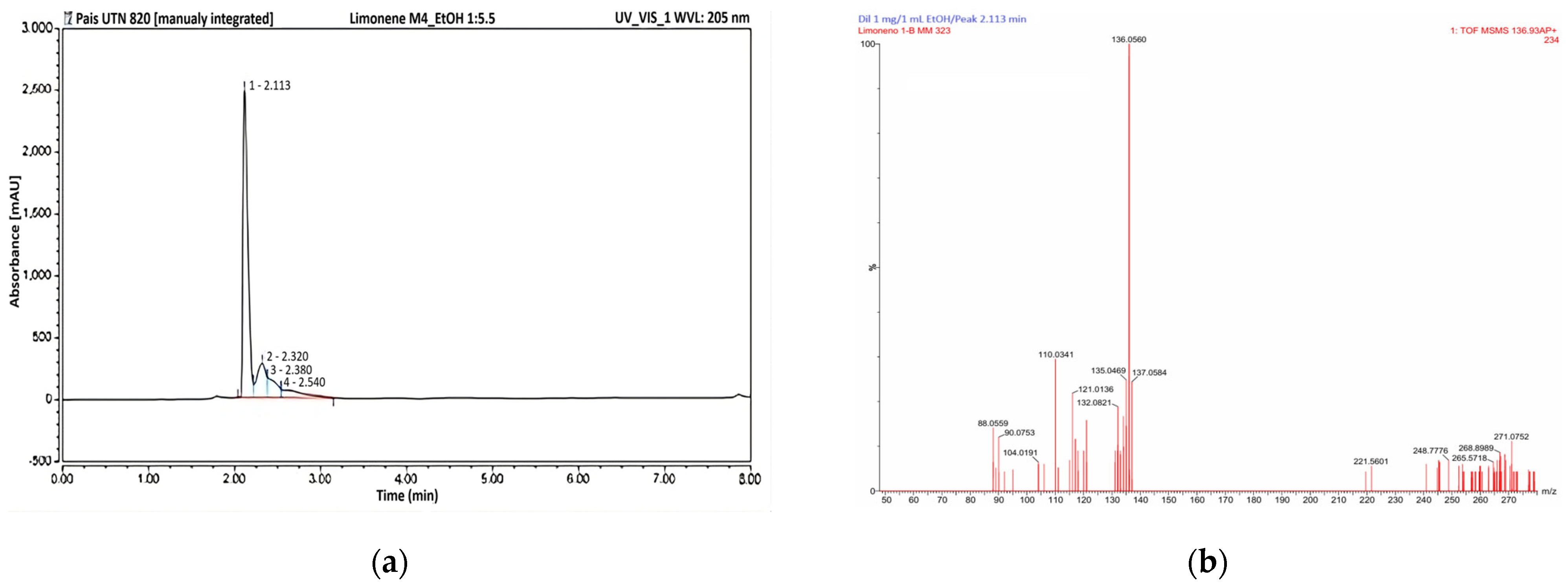
2.3.3. Reversed-Phase HPLC
2.3.4. Atmospheric Pressure Gas Chromatography–Mass Spectrometry (APGC-MS)
2.4. Toxicity Evaluation Using the Caenorhabditis Elegans Model
2.4.1. Caenorhabditis elegans Strain Culture
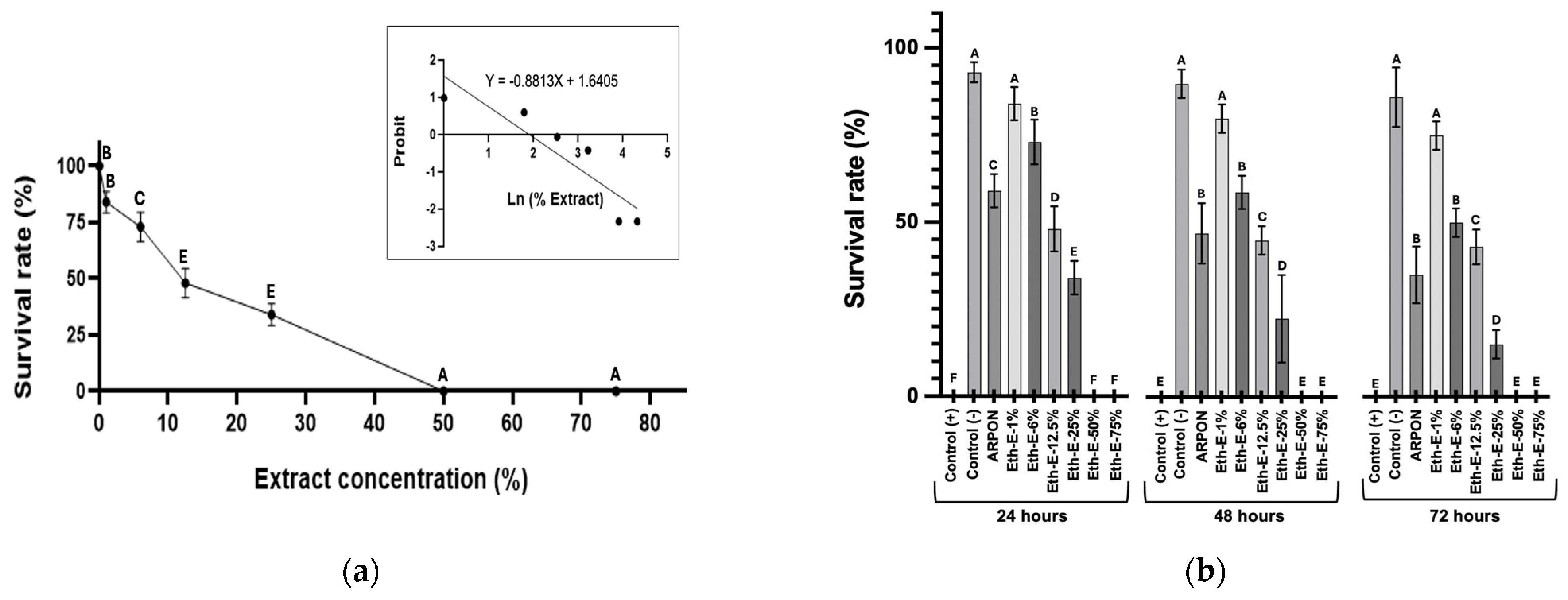
2.4.2. Toxicity Assay and LC50 Estimation
2.5. Field Experiments on Potato (Solanum tuberosum L.) Cultivation
2.6. Post-Harvest Analysis
2.7. Statistical Analysis of Experiments
3. Results
3.1. Pilot-Scale Solid–Liquid Extraction
3.2. Characterisation of Ethanolic Extract from Tangerine Peels
3.3. Toxicity Evaluation of Ethanolic Extracts of Tangerine Peels on Caenorhabditis elegans Model
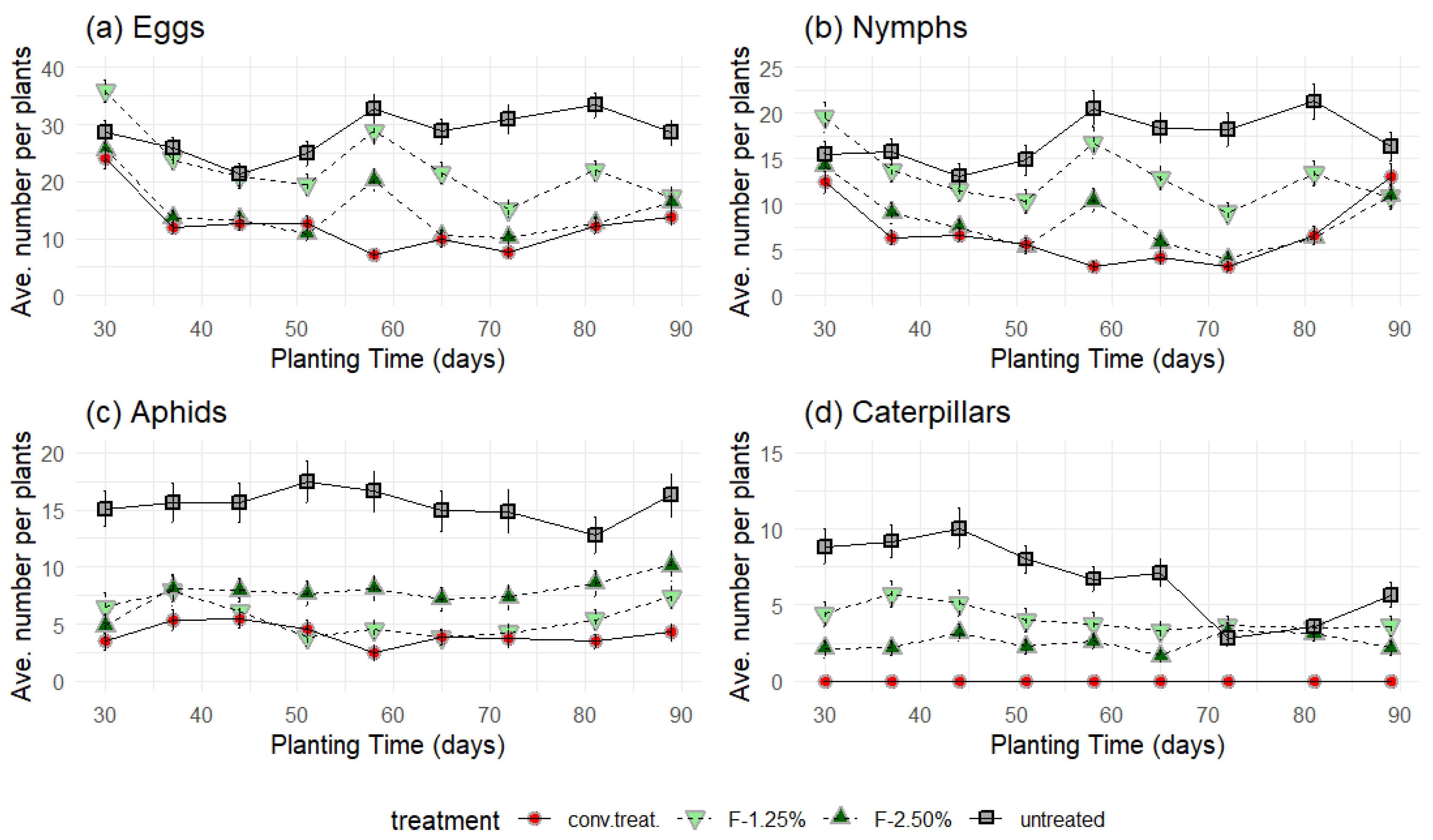
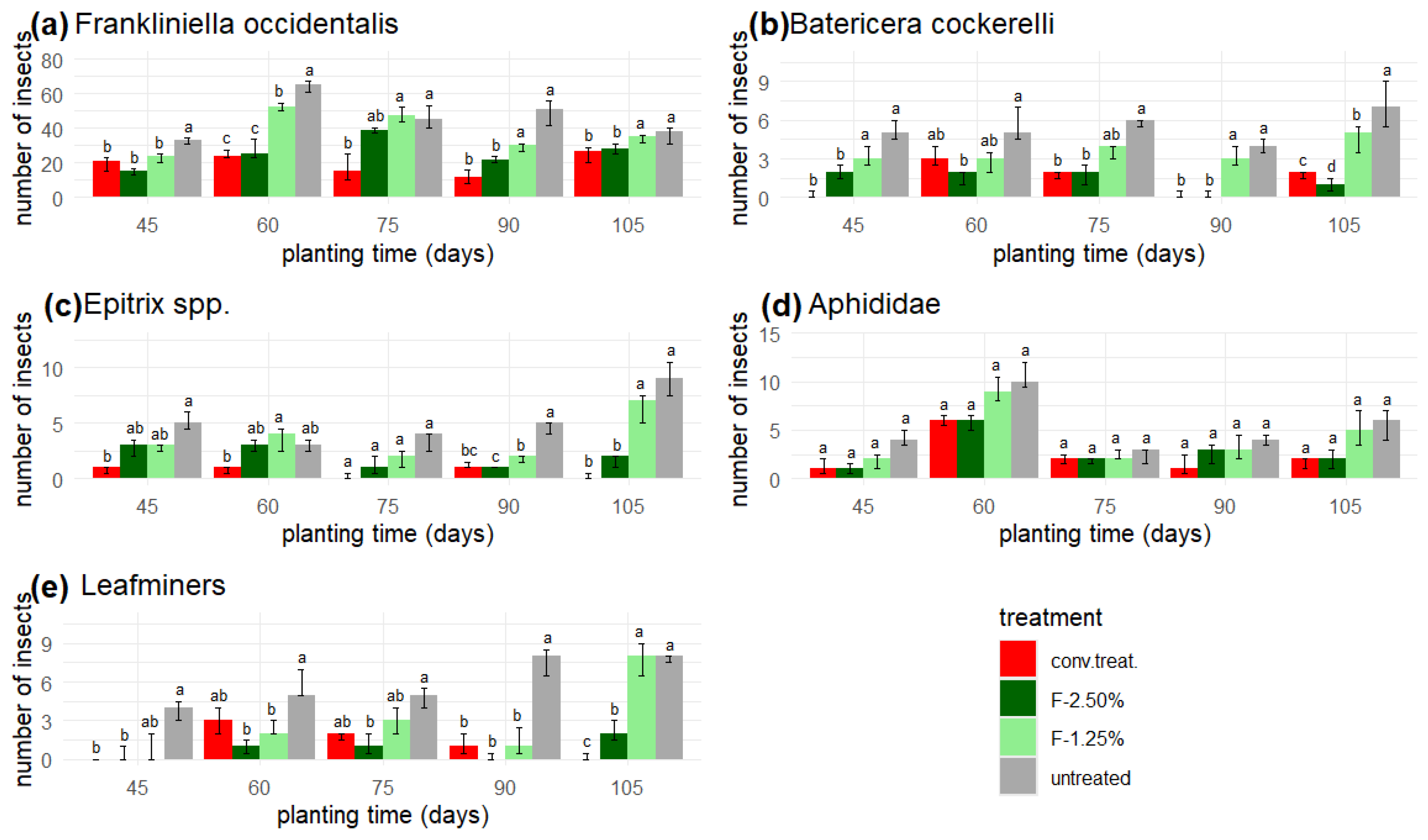
3.4. Field Experiments
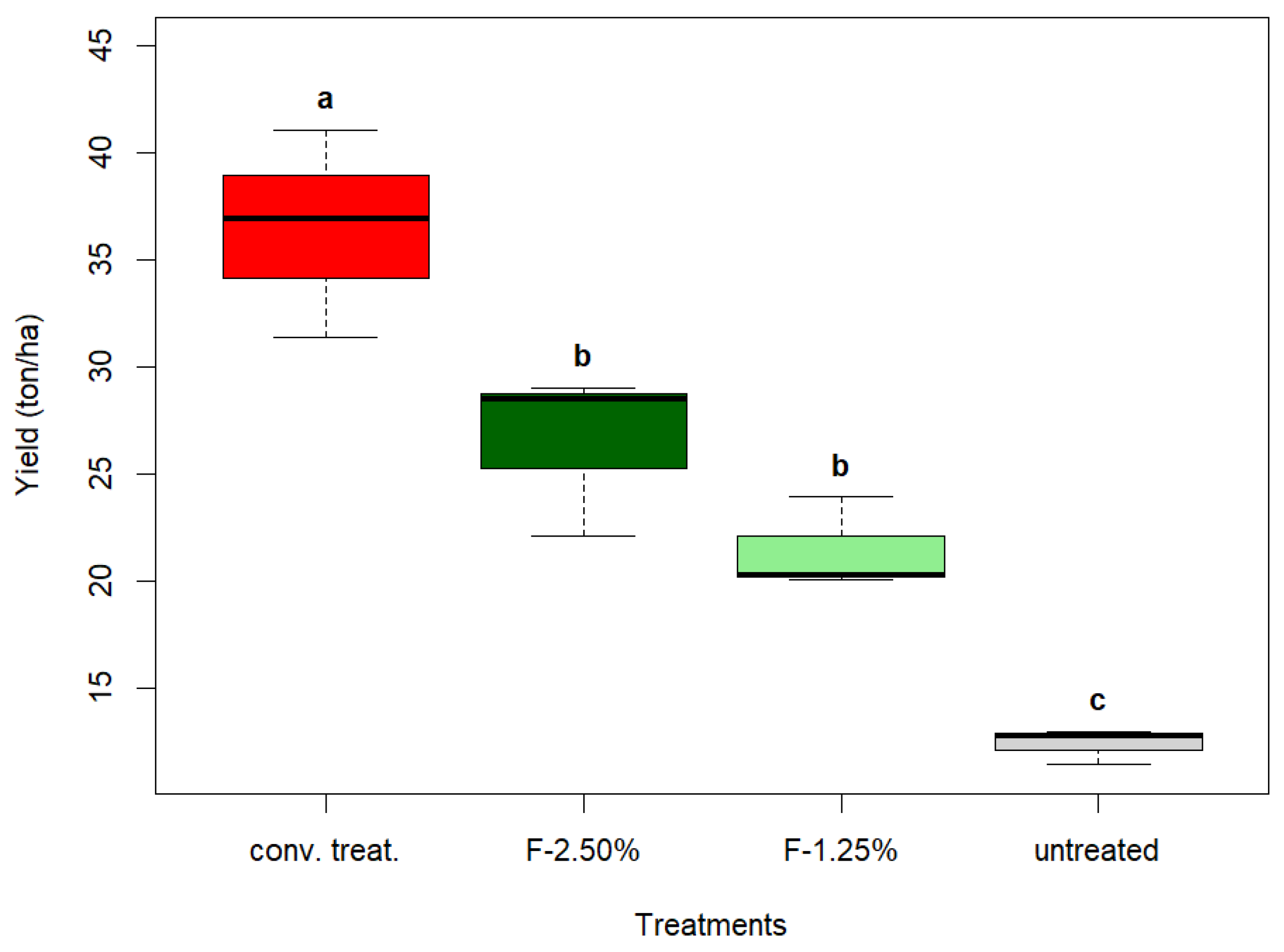
3.5. Post-Harvest Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeschke, P. Progress of Modern Agricultural Chemistry and Future Prospects. Pest Manag. Sci. 2015, 72, 433–455. [Google Scholar] [CrossRef]
- Kutluay Sahin, D.; Şahin, L. Economic Value of the Use of Chemicals in Agriculture: The Case of European Countries. Hacet. Üniversitesi İktisadi Ve İdari Bilim. Fakültesi Derg. 2023, 41, 98–110. [Google Scholar] [CrossRef]
- Kaur, R.; Choudhary, D.; Bali, S.; Bandral, S.S.; Singh, V.; Ahmad, M.A.; Rani, N.; Singh, T.G.; Chandrasekaran, B. Pesticides: An Alarming Detrimental to Health and Environment. Sci. Total Environ. 2024, 915, 170113. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.P. Pesticides, Environment, and Food Safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Hawkins, N.J.; Bass, C.; Dixon, A.; Neve, P. The Evolutionary Origins of Pesticide Resistance. Biol. Rev. 2019, 94, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wu, S.; Wu, C.; Wei, Z.; Ning, J.; She, D. Risk assessment of airborne agricultural pesticide exposure in humans in rural China. Environ. Geochem. Health 2024, 46, 117. [Google Scholar] [CrossRef] [PubMed]
- Suchail, S.; Guez, D.; Belzunces, L.P. Toxicity of Imidacloprid and Its Metabolites in Apis Mellifera. In Hazards of Pesticides to Bees; INRA Editions: Paris, France, 2001; ISBN 2-7380-0966-2. [Google Scholar]
- Rondeau, G.; Sánchez-Bayo, F.; Tennekes, H.A.; Decourtye, A.; Ramírez-Romero, R.; Desneux, N. Delayed and Time-Cumulative Toxicity of Imidacloprid in Bees, Ants and Termites. Sci. Rep. 2014, 4, srep05566. [Google Scholar] [CrossRef]
- Laxmishree, C.; Nandita, S. Botanical Pesticides—A Major Alternative to Chemical Pesticides: A Review. Int. J. Life Sci. 2017, 5, 722–729. [Google Scholar]
- Nagarjuna Reddy, D.; Al-Rajab, A.J. Botanical Pesticides for Organic Farming and Sustainable Agriculture. In Biopesticides in Organic Farming; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Damalas, C.A.; Koutroubas, S.D. Botanical Pesticides for Eco-friendly Pest Management: Drawbacks and Limitations. In Pesticides in Crop Production: Physiological and Biochemical Action; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Riyaz, M.; Mathew, P.; Zuber, S.M.; Rather, G.A. Botanical Pesticides for an Eco-Friendly and Sustainable Agriculture: New Challenges and Prospects. In Sustainable Agriculture; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Ahmed, N.; Alam, M.; Saeed, M.; Ullah, H.; Iqbal, T.; Awadh Al-Mutairi, K.; Shahjeer, K.; Ullah, R.; Ahmed, S.; Abd Aleem Hassan Ahmed, N.; et al. Botanical Insecticides Are a Non-Toxic Alternative to Conventional Pesticides in the Control of Insects and Pests. In Global Decline of Insects; IntechOpen: London, UK, 2022. [Google Scholar]
- Kato-Noguchi, H.; Kato, M. Pesticidal Activity of Citrus Fruits for the Development of Sustainable Fruit-Processing Waste Management and Agricultural Production. Plants 2025, 14, 754. [Google Scholar] [CrossRef]
- Papanastasiou, S.A.; Ioannou, C.S.; Papadopoulos, N.T. Oviposition-Deterrent Effect of Linalool—A Compound of Citrus Essential Oils—On Female Mediterranean Fruit Flies, Ceratitis Capitata (Diptera: Tephritidae). Pest Manag. Sci. 2020, 76, 3066–3077. [Google Scholar] [CrossRef]
- Flores, N.; Prado, J.; Espin, R.; Rodríguez, H.; Pais-Chanfrau, J.-M. Laboratory Evaluation of a Bio-Insecticide Candidate from Tangerine Peel Extracts against Trialeurodes Vaporariorum (Homoptera: Aleyrodidae). PeerJ 2024, 12, e16885. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mediavilla, N.A.; Prado-Beltrán, J.K.; Espín-Valladares, R.C.; Rodríguez-Cabrera, H.M.; Pais-Chanfrau, J.M. Bio-Insecticidal Potential of Tangerine Peel Oil Against Greenhouse Whitefly: A Green Biopesticide Candidate. Preprints 2023, 10, 11. [Google Scholar]
- Capello, C.; Fischer, U.; Hungerbühler, K. What Is a Green Solvent? A Comprehensive Framework for the Environmental Assessment of Solvents. Green Chem. 2007, 9, 927–934. [Google Scholar] [CrossRef]
- Boudries, H.; Loupassaki, S.; Ladjal Ettoumi, Y.; Souagui, S.; Bachir Bey, M.; Nabet, N.; Chikhoune, A.; Madani, K.; Chibane, M. Chemical Profile, Antimicrobial and Antioxidant Activities of Citrus Reticulata and Citrus Clementina (L.) Essential Oils. Int. Food Res. J. 2017, 24, 1782. [Google Scholar]
- Jayaprakasha, G.K.; Negi, P.S.; Sikder, S.; Mohanrao, L.J.; Sakariah, K.K. Antibacterial Activity of Citrus Reticulata Peel Extracts. Z. Fur Naturforschung—Sect. C J. Biosci. 2000, 55, 1030–1034. [Google Scholar] [CrossRef]
- Shahzad, K.; Nawaz, S.; Ahmad, R.; Akram, N.; Iqbal, Z. Evaluation of Antbacterial, Antifungal and Antioxidant Activity of Essentail Oil of Citrus Reticulata Fruit (Tangerine Fruit Peel). Pharmacologyonline 2009, 3, 614–622. [Google Scholar]
- Tao, N.G.; Liu, Y.J.; Tang, Y.F.; Zhang, J.H.; Zhang, M.L.; Zeng, H.Y. Essential Oil Composition and Antimicrobial Activity of Citrus Reticulata. Chem. Nat. Compd. 2009, 45, 437–438. [Google Scholar] [CrossRef]
- Khaing, T.; Win, K.H.; Khaing, Y.K. Phytochemical Screening, Antimicrobial Activities and Extraction of Essential Oil from the Peel of Citrus Reticulata Blanco. Int. J. Sci. Res. Publ. 2019, 9, 750–754. [Google Scholar] [CrossRef]
- Wang, T.; Li, Q.; Zhang, H.; Chen, J. Flavonoids from Citrus Reticulata: Inhibitory Activity against Pathogenic Fungi and Biocontrol Potential. Physiol. Mol. Plant Pathol. 2024, 131, 102250. [Google Scholar] [CrossRef]
- Chutia, M.; Deka Bhuyan, P.; Pathak, M.G.; Sarma, T.C.; Boruah, P. Antifungal Activity and Chemical Composition of Citrus Reticulata Blanco Essential Oil against Phytopathogens from North East India. LWT—Food Sci. Technol. 2009, 42, 777–780. [Google Scholar] [CrossRef]
- Hadia Mohammed, A. Insecticidal and Antibacterial Activity of Citrus Fruits’ Peels and Juices; UOFK: Lexington, KY, USA, 2015. [Google Scholar]
- Shin, S.D.; Kim, C.S.; Lee, J.H. Compositional Characteristics and Antibacterial Activity of Essential Oils in Citrus Hybrid Peels. Food Sci. Technol. 2022, 42, e95921. [Google Scholar] [CrossRef]
- Mahmoud, E. Essential Oils of Citrus Fruit Peels Antioxidant, Antibacterial and Additive Value as Food Preservative. J. Food Dairy Sci. 2017, 8, 111–116. [Google Scholar] [CrossRef]
- Zhang, T.; Li, J.; Shen, A.; Li, S. Effect of Polymethoxy Flavones from Citrus Peels on Antibacterial and Fresh-Keeping of Red Grape. Sci. Technol. Food Ind. 2023, 44, 143–150. [Google Scholar] [CrossRef]
- Manuranjan, G.; Lalduhsanga, P.; Lalhlenmawia, H.; Bibhuti, K.; Thanzami, K. Physicochemical, Antibacterial and Antioxidant Properties of Fixed and Essential Oils Extracted from the Peels of Citrus Macroptera Fruit. Indian J. Pharm. Sci. 2019, 81, 82–88. [Google Scholar] [CrossRef]
- Azghar, A.; Dalli, M.; Azizi, S.; Benaissa, E.M.; Ben Lahlou, Y.; Elouennass, M.; Maleb, A. Chemical Composition and Antibacterial Activity of Citrus Peels Essential Oils Against Multidrug-Resistant Bacteria: A Comparative Study. J. Herb. Med. 2023, 42, 100799. [Google Scholar] [CrossRef]
- Jing, L.; Lei, Z.; Li, L.; Xie, R.; Xi, W.; Guan, Y.; Sumner, L.W.; Zhou, Z. Antifungal Activity of Citrus Essential Oils. J. Agric. Food Chem. 2014, 62, 3011–3033. [Google Scholar] [CrossRef] [PubMed]
- Hajji-Hedfi, L.; Rhouma, A.; Tawfeeq Al-Ani, L.K.; Bargougui, O.; Tlahig, S.; Jaouadi, R.; Zaouali, Y.; Degola, F.; Abdel-Azeem, A.M. The Second Life of Citrus: Phytochemical Characterization and Antifungal Activity Bioprospection of C. Limon and C. Sinensis Peel Extracts Against Potato Rot Disease. Waste Biomass Valorization 2025, 1–13. [Google Scholar] [CrossRef]
- Trabelsi, D.; Hamdane, A.M.; Said, M.B.; Abdrrabba, M. Chemical Composition and Antifungal Activity of Essential Oils from Flowers, Leaves and Peels of Tunisian Citrus Aurantium Against Penicillium Digitatum and Penicillium Italicum. J. Essent. Oil-Bear. Plants 2016, 19, 1660–1674. [Google Scholar] [CrossRef]
- Uwidia, I.E.; Owolabi, B.J.; Okafor, R.C. Extraction, Derivatization, Characterization and Antifungal Investigation of Limonene from Citrus Sinensis Peels. Tanzan. J. Sci. 2020, 46, 419–429. [Google Scholar]
- Bagheri, G.; Martorell, M.; Ramírez-Alarcón, K.; Salehi, B.; Sharifi-Rad, J. Phytochemical Screening of Moringa Oleifera Leaf Extracts and Their Antimicrobial Activities. Cell. Mol. Biol. 2020, 66, 20–26. [Google Scholar] [CrossRef]
- Yadav, R.; Agarwala, M. Phytochemical Analysis of Some Medicinal Plants | Journal of Phytology. J. Phytol. 2011, 3, 10–14. [Google Scholar]
- Usman, A.; Abdulrahman, F.I.; Usman, A. Qualitative Phytochemical Screening and in Vitro Antimicrobial Effects of Methanol Stem Bark Extract of Ficus Thonningii (Moraceae). Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Patil, D.K.; Jain, A.P. Extraction, Qualitative and Quantitative Determination of Secondary Metabolites of Corchorus Olitorius. J. Drug Deliv. Ther. 2019, 9, 252–255. [Google Scholar] [CrossRef]
- Stiernagle, T. Maintenance of Caenorhabditis Elegans; WormBook: New York, NY, USA, 2015; Volume 16. [Google Scholar]
- Mishra, S.; Gaur, A.V.; Agarwal, R. Standardization of Synchronization Procedure to Collect the Similar Aged C. Elegans. Peer Rev. J. Forens. Gen. Sci. 2020, 4, 268–271. [Google Scholar] [CrossRef]
- Porta-de-la-Riva, M.; Fontrodona, L.; Villanueva, A.; Cerón, J. Basic Caenorhabditis Elegans Methods: Synchronization and Observation. J. Vis. Exp. 2012, 10, 4019. [Google Scholar] [CrossRef]
- Hart, A. Behavior. WormBook 2006. [Google Scholar] [CrossRef]
- D’Almeida, R.E.; Sued, N.; Arena, M.E. Citrus Paradisi and Citrus Reticulata Essential Oils Interfere with Pseudomonas Aeruginosa Quorum Sensing in Vivo on Caenorhabditis Elegans. Phytomedicine Plus 2022, 2, 100160. [Google Scholar] [CrossRef]
- Fuentes, C.; Verdú, S.; Fuentes, A.; Ruiz, M.J.; Barat, J.M. Effects of Essential Oil Components Exposure on Biological Parameters of Caenorhabditis Elegans. Food Chem. Toxicol. 2022, 159, 112763. [Google Scholar] [CrossRef]
- Perez-Alvarez, R.; Arguelles-Cardenas, J.; Aguilera Garramuno, E. Spatial Distribution of Premnotrypes Vorax (Hustache) (Coleoptera: Curculionidae) in Potato Crops. Rev. Corpoica—Cienc. Y Tecnol. Agropecu. 2010, 11, 11–20. [Google Scholar] [CrossRef]
- Budiarto, K.; Andrini, A.; Budiyati, E.; Mariana, B.D.; Chaireni; Martasari; Mas’udah, S.; Yulia, N.D.; Ikarini, I.; Yulianti, F. Bioactive Phytochemical Contents on Fruit Peel of Several Citrus Species. In Proceedings of the BIO Web of Conferences; EDP Sciences: London, UK, 2024; Volume 91. [Google Scholar]
- Isman, M.B. Botanical Insecticides in the Twenty-First Century-Fulfilling Their Promise? Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar] [CrossRef]
- Campos, E.V.R.; Proença, P.L.F.; Oliveira, J.L.; Bakshi, M.; Abhilash, P.C.; Fraceto, L.F. Use of Botanical Insecticides for Sustainable Agriculture: Future Perspectives. Ecol. Indic. 2019, 105, 483–495. [Google Scholar] [CrossRef]
- Gomes da Câmara, C.; Ramos de Melo, J.; Correia da Silva, M. Insecticidal Activity of Melaleuca Leucadendron and Citrus Reticulata Essential Oils against Larvae of Plutella Xylostella. Rev. Prot. Veg. 2015, 30, 39. [Google Scholar]
- Author, C.; Kosar Abbas, S.; Ahmad, F.; Sagheer, M.; Yasir, M.; Ahmad, S.; Muhammad, W. Insecticidal and Growth Inhibition Activities of Citrus Paradisi and Citrus Reticulata Essential Oils Against Lesser Grain Borer, Rhyzopertha Dominica (F.) (Coleoptera: Bostrichidae). World J. Zool. 2012, 7, 289–294. [Google Scholar] [CrossRef]
- Safavi, S.A.; Mobki, M. Fumigant Toxicity of Essential Oils from Citrus Reticulata Blanco Fruit Peels against Tribolium Castaneum Herbst (Coleoptera: Tenebrionidae). J. Crop. Prot. 2012, 1, 115–120. [Google Scholar]
- Fouad, H.A.; da Camara, C.A.G. Chemical Composition and Bioactivity of Peel Oils from Citrus Aurantiifolia and Citrus Reticulata and Enantiomers of Their Major Constituent against Sitophilus Zeamais (Coleoptera: Curculionidae). J. Stored Prod. Res. 2017, 73, 30–36. [Google Scholar] [CrossRef]
- Safavi, S.A.; Mobki, M. Susceptibility of Tribolium Castaneum (Herbst, 1797) Larvae to Essential Oils of Citrus Reticulata Blanco Fruit Peels and the Synergist, Diethyl Maleate. Biharean Biol. 2016, 10, 82–85. [Google Scholar]
- Isman, M.B. Bridging the Gap: Moving Botanical Insecticides from the Laboratory to the Farm. Ind. Crops. Prod. 2017, 110, 10–14. [Google Scholar] [CrossRef]
- Villamizar-Roa, É.J.; Espitia-Cruz, L.V.; Arenas-Díaz, G. Intrinsic Growth Rate and Evolution of the Premnotrypes Vorax Population Using Fuzzy Information. BioSystems 2024, 237, 105161. [Google Scholar] [CrossRef]
- Valencia, L. El Gusano Blanco de La Papa. Premnotrypes Vorax (Hustache) En Colombia. I.- Comportamiento de Adultos En El Campo. Rev. Latinoam. De La Papa 2016, 2, 57–70. [Google Scholar] [CrossRef]
- Rane Zab Anish Kumar, P.; Bhaskar, A. Determination of Bioactive Components from the Ethanolic Peel Extract of Citrus Reticulata by Gas Chromatography—Mass Spectrometry. Int. J. Drug Dev. Res. 2012, 4, 166–174. [Google Scholar]
- Biondi, A.; Zappalà, L.; Stark, J.D.; Desneux, N. Do Biopesticides Affect the Demographic Traits of a Parasitoid Wasp and Its Biocontrol Services through Sublethal Effects? PLoS ONE 2013, 8, e76548. [Google Scholar] [CrossRef]
- Xavier, V.M.; Message, D.; Picanço, M.C.; Chediak, M.; Santana Júnior, P.A.; Ramos, R.S.; Martins, J.C. Acute Toxicity and Sublethal Effects of Botanical Insecticides to Honey Bees. J. Insect Sci. 2015, 15, 137. [Google Scholar] [CrossRef]
- El Aalaoui, M.; Bouharroud, R.; Sbaghi, M.; El Bouhssini, M.; Hilali, L.; Dari, K. Comparative Toxicity of Different Chemical and Biological Insecticides against the Scale Insect Dactylopius Opuntiae and Their Side Effects on the Predator Cryptolaemus Montrouzieri. Arch. Phytopathol. Plant Prot. 2019, 52, 155–169. [Google Scholar] [CrossRef]
- Wiyono; Budiyono, A.; Supriyadi, T. Siti mardhika sari Bokashi And Botanical Pesticides Production Training in Support Organic Farming for Sustainable Agriculture. J. Community Capacit. Empower. 2024, 2, 1–6. [Google Scholar] [CrossRef]
- Ndakidemi, B.; Mtei, K.; Ndakidemi, P.A. Impacts of Synthetic and Botanical Pesticides on Beneficial Insects. Agric. Sci. 2016, 07, 364–372. [Google Scholar] [CrossRef]
- Ngegba, P.M.; Cui, G.; Khalid, M.Z.; Zhong, G. Use of Botanical Pesticides in Agriculture as an Alternative to Synthetic Pesticides. Agriculture 2022, 12, 600. [Google Scholar] [CrossRef]
- Wagh, M.S.; Sowjanya, S.; Nath, P.C.; Chakraborty, A.; Amrit, R.; Mishra, B.; Mishra, A.K.; Mohanta, Y.K. Valorisation of Agro-Industrial Wastes: Circular Bioeconomy and Biorefinery Process—A Sustainable Symphony. Process Saf. Environ. Prot. 2024, 183, 708–725. [Google Scholar] [CrossRef]
- Sharma, P.; Gaur, V.K.; Sirohi, R.; Varjani, S.; Hyoun Kim, S.; Wong, J.W.C. Sustainable Processing of Food Waste for Production of Bio-Based Products for Circular Bioeconomy. Bioresour. Technol. 2021, 325, 124684. [Google Scholar] [CrossRef]
- Pimentel, D.; Burgess, M. Environmental and Economic Costs of the Application of Pesticides Primarily in the United States. In Integrated Pest Management; Springer: Berlin/Heidelberg, Germany, 2014; Volume 3. [Google Scholar]
- FAO. The State of Food and Agriculture 2019. In Moving Forward on Food Loss and Waste Reduction; FAO: Rome, Italy, 2019. [Google Scholar]
- Cherubini, F.; Ulgiati, S. Crop Residues as Raw Materials for Biorefinery Systems—A LCA Case Study. Appl. Energy 2010, 87, 47–57. [Google Scholar] [CrossRef]
- Cherubini, F. The Biorefinery Concept: Using Biomass Instead of Oil for Producing Energy and Chemicals. Energy Convers Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Reganold, J.P.; Wachter, J.M. Organic Agriculture in the Twenty-First Century. Nat. Plants 2016, 2, 15221. [Google Scholar] [CrossRef] [PubMed]



| Name | Function | ‘conv. treat.’ | F-2.50% | F-1.25% | ‘untreated’ | Dose | Frequency | Mode of Action ‡ |
|---|---|---|---|---|---|---|---|---|
| ‘Biol’ | organic fert. | + | + | + | + | 5 L † | Every month | - |
| Compost | organic fert. | + | + | + | + | 300 kg † | Start of cultivation | - |
| Calcium carbonate | Soil pH adjust. | + | + | + | + | 50 kg † | Start of cultivation | - |
| NPK (13-40-13) | inorganic fert. | + | + | + | + | 7.46 kg † | Every two months | - |
| Imidacloprid | Insecticide | + | − | − | − | 0.5 mL·L−1 | Every two weeks | (i) |
| Thiamethoxam + lambda cyhalothrin | Insecticide | + | − | − | − | 125 mL·L−1 | (ii) | |
| Acephate | Insecticide | + | − | − | − | 100 g·L−1 | (iii) | |
| Fipronil | Insecticide | + | − | − | − | 2 mL·L−1 | (iv) | |
| Methomyl | Insecticide | + | − | − | − | 3 mL·L−1 | (iii) | |
| Malathion | Insecticide | + | − | − | − | 2 mL·L−1 | (iii) | |
| Fluopicolide + Propamocarb chlorhydrate | Fungicide | + | − | − | − | 1.6 mL·L−1 | Every two weeks | (v) |
| Propineb + Fluopicolide | Fungicide | + | − | − | − | 2 mL·L−1 | (vi) | |
| Carboxin + Captan | Fungicide | + | − | − | − | 3 g·L−1 | (vii) |
| Test | Tangerine Peel Ethanolic Extract † |
|---|---|
| Amino Acids and Proteins | − |
| Phenols and Tannins | + |
| Carbohydrates | − |
| Flavonoids (Alkaline test) | + |
| Flavonoids (Shinoda test) | + |
| Steroids | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pais-Chanfrau, J.-M.; Quiñonez-Montaño, L.J.; Núñez-Pérez, J.; Prado-Beltrán, J.K.; Cañarejo-Antamba, M.; Burbano-García, J.L.; Chiliquinga-Quispe, A.J.; Rodríguez Cabrera, H.M. Extract of Tangerine Peel as a Botanical Insecticide Candidate for Smallholder Potato Cultivation. Insects 2025, 16, 680. https://doi.org/10.3390/insects16070680
Pais-Chanfrau J-M, Quiñonez-Montaño LJ, Núñez-Pérez J, Prado-Beltrán JK, Cañarejo-Antamba M, Burbano-García JL, Chiliquinga-Quispe AJ, Rodríguez Cabrera HM. Extract of Tangerine Peel as a Botanical Insecticide Candidate for Smallholder Potato Cultivation. Insects. 2025; 16(7):680. https://doi.org/10.3390/insects16070680
Chicago/Turabian StylePais-Chanfrau, José-Manuel, Lisbeth J. Quiñonez-Montaño, Jimmy Núñez-Pérez, Julia K. Prado-Beltrán, Magali Cañarejo-Antamba, Jhomaira L. Burbano-García, Andrea J. Chiliquinga-Quispe, and Hortensia M. Rodríguez Cabrera. 2025. "Extract of Tangerine Peel as a Botanical Insecticide Candidate for Smallholder Potato Cultivation" Insects 16, no. 7: 680. https://doi.org/10.3390/insects16070680
APA StylePais-Chanfrau, J.-M., Quiñonez-Montaño, L. J., Núñez-Pérez, J., Prado-Beltrán, J. K., Cañarejo-Antamba, M., Burbano-García, J. L., Chiliquinga-Quispe, A. J., & Rodríguez Cabrera, H. M. (2025). Extract of Tangerine Peel as a Botanical Insecticide Candidate for Smallholder Potato Cultivation. Insects, 16(7), 680. https://doi.org/10.3390/insects16070680











