First Record of Dioryctria simplicella (Lepidoptera: Pyralidae) in China: Morphology, Molecular Identification, and Phylogenetic Position
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimens Collection
2.2. Morphological Study
2.3. Molecular and Phylogenetic Study
3. Results
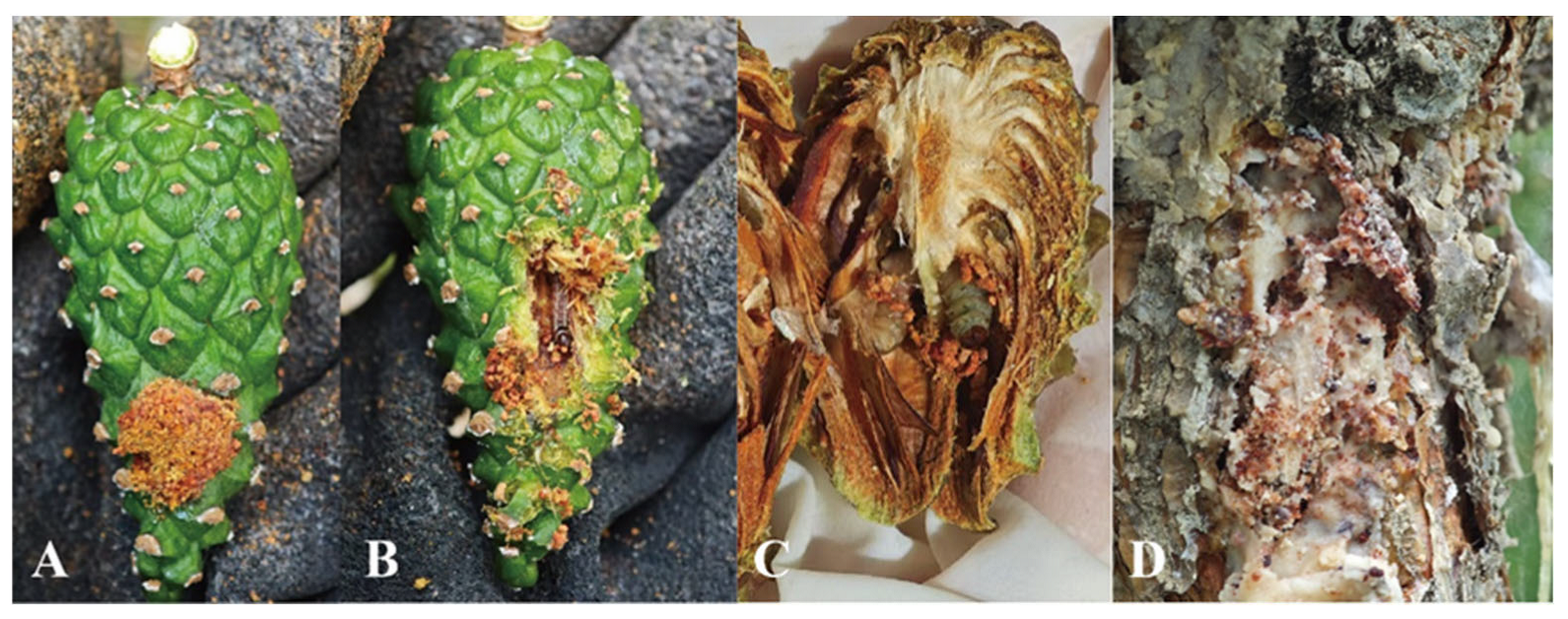
3.1. Subsection Species Descriptions
- Dioryctria simplicella Heinemann
- Material examined.
- Diagnosis. The genital morphology provides reliable diagnostic characters for distinguishing D. simplicella. In male genitalia, valva relatively narrow, costa of valva extending distally into a beak-shaped apex bearing a minute spine.
- Description.
3.2. Molecular and Phylogenetic Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neunzig, H.H.; Dow, L.C. The Phycitinae of Belize (Lepidoptera: Pyralidae); North Carolina Agricultural Research Service: Raleigh, NC, USA, 1993. [Google Scholar]
- Neunzig, H.H. New species of Phycitinae (Lepidoptera: Pyralidae) from the Dominican Republic. Proc. Entomol. Soc. Wash. 1996, 98, 774–801. [Google Scholar]
- Turgeon, J.J.; Roques, A.; Groot, P. Insect fauna of coniferous seed cones: Diversity, host plant interactions, and management. Annu. Rev. Entomol. 1994, 39, 179–212. [Google Scholar] [CrossRef]
- Neunzig, H.H. Pyraloidea, Pyralidae (part), Phycitinae (part). In The Moths of America North of Mexico fasc. 15.5; Wedge Entomological Research: Bakersfield, CA, USA, 2003. [Google Scholar]
- Lyons, L.A. Insects affecting seed production in red pines. II. Dioryctria disclusa Heinrich, D. abietella (D and S) and D. cambiicola (Dyar) (Lepidoptera: Phycitidae). Can. Entomol. 1957, 89, 70–79. [Google Scholar] [CrossRef]
- Hedlin, A.F.; Yates, H.O., III; Tovar, D.C.; Ebel, B.H.; Koerber, T.W.; Merkel, E.P. Cone and Seed Insects of North American Conifers; Canadian Forestry Service: Ottawa, Canada, 1980. [Google Scholar] [CrossRef]
- Hainze, J.H.; Benjamin, D.M. Impact of the red pine shoot moth, Dioryctria resinosella (Lepidoptera: Pyralidae), on height and radial growth in Wisconsin red pine plantations. J. Econ. Entomol. 1984, 77, 36–42. [Google Scholar] [CrossRef]
- Blake, E.A.; Wagner, M.R.; Koerber, T.W. Relative effect of seed and cone insects on ponderosa pine in northern Arizona. J. Econ. Entomol. 1989, 82, 1691–1694. [Google Scholar] [CrossRef]
- Mosseler, A.; Roberts, B.A.; Tricco, P. The effects of fir coneworm, Dioryctria abietivorella (Grote) (Lepidoptera: Pyralidae), on seed production in small isolated populations of red pine, Pinus resinosa Ait. For. Ecol. Manag. 1992, 53, 15–27. [Google Scholar] [CrossRef]
- Heinrich, C. American Moths of the Subfamily Phycitinae; Smithsonian Institution: Washington, DC, USA, 1956; Volume 207, pp. 1–581. [Google Scholar]
- Mutuura, A.; Munroe, E. American species of Dioryctria (Lepidoptera: Pyralidae) III. Grouping of species: Species of the auranticella group, including the Asian species, with the description of a new species. Can. Entomol. 1972, 104, 609–625. [Google Scholar] [CrossRef]
- Mutuura, A.; Munroe, E. A new genus related to Dioryctria Zeller (Lepidoptera: Pyralidae: Phycitinae), with definition of an additional species group in Dioryctria. Can. Entomol. 1974, 106, 937–940. [Google Scholar] [CrossRef]
- Wang, P.Y.; Sung, S.M. Description of a new species of Dioryctria Zeller on Pinus sylvestris var. Mongolica from north-east China, with establishment of a new species group. Acta Entomol. Sin. 1982, 25, 324–327. [Google Scholar]
- Speidel, W.; Asselbergs, J.E.F. The status of Ocrisia Ragonot, 1893, and notes on Dioryctria Zeller, 1846 (Lepidoptera: Pyralidae, Phycitinae). Entomol. Z. 2000, 110, 144–146. [Google Scholar]
- Segerer, A.H.; Prose, H. Dioryctria resiniphila sp. n., eine neue Pyralidae auf Abies cephalonica LOUD, in Griechenland. NachrBl. Bayer. Entomol. 1997, 46, 57–67. [Google Scholar]
- Knölke, S. A Revision of the European Representatives of the Microlepidopteran Genus Dioryctria Zeller, 1846 (Insecta: Lepidoptera: Pyralidae: Phycitinae). Ph.D. Thesis, Ludwig-Maximilians-Universität München, Munich, Germany, 2008. Available online: http://hdl.handle.net/10.5282/edoc.8425 (accessed on 5 May 2025).
- Du, Y.; Roe, A.D.; Sperling, F.A.H. Phylogenetic framework for Dioryctria (Lepidoptera: Pyralidae: Phycitinae) based on combined analysis of mitochondrial DNA and morphology. Can. Entomol. 2005, 137, 685–711. [Google Scholar] [CrossRef]
- Du, Y.L. A Taxonomic Study on Subfamily Phycitinae of Northern China (Lepidoptera: Pyralidae). Ph.D. Thesis, Nankai University, Tianjin, China, 2002. [Google Scholar]
- Kuang, D.H.; Li, H.H. One new species and three new record species of the genus Dioryctria Zeller in China (Lepidoptera, Pyralidae, Phycitinae). Acta Zootaxon. Sin. 2009, 34, 42–45. [Google Scholar]
- Wang, P.Y. Economic Insects of China; Wang, P.Y., Ed.; Science Press: Beijing, China, 1980; pp. 77–79. [Google Scholar]
- Wang, P.Y.; Sung, S.M. Revision of Chinese coneworms Dioryctria of the sylvestrella group (Lepidoptera: Pyralidae, Phyeitinae). Acta Entomol. Sin. 1985, 28, 302–313. [Google Scholar] [CrossRef]
- Rebel, H. Famil. Pyralidae—Micropterigidae. In Catalog der Lepidopteren des paläarctischen Faunengebietes; Staudinger, O., Rebel, H., Eds.; Friedländer & Sohn: Berlin, Germany, 1901; Volume 2, pp. 1–368. [Google Scholar]
- Roesler, R.U. Phycitinen Studien IV (Lep., Pyralidae). Entomol. Z. 1968, 78, 225–239. [Google Scholar]
- Petersen, G.; Gaedike, R. Zur Taxonomie der einheimischen Dioryctria-Arten (Lep., Phycitinae). Entomol. Ber. 1980, 40, 21–35. [Google Scholar]
- Fuchs, A. Zwei neue kleinschmetterlinge. Stettin. Entomol. Ztg. Stettin 1899, 60, 180–183. [Google Scholar]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; DeWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. B 2003, 270, 313–322. [Google Scholar] [CrossRef]
- Simon, C.; Frati, F.; Beckenbach, A.; Crespi, B.; Liu, H.; Flook, P. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 1994, 87, 651–701. [Google Scholar] [CrossRef]
- Caterino, M.S.; Cho, S.; Sperling, F.A.H. The current state of insect molecular systematics: A thriving tower of Babel. Annu. Rev. Entomol. 2000, 45, 1–54. [Google Scholar] [CrossRef]
- Piao, M.; Zheng, Y.; Feng, Z. Larval descriptions of three species of Phycitinae (Lepidoptera: Pyralidae) from China. Entomotaxonomia 2009, 31, 194–200. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Fuchs, A. Alte und neue Kleinfalter der europäischen Fauna. Stettin. Entomol. Z. 1903, 64, 227–247. [Google Scholar]
- Heinemann, H.V. Die Schmetterlinge Deutschlands und der Schweiz. Zweite Abteilung. Kleinschmetterlinge. Band I, Heft II. Die Zünsler; C. A. Schwetschke und Sohn: Braunschweig, Germany, 1865. [Google Scholar]
- Derzhinsky, Y.A.; Sinev, S.Y.; Streltzov, A.N.; Tatun, Y.V.; Murashkevich, K.D. The pyralid moths of the genus Dioryctria Z. (Lepidoptera: Pyralidae, Phycitinae) in the fauna of Belarus. Amurian Zool. J. 2023, 15, 798–812. [Google Scholar] [CrossRef]
- Hassler, M.; Speidel, W. Beitrag zur Verbreitung von Dioryctria simplicella (Heinemann) (Lepidoptera: Pyralidae: Phycitinae). Entomol. Z. 1986, 96, 321–336. [Google Scholar]
- Parsons, M.; Clancy, S. Dioryctria sylvestrella (Ratz.)—New to Britain and Ireland, and the identification of the British Dioryctria species. Atropos 2002, 15, 16–19. [Google Scholar]



| Species Group | Species | GenBank NO. |
|---|---|---|
| abietella-group | Dioryctria simplicella | PV668953.1 |
| Dioryctria simplicella | PV668954.1 | |
| Dioryctria simplicella | PV668955.1 | |
| Dioryctria simplicella | PV668956.1 | |
| Dioryctria simplicella | PV668957.1 | |
| Dioryctria simplicella | PV668958.1 | |
| Dioryctria simplicella | HM875123.1 | |
| Dioryctria simplicella | HM872148.1 | |
| Dioryctria simplicella | HM872031.1 | |
| Dioryctria simplicella | GU707355.1 | |
| Dioryctria abietella | GU706362.1 | |
| Dioryctria abietella | KX048878.1 | |
| Dioryctria abietivorella | MG468430.1 | |
| Dioryctria abietivorella | JF852743.1 | |
| Dioryctria mendacella | OR449782.1 | |
| Dioryctria mendacella | OQ563613.1 | |
| Dioryctria pineae | HQ563610.1 | |
| zimmermani-group | Dioryctria amatella | GU801522.1 |
| Dioryctria banksiella | HM869672.1 | |
| Dioryctria banksiella | KT147303.1 | |
| auranticella-group | Dioryctria auranticella | HM868369.1 |
| Dioryctria auranticella | KF492320.1 | |
| Dioryctria disclusa | KT143314.1 | |
| Dioryctria pryeri | MF052103.1 | |
| Dioryctria pryeri | MF049781.1 | |
| Dioryctria yiai | MF055629.1 | |
| Dioryctria yiai | MF055620.1 | |
| ponderosae-group | Dioryctria okanaganella | HM867929.1 |
| baumhoferi-group | Dioryctria pentictonella | HM426749.1 |
| Dioryctria subtracta | HM406370.1 | |
| schuetzeella-group | Dioryctria pseudotsugella | KM554558.1 |
| Dioryctria pseudotsugella | KM554272.1 | |
| Dioryctria reniculelloides | KM553829.1 | |
| Dioryctria reniculelloides | KM548677.1 | |
| Dioryctria schuetzeella | HM874495.1 | |
| Dioryctria schuetzeella | HM871547.1 | |
| Dioryctria sylvestrella | HM875194.1 | |
| Dioryctria sylvestrella | KC135937.1 | |
| outgroup | Pyralis lienigialis | HM874786.1 |
| outgroup | Orybina regalis | PQ298688.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, N.; Ding, X.; Xie, D.; Chen, H.; Chi, D.; Yu, J. First Record of Dioryctria simplicella (Lepidoptera: Pyralidae) in China: Morphology, Molecular Identification, and Phylogenetic Position. Insects 2025, 16, 664. https://doi.org/10.3390/insects16070664
Jia N, Ding X, Xie D, Chen H, Chi D, Yu J. First Record of Dioryctria simplicella (Lepidoptera: Pyralidae) in China: Morphology, Molecular Identification, and Phylogenetic Position. Insects. 2025; 16(7):664. https://doi.org/10.3390/insects16070664
Chicago/Turabian StyleJia, Niya, Xiyao Ding, Dan Xie, Huanwen Chen, Defu Chi, and Jia Yu. 2025. "First Record of Dioryctria simplicella (Lepidoptera: Pyralidae) in China: Morphology, Molecular Identification, and Phylogenetic Position" Insects 16, no. 7: 664. https://doi.org/10.3390/insects16070664
APA StyleJia, N., Ding, X., Xie, D., Chen, H., Chi, D., & Yu, J. (2025). First Record of Dioryctria simplicella (Lepidoptera: Pyralidae) in China: Morphology, Molecular Identification, and Phylogenetic Position. Insects, 16(7), 664. https://doi.org/10.3390/insects16070664






