Overview and Recent Advances in Bioassays to Evaluate the Potential of Entomopathogenic Fungi Against Ambrosia Beetles
Simple Summary
Abstract
1. Introduction
2. Entomopathogenic Fungi
3. Ambrosia Beetles
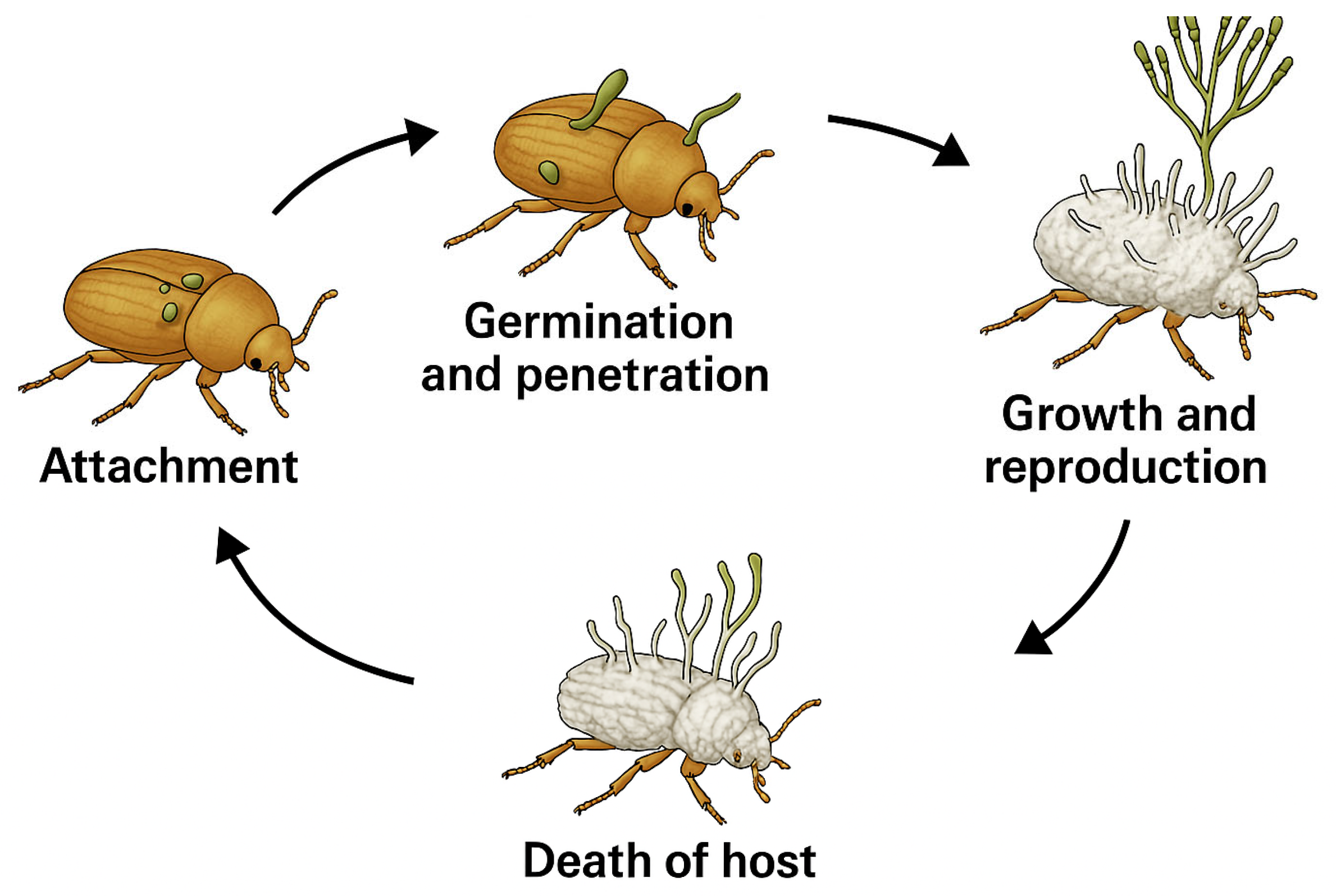
4. Entomopathogenic Fungi Against Ambrosia Beetles
5. Bioassay Systems
5.1. In Vivo Bioassays on Ambrosia Beetles
5.1.1. Origin of Beetles
5.1.2. Inoculation Methods
5.2. Post-Inoculation Conditions
5.3. In Situ Bioassays on Ambrosia Beetles
6. Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vega, F.E.; Hofstetter, R.W. (Eds.) Bark Beetles: Biology and Ecology of Native and Invasive Species; Academic Press: London, UK, 2014; ISBN 978-0-124-17156-5. [Google Scholar]
- Six, D.L. Ecological and evolutionary determinants of bark beetle—Fungus symbioses. Insects 2012, 3, 339–366. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Hulcr, J. Scolytus and other economically important bark and ambrosia beetles. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Academic Press: New York, NY, USA, 2015; pp. 495–531. [Google Scholar]
- Haack, R.A.; Rabaglia, R.J. Exotic bark and ambrosia beetles in the USA: Potential and current invaders. In Potential Invasive Pest of Agricultural Crops; Peña, J., Ed.; CAB International: London, UK, 2013; pp. 48–74. [Google Scholar]
- Baniszewski, J.A.; Barnett, J.; Reding, M.E.; Ranger, C.M. Seasonal dominance of exotic ambrosia beetles compared to native species within deciduous and coniferous woodlots. Biol. Invasions 2024, 26, 1651–1668. [Google Scholar] [CrossRef]
- Fraedrich, S.W.; Harrington, T.C.; Rabaglia, R.J.; Ulyshen, M.D.; Mayfiel, A.E.; Hanula, J.L.; Eickwort, J.M.; Miller, D.R. A fungal symbiont of the redbay ambrosia beetle causes a lethal wilt in redbay and other Lauraceae in the southeastern United States. Plant Dis. 2008, 92, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Peña, J.E.; Crane, J.H.; Capinera, J.L.; Duncan, R.E.; Kendra, P.E.; Ploetz, R.C.; McLean, S.; Brar, G.; Thomas, M.C.; Cave, R.D. Chemical Control of the Redbay Ambrosia Beetle, Xyleborus glabratus, and Other Scolytinae (Coleoptera: Curculionidae). Fla. Entomol. 2011, 94, 882–896. [Google Scholar] [CrossRef]
- Cueva, F.D. El control biológico como estrategia para apoyar las exportaciones agrícolas no tradicionales en Perú: Un análisis empírico. Contab. Neg. 2012, 7, 81–100. (In Spanish) [Google Scholar]
- Mendell, B.C.; Lang, A.H.; Caldwell, W.; Garrett, D.L. Chemical use and forest certification: Productivity and economic implications. J. For. 2015, 113, 367–371. [Google Scholar] [CrossRef]
- Holmes, S.B.; MacQuarrie, C.J. Chemical control in forest pest management. Can. Entomol. 2016, 148, S270–S295. [Google Scholar] [CrossRef]
- Hejazi, M.; Grant, J.H.; Peterson, E. Trade impact of maximum residue limits in fresh fruits and vegetables. Food Policy 2022, 106, 102203. [Google Scholar] [CrossRef]
- Gugliuzzo, A.; Biedermann, P.H.; Carrillo, D.; Castrillo, L.A.; Egonyu, J.P.; Gallego, D.; Khalid, H.; Hulcr, J.; Jactel, H.; Kajimura, H.; et al. Recent advances toward the sustainable management of invasive Xylosandrus ambrosia beetles. J. Pest Sci. 2021, 94, 615–637. [Google Scholar] [CrossRef]
- Martini, X.; Hughes, M.A.; Conover, D.; Smith, J. Use of semiochemicals for the management of the redbay ambrosia beetle. Insects 2020, 11, 796. [Google Scholar] [CrossRef]
- Peña, J.E.; Weihman, S.W.; McLean, S.; Cave, R.D.; Carrillo, D.; Duncan, R.E.; Evans, G.; Krauth, S.; Thomas, M.C.; Lu, S.S.; et al. Predators and parasitoids associated with Scolytinae in Persea species (Laurales: Lauraceae) and other Lauraceae in Florida and Taiwan. Fla. Entomol. 2015, 98, 903–910. [Google Scholar] [CrossRef]
- Fuentes-Guardiola, L.T.; Sánchez-González, J.A.; Birrueta-Valencia, M.M.; Arredondo-Bernal, H.C. Hymenoptera parasítica asociada a especies de Xyleborus Eichhoff en aguacate en Colima, México. Southwest. Entomol. 2019, 44, 271–279. (In Spanish) [Google Scholar] [CrossRef]
- Reverchon, F.; Contreras-Ramos, S.M.; Eskalen, A.; Guerrero-Analco, J.A.; Quiñones-Aguilar, E.E.; Rios-Velasco, C.; Velázquez- Fernández, J.B. Microbial biocontrol strategies for ambrosia beetles and their associated phytopathogenic fungi. Food Syst. 2021, 5, 737977. [Google Scholar] [CrossRef]
- Jacobo-Macías, E.R.; Robles-Bermúdez, A.; Cambero-Campos, O.J.; Coronado-Blanco, J.M.; Isiordia-Aquino, N.; Ruíz-Cancino, E.; Robles-Navarrete, A.P. Parasitoides himenópteros presentes en especies ambrosiaes y descortezadores recolectados de Persea americana Mill. (Laurales: Lauraceae), en Nayarit, México. Acta Zool. Mex 2022, 38, 1–12. (In Spanish) [Google Scholar]
- Castrillo, L.A.; Griggs, M.H.; Vandenberg, J.D. Granulate ambrosia beetle, Xylosandrus crassiusculus (Coleoptera: Curculionidae), survival and brood production following exposure to entomopathogenic and mycoparasitic fungi. Biol. Control 2013, 67, 220–226. [Google Scholar] [CrossRef]
- Nel, W.J.; Slippers, B.; Wingfield, M.J.; Yilmaz, N.; Hurley, B.P. Efficacy of commercially available entomopathogenic agents against the polyphagous shot hole borer in South Africa. Insects 2023, 14, 361. [Google Scholar] [CrossRef]
- Mann, A.J.; Davis, T.S. Entomopathogenic fungi to control bark beetles: A review of ecological recommendations. Pest Manag. Sci. 2021, 77, 3841–3846. [Google Scholar] [CrossRef]
- Singh, D.; Raina, T.K.; Singh, J. Entomopathogenic fungi: An effective biocontrol agent for management of insect populations naturally. J. Pharm. Sci. Res. 2017, 9, 833. [Google Scholar]
- Zimowska, B.; Krol, E.D. Entomopathogenic fungi and their biocenotic importance. Adv. Microbiol. 2019, 58, 471–483. [Google Scholar] [CrossRef]
- Butt, T.M.; Coates, C.J.; Dubovskiy, I.M.; Ratcliffe, N.A. Entomopathogenic fungi: New insights into host–pathogen interactions. Adv. Genet. 2016, 94, 307–364. [Google Scholar]
- Shahid, A.A.; Rao, Q.A.; Bakhsh, A.; Husnain, T. Entomopathogenic fungi as biological controllers: New insights into their virulence and pathogenicity. Arch. Biol. Sci. Belgrade 2012, 64, 21–42. [Google Scholar] [CrossRef]
- Berestetskiy, A.; Hu, Q. The chemical ecology approach to reveal fungal metabolites for arthropod pest management. Microorganisms 2021, 9, 1379. [Google Scholar] [CrossRef] [PubMed]
- Bugti, G.A.; Bin, W.; Memon, S.A.; Khaliq, G.; Jaffar, M.A. Entomopathogenic fungi: Factors involved in successful microbial control of insect pests. J. Entomol. 2020, 17, 74–83. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; González-Mas, N.; Yousef-Yousef, M.; Garrido-Jurado, I.; Fernández-Bravo, M. Key role of environmental competence in successful use of entomopathogenic fungi in microbial pest control. J. Pest Sci. 2024, 97, 1–15. [Google Scholar] [CrossRef]
- Jaronski, S.T. Ecological factors in the inundative use of fungal entomopathogens. BioControl 2010, 55, 159–185. [Google Scholar] [CrossRef]
- Rajula, J.; Rahman, A.; Krutmuang, P. Entomopathogenic fungi in Southeast Asia and Africa and their possible adoption in biological control. Biol. Control 2020, 151, 104399. [Google Scholar] [CrossRef]
- Maina, U.M.; Galadima, I.B.; Gambo, F.M.; Zakaria, D.J.J.O.E. A review on the use of entomopathogenic fungi in the management of insect pests of field crops. J. Entomol. Zool. Stud. 2018, 6, 27–32. [Google Scholar]
- Kidanu, S.; Hagos, L. Research and application of entomopathogenic fungi as pest management option: A review. J. Env. Earth Sci. 2020, 10, 31–39. [Google Scholar]
- Dar, S.A.; Rather, B.A.; Kandoo, A.A. Insect pest management by entomopathogenic fungi. J. Entomol. Zool. Stud. 2017, 5, 1185–1190. [Google Scholar]
- Mayers, C.G.; Harrington, T.C.; Biedermann, P.H. Mycangia define the diverse ambrosia beetle–fungus symbioses. In The Convergent Evolution of Agriculture in Humans and Insects; MIT Press: Cambridge, MA, USA, 2022; pp. 105–142. [Google Scholar]
- Klepzig, K.D.; Six, D.L. Bark beetle-fungal symbiosis: Context dependency in complex associations. Symbiosis 2004, 37, 189–205. [Google Scholar]
- Farrell, B.D.; Sequeira, A.S.; O'Meara, B.C.; Normark, B.B.; Chung, J.H.; Jordal, B.H. The evolution of agriculture in beetles (Curculionidae: Scolytinae and Platypodinae). Evolution 2001, 55, 2011–2027. [Google Scholar] [CrossRef] [PubMed]
- Hulcr, J.; Stelinski, L.L. The ambrosia symbiosis: From evolutionary ecology to practical management. Annu. Rev. Entomol. 2017, 62, 285–303. [Google Scholar] [CrossRef] [PubMed]
- Joseph, R.; Keyhani, N.O. Fungal mutualisms and pathosystems: Life and death in the ambrosia beetle mycangia. Appl. Microbiol. Biotechnol. 2021, 105, 3393–3410. [Google Scholar] [CrossRef] [PubMed]
- Kirkendall, L.R.; Biedermann, P.H.W.; Jordal, B.H. Evolution and diversity of bark and ambrosia beetles. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 85–156. ISBN 9780124171732. [Google Scholar]
- Harrington, T.; Fraedrich, S.; Aghayeva, D. Raffaelea lauricola a new ambrosia beetle symbiont and pathogen on the Lauraceae. Mycotaxon 2008, 104, 399–404. [Google Scholar]
- Schuler, H.; Witkowski, R.; Van de Vossenberg, B.; Hoppe, B.; Mittelbach, M.; Bukovinszki, T.; Schwembacher, S.; van de Meulengraaf, B.; Lange, U.; Rode, S.; et al. Recent invasion and eradication of two members of the Euwallacea fornicatus species complex (Coleoptera: Curculionidae: Scolytinae) from tropical greenhouses in Europe. Biol. Invasions 2023, 25, 299–307. [Google Scholar] [CrossRef]
- Landi, L.; Braccini, C.L.; Knížek, M.; Pereyra, V.A.; Marvaldi, A.E. A newly detected exotic ambrosia beetle in Argentina: Euwallacea interjectus (Coleoptera: Curculionidae: Scolytinae). Flor. Entomol. 2019, 102, 240–242. [Google Scholar] [CrossRef]
- Stouthamer, R.; Rugman-Jones, P.; Thu, P.Q.; Eskalen, A.; Thibault, T.; Hulcr, J.; Liangjomg, W.; Jordal, B.H.; ChiYu, C.; Cooperband, M.; et al. Tracing the origin of a cryptic invader: Phylogeography of the Euwallacea fornicatus (Coleoptera: Curculionidae: Scolytinae) species complex. Agric. For. Entomol. 2017, 19, 366–375. [Google Scholar] [CrossRef]
- Grégoire, J.C.; Jactel, H.; Hulcr, J.; Battisti, A.; Inward, D.; Petter, F.; Grousset, F. Cosmopolitan Scolytinae: Strong common drivers but too many singularities for accurate prediction. NeoBiota 2023, 84, 81–105. [Google Scholar] [CrossRef]
- Bentz, B.J.; Jönsson, A.M. Modeling Bark Beetles responses to climate change. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 533–553. ISBN 9780124171732. [Google Scholar]
- Fettig, C.J.; Asaro, C.; Nowak, J.T.; Dodds, K.J.; Gandhi, K.J.; Moan, J.E.; Robert, J. Trends in bark beetle impacts in North America during a period (2000–2020) of rapid environmental change. J. For. 2022, 120, 693–713. [Google Scholar] [CrossRef]
- Kendra, P.E.; Montgomery, W.S.; Niogret, J.; Epsky, N.D. An uncertain future for American Lauraceae: A lethal threat from redbay ambrosia beetle and laurel wilt disease (a review). Am. J. Plant Sci. 2013, 4, 727–738. [Google Scholar] [CrossRef]
- Fraedrich, S.W.; Johnson, C.W.; Menard, R.D.; Harrington, T.C.; Olatinwo, R.; Best, G.S. First report of Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae) and laurel wilt in Louisiana, USA: The disease continues westward on sassafras. Fla. Entomol. 2015, 98, 1266–1268. Available online: https://www.jstor.org/stable/24587650 (accessed on 17 April 2025). [CrossRef]
- Hughes, M.A.; Smith, J.A.; Ploetz, R.C.; Kendra, P.E.; Mayfield, A.E., III; Hanula, J.L.; Hulcr, J.; Stelinski, L.L.; Cameron, S.; Riggins, J.J. Recovery plan for laurel wilt on redbay and other forest species caused by Raffaelea lauricola and disseminated by Xyleborus glabratus. Plant Health Progr. 2015, 16, 173–210. [Google Scholar] [CrossRef]
- Cloonan, K.R.; Montgomery, W.S.; Narvaez, T.I.; Carrillo, D.; Kendra, P.E. Community of bark and ambrosia beetles (Coleoptera: Curculionidae: Scolytinae and Platypodinae) in agricultural and forest ecosystems with laurel wilt. Insects 2022, 13, 971. [Google Scholar] [CrossRef] [PubMed]
- Lira-Noriega, A.; Soberón, J.; Equihua, J. Potential invasion of exotic ambrosia beetles Xyleborus glabratus and Euwallacea sp. in Mexico: A major threat for native and cultivated forest ecosystems. Sci. Rep. 2018, 8, 10179. [Google Scholar] [CrossRef]
- Carrillo, D.; Dunca, R.; Ploetz, R.; Peña, J.E. Lateral transfer of a phytopathogenic symbiont among native and exotic ambrosia beetles. Plant Pathol. 2014, 63, 54–62. [Google Scholar] [CrossRef]
- Castrejón-Antonio, J.E.; Montesinos-Matías, R.; Acevedo-Reyes, N.; Tamez-Guerra, P.; Ayala-Zermeño, M.Á.; Berlanga-Padilla, A.M.; Arredondo-Bernal, H.C. Especies de Xyleborus (Coleoptera: Curculionidae: Scolytinae) asociados a huertos de aguacate en Colima, México. Acta Zool. Mex. 2017, 33, 146–150. (In Spanish) [Google Scholar] [CrossRef]
- Angeles-Restrepo, M.; Ochoa-Ascencio, S.; Fernández-Pavía, S.; Vázquez-Marrufo, G.; Equihua-Martínez, A.; Barrieto-Priego, A.F.; Correa-Suarez, M.; Saucedo-Carabez, J.R. Identificación de escarabajos ambrosiaes (Coleópteros: Curculionidae) asociados a árboles de aguacate en Michoacán, México. Folia Entomol. Mex. 2019, 5, 80–88. (In Spanish) [Google Scholar]
- Kendra, P.E.; Guillén, L.; Tabanca, N.; Montgomery, W.S.; Schnell, E.Q.; Deyrup, M.A.; Cloonan, K.R. Risk assessment of Hass avocado and Mexican Lauraceae for attack by redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae). Agric. For. Entomol. 2023, 25, 285–302. [Google Scholar] [CrossRef]
- Castrillo, L.A.; Griggs, M.H.; Ranger, C.M.; Reding, M.E.; Vandenberg, J.D. Virulence of commercial strain of Beauveria bassiana and Metarhizium brunneum (Ascomycota: Hypocreales) against adult Xylosandrus germanus (Coleoptera: Curculionidae) and impact on brood. Biol. Control 2011, 58, 121–126. [Google Scholar] [CrossRef]
- Carrillo, D.; Dunlap, C.; Avery, P.; Navarrete, J.; Dunca, R.; Jackson, M.; Peña, J.E. Entomopathogenic fungi as biological control agents for the vector of the laurel wilt disease, the redbay ambrosia beetle, Xyleborus glabratus (Coleoptera: Curculionidae). Biol. Control 2015, 81, 44–50. [Google Scholar] [CrossRef]
- Avery, P.B.; Bojorque, V.; Gámez, C.; Duncan, R.E.; Carrillo, D.; Cave, R.D. Spore acquisition and survival of ambrosia beetles associated with the laurel wilt pathogen in avocados after exposure to entomopathogenic fungi. Insects 2018, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Toksöz, S.; Oksal, E.; Saruhan, I.; Kepenekci, I. Pathogenicity of the entomopathogenic fungus, Purpureocillium lilacinum TR1 against ambrosia beetles, Xylosandrus germanus (Blandford) and Xyleborus dispar (Fabricius) (Coleoptera: Curculionidae: Scolytinae). Mun. Ent. Zool. 2018, 13, 471–481. [Google Scholar]
- Kushiyev, R.; Tuncer, C.; Erper, I.; Ozdemir, I.O.; Saruhan, I. Efficacy of native entomopathogenic fungus, Isaria fumosorosea, against bark and ambrosia beetles, Anisandrus dispar Fabricius and Xylosandrus germanus Blandford (Coleoptera: Curculionidae: Scolytinae). Egypt. J. Biol. Pest Control 2018, 28, 55. [Google Scholar] [CrossRef]
- Tuncer, C.; Kushiyev, R.; Erper, I.; Ozdemir, I.O.; Saruhan, I. Efficacy of native isolates of Metarhizium anisopliae and Beauveria bassiana against the invasive ambrosia beetle, Xylosandrus germanus Blandford (Coleoptera: Curculionidae: Scolytinae). Egypt. J. Biol. Pest Control 2019, 29, 28. [Google Scholar] [CrossRef]
- Castrejón-Antonio, J.E.; Tamez-Guerra, P.; Montesinos-Matías, R.; Ek-Ramos, M.J.; Garza-López, P.M.; Arredondo-Bernal, H.C. Selection of Beauveria bassiana (Hypocreales: Cordycipitaceae) strains to control Xyleborus affinis (Curculionidae: Scolytinae) females. PeerJ 2020, 8, e9472. [Google Scholar] [CrossRef]
- Reynoso-López, E.A.; Méndez-Hernández, J.E.; Ek-Ramos, J.; Montesinos-Matías, R.; Loera, O. Metarhizium robertsii in combination with Trichoderma asperellum reduce the malathion doses used to control ambrosia beetles: The case of Xyleborus affinis. Biocontrol Sci. Technol. 2021, 31, 1080–1097. [Google Scholar] [CrossRef]
- Castrejón-Antonio, J.E.; Tamez-Guerra, P.; García-Ortiz, N.; Muñiz-Paredes, F.; Sánchez-Rangel, J.C.; Montesinos-Matías, R. Biocontrol of Xyleborus affinis (Curculionidae: Scolitinae) females and progeny by Beauveria bassiana (Hypocreales: Cordycipitaceae) in a sawdust artificial diet model. Insects 2023, 14, 477. [Google Scholar] [CrossRef]
- Castrejón-Antonio, J.E.; Tamez-Guerra, P.; Montesinos-Matías, R.; Sánchez-Rangel, J.C.; Mendoza-Muñoz, N. Compatibility of Beauveria bassiana with Inverted Emulsions in Contaminated Surface Tests Against Xyleborus affinis. Southwest. Entomol. 2025, 50, 210–227. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, N.; Gao, H.; Lai, S.; Zhou, Y.; Hao, D.; Dai, L. Laboratory Exploration of Several Potential Biocontrol Methods Against the Ambrosia Beetle, Euwallacea interjectus. Insects 2025, 16, 56. [Google Scholar] [CrossRef]
- Sibao, W.A.N.G.; Xuexia, M.I.A.O.; Weiguo, Z.H.A.O.; Huang, B.; Meizhen, F.A.N.; Zengzhi, L.I.; Huang, Y. Genetic diversity and population structure among strains of the entomopathogenic fungus, Beauveria bassiana, as revealed by inter-simple sequence repeats (ISSR). Mycol. Res. 2025, 109, 1364–1372. [Google Scholar] [CrossRef]
- Rohrlich, C.; Merle, I.; Mze Hassani, I.; Verger, M.; Zuin, M.; Besse, S.; Robène, I.; Nibouche, S.; Costet, L. Variation in physiological host range in three strains of two species of the entomopathogenic fungus Beauveria. PLoS ONE 2018, 13, e0199199. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Raza, A.B.M.; Alkafafy, M.; Sayed, S.; Hamid, M.I.; Majeed, M.Z.; Riaz, M.A.; Gaber, N.M.; Asim, M. Isolation, identification and virulence of indigenous entomopathogenic fungal strains against the peach-potato aphid, Myzus persicae Sulzer (Hemiptera: Aphididae), and the fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae). Egypt. J. Biol. Pest Control 2022, 32, 2. [Google Scholar] [CrossRef]
- Hussain, A.; Tian, M.Y.; He, Y.R.; Ruan, L.; Ahmed, S. In vitro and in vivo culturing impacts on the virulence characteristics of serially passed entomopathogenic fungi. J. Food Agric. Environ. 2010, 8, 481–487. [Google Scholar]
- Robertson, J.L.; Jones, M.M.; Olguin, E.; Alberts, B. Bioassays with Arthropods, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 1–10. ISBN 978-1-4822-1708-7. [Google Scholar]
- Beris, E. Evaluation and environmental testing of entomopathogenic fungi for their effectiveness as bio-control agents of major vineyard pests. Plant Prot. 2021, 5, 13–23. Available online: https://esciencepress.net/journals/index.php/PP/article/view/3571 (accessed on 17 April 2025).
- Liu, Y.C.; Ni, N.T.; Chang, J.C.; Li, Y.H.; Lee, M.R.; Kim, J.S.; Nai, Y.S. Isolation and selection of entomopathogenic fungi from soil samples and evaluation of fungal virulence against insect pests. J. Vis. Exp. 2021, 175, 62882. [Google Scholar] [CrossRef]
- Haverty, M.I.; Robertson, J.L. Laboratory bioassays for selecting candidate insecticides and application rates for field tests on the western spruce budworm. J. Econ. Entomol. 1982, 75, 179. [Google Scholar] [CrossRef]
- Draganova, S.A.; Doychev, D.D.; Pilarska, D.K.; Takov, D.I. Bioassays of entomopathogenic fungi against xylophagous insects in Bulgaria: Laboratory and field experiments. Acta Zool. Bulg. 2017, 69, 411–419. [Google Scholar]
- Graf, T.; Scheibler, F.; Niklaus, P.A.; Grabenweger, G. From lab to field: Biological control of the Japanese beetle with entomopathogenic fungi. Front. Insect Sci. 2023, 3, 1138427. [Google Scholar] [CrossRef]
- Abalo, M.; Scorsetti, A.C.; Vianna, M.F.; Russo, M.L.; De Abajo, J.M.; Pelizza, S.A. Field evaluation of entomopathogenic fungi formulations against Rachiplusia nu (Lepidoptera: Noctuidae) in soybean crop. J. Plant Prot. Res. 2022, 62, 403–410. [Google Scholar] [CrossRef]
- Wakil, W.; Kavallieratos, N.G.; Nika, E.P.; Qayyum, M.A.; Yaseen, T.; Ghazanfar, M.U.; Yasin, M. Combinations of Beauveria bassiana and spinetoram for the management of four important stored-product pests: Laboratory and field trials. Environ. Sci. Pollut. Res. 2023, 30, 27698–27715. [Google Scholar] [CrossRef]
- Foster, R.N.; Jaronski, S.; Reuter, K.C.; Black, L.R.; Schlothauer, R.; Harper, J.; Jech, L.E. Simulated aerial sprays for field cage evaluation of Beauveria bassiana and Metarhizium brunneum (Ascomycetes: Hypocreales) against Anabrus simplex (Orthoptera: Tettigoniidae) in Montana. Biocontrol Sci. Technol. 2011, 21, 1331–1350. [Google Scholar] [CrossRef]
- Mann, A.J.; Davis, T.S. Plant secondary metabolites and low temperature are the major limiting factors for Beauveria bassiana (Bals.-Criv.) Vuill. (Ascomycota: Hypocreales) growth and virulence in a bark beetle system. BioControl 2020, 141, 104130. [Google Scholar] [CrossRef]
- Draganova, S.A.; Takov, D.I.; Doychev, D.D. Naturally-occurring entomopathogenic fungi on three bark beetle species (Coleoptera: Curculionidae) in Bulgaria. Pestic. Fitomed. 2010, 25, 59–63. [Google Scholar] [CrossRef]
- Katumanyane, A.; Slippers, B.; Wondafrash, M.; Malan, A.P.; Hurley, B.P. Natural infection of white grubs (Coleoptera: Scarabaeidae) with entomopathogenic nematodes in the KwaZulu-Natal province of South Africa. J. Helminthol. 2023, 97, e54. [Google Scholar] [CrossRef]
- Cohen, A.C. Insect Diets: Science and Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 1–19. [Google Scholar]
- Castrillo, L.A.; Griggs, M.H.; Vandenberg, J.D. Brood production by Xylosandrus germanus (Coleoptera: Curculionidae) and growth of its fungal symbiont on artificial diet based on sawdust of different tree species. Environ. Entomol. 2012, 41, 822–827. [Google Scholar] [CrossRef]
- Cruz, L.F.; Rocio, S.A.; Duran, L.G.; Menocal, O.; Garcia-Avila, C.D.J.; Carrillo, D. Developmental biology of Xyleborus bispinatus (Coleoptera: Curculionidae) reared on an artificial medium and fungal cultivation of symbiotic fungi in the beetle's galleries. Fungal Ecol. 2018, 35, 116–126. [Google Scholar] [CrossRef]
- Maner, M.L.; Hanula, J.L.; Braman, S.K. Rearing redbay ambrosia beetle, Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae), on semi-artificial media. Fla. Entomol. 2013, 96, 1042–1051. [Google Scholar] [CrossRef]
- Mizuno, T.; Kajimura, H. Effects of ingredients and structure of semi-artificial diet on the reproduction of an ambrosia beetle, Xyleborus pfeili (Ratzeburg) (Coleoptera: Curculionidae: Scolytinae). Appl. Entomol. Zool. 2009, 44, 363–370. [Google Scholar] [CrossRef]
- Biedermann, P.H.; Klepzig, K.D.; Taborsky, M. Fungus cultivation by ambrosia beetles: Behavior and laboratory breeding success in three xyleborine species. Environ. Entomol. 2009, 38, 1096–1105. [Google Scholar] [CrossRef]
- Gokulakrishnaa, R.K.; Thirunavukkarasu, S. Bioassay techniques in entomological research. Int. J. Plant Soil Sci. 2023, 35, 363–373. [Google Scholar] [CrossRef]
- Mannino, M.C.; Huarte-Bonnet, C.; Davyt-Colo, B.; Pedrini, N. Is the insect cuticle the only entry gate for fungal infection? Insights into alternative modes of action of entomopathogenic fungi. J. Fungi 2019, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Skrzecz, I.; Sierpińska, A.; Tumialis, D. Entomopathogens in the integrated management of forest insects: From science to practice. Pest Manag. Sci. 2024, 80, 2503–2514. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Huang, Y.; Thomson, S.J.; Elliott, R.B. Effects of conidial densities and spray volume of Metarhizium anisopliae and Beauveria bassiana fungal suspensions on conidial viability, droplet size and deposition coverage in bioassay using a novel bioassay spray system. Biocontrol Sci. Technol. 2013, 23, 362–366. [Google Scholar] [CrossRef]
- Behle, R.W. Importance of direct spray and spray residue contact for infection of Trichoplusia ni larvae by field applications of Beauveria bassiana. J. Econ. Entomol. 2006, 99, 1120–1128. [Google Scholar] [CrossRef]
- Dara, S.K.; Montalva, C.; Barta, M. Microbial control of invasive forest pests with entomopathogenic fungi: A review of the current situation. Insects 2019, 10, 341. [Google Scholar] [CrossRef]
- Hulcr, J.; Rountree, N.R.; Diamond, S.E.; Stelinski, L.L.; Fierer, N.; Dunn, R.R. Mycangia of ambrosia beetles host communities of bacteria. Microb. Ecol. 2012, 64, 784–793. [Google Scholar] [CrossRef]
- Donegan, K.; Lighthart, B. Effect of several stress factors on the susceptibility of the predatory insect, Chrysoperla carnea (Neuroptera: Chrysopidae), to the fungal pathogen Beauveria bassiana. J. Invertebr. Pathol. 1989, 54, 79–84. [Google Scholar] [CrossRef]
- Lord, J.C. Dietary stress increases the susceptibility of Tribolium castaneum to Beauveria bassiana. J. Econ. Entomol. 2010, 103, 1542–1546. [Google Scholar] [CrossRef]
- Brar, G.S.; Capinera, J.L.; Kendra, P.E.; McLean, S.; Peña, J.E. Life cycle, development, and culture of Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae). Fla. Entomol. 2013, 96, 1158–1167. [Google Scholar] [CrossRef]
- Ranger, C.M.; Reding, M.E.; Persad, A.B.; Herms, D.A. Ability of stress-related volatiles to attract and induce attacks by Xylosandrus germanus and other ambrosia beetles. Agric. For. Entomol. 2010, 12, 177–185. [Google Scholar] [CrossRef]
- Castrillo, L.A.; Mayfield, A.E., III; Griggs, M.H.; Camp, R.; Mudder, B.; Taylor, A.; Vandenberg, J.D. Mortality and reduced brood production in walnut twig beetles, Pityophthorus juglandis (Coleoptera: Curculionidae), following exposure to commercial strains of entomopathogenic fungi Beauveria bassiana and Metarhizium brunneum. Biol. Control 2017, 114, 79–86. [Google Scholar] [CrossRef]
- Olatinwo, R.; Walters, S.; Strom, B. Impact of Beauveria bassiana (Ascomycota: Hypocreales) on the Small Southern Pine Engraver (Coleoptera: Scolytidae) in a Loblolly Pine Bolt Assay. J. Entomol. Sci. 2018, 53, 180–191. [Google Scholar] [CrossRef]
- Kreutz, J.; Zimmermenn, G.; Mahron, H.; Vaupel, O.; Mosbacher, G. Preliminary investigations on the use of Beauveria bassiana (Bals.) Vuill. and other control methods against the bark beetle Ips typographus L. (Col., Scolytidae) in the Weld. IOBC/WPRS Bull. 2000, 23, 167–173. [Google Scholar]
- Kreutz, J.; Vaupel, O.; Zimmermann, G. Efficacy of Beauveria bassiana (Bals.) Vuill. against the spruce bark beetle, Ips typographus L., in the laboratory under various conditions. J. Appl. Entomol. 2004, 128, 384–389. [Google Scholar] [CrossRef]
- Davis, T.S.; Mann, A.J.; Malesky, D.; Jankowski, E.; Bradley, C. Laboratory and field evaluation of the entomopathogenic fungus Beauveria bassiana (Deuteromycotina: Hyphomycetes) for population management of spruce beetle, Dendroctonus rufipennis (Coleoptera: Scolytinae), in felled trees and factors limiting pathogen success. Environ. Entomol. 2018, 47, 594–602. [Google Scholar] [CrossRef]
- Grodzki, W.; Kosibowicz, M. An attempt to use the fungus Beauveria bassiana (Bals.) Vuill. in forest protection against the bark beetle Ips typographus (L.) in the field. For. Res. Pap. 2015, 76, 5–17. [Google Scholar] [CrossRef]
- Batta, Y.A. Biocontrol of almond bark beetle (Scolytus amygdali Geurin-Meneville, Coleoptera: Scolytidae) using Beauveria bassiana (Bals.) Vuill. (Deuteromycotina: Hyphomycetes). J. Appl. Microbiol. 2007, 103, 1406–1414. [Google Scholar] [CrossRef]
- Fernandez, K.X.; Pokorny, S.; Ishangulyeva, G.; Ullah, A.; Todorova, S.I.; Erbilgin, N.; Carroll, A.; Vederas, J.C. Beauveria bassiana exhibits strong virulence against Dendroctonus ponderosae in greenhouse and field experiments. Appl. Microbiol. Biotechnol. 2023, 107, 3341–3352. [Google Scholar] [CrossRef]
- Fora, C.G.; Boja, N.; Moatăr, M.; Tóth, F.; Balog, A. Effect of entomopathogenic fungi, Beauveria bassiana (Cordycipitaceae), on the bark beetle, Ips typographus (L.), under field conditions. Insects 2022, 13, 885. [Google Scholar] [CrossRef]
- Srei, N.; Lavallée, R.; Guertin, C. Susceptibility of Dendroctonus simplex to Hypocreales fungi: Towards the development of a biological control strategy. J. Appl. Entomol. 2017, 141, 487–495. [Google Scholar] [CrossRef]
| Fungal Species/Strain | Target Species | Efficacy Level | Inoculation | Post-Inoculation Conditions |
|---|---|---|---|---|
| Metarhizium brunneum F52, Beauveria bassiana Naturalist, B. bassiana GHA | Xylosandrus germanus | 6.7–61.7% [55] | Direct | Wet chamber/Artificial diet |
| M. brunneum F52, B. bassiana Naturalist, B. bassiana GHA | Xylosandrus crassiusculus | 76.7–95.6% [18] | Direct and Indirect | Wet chamber/ Natural diet |
| Isaria fumosorosea Ifr, I. fumosorosea PFR, B. bassiana GHA | Xyleborus glabratus | 54.7–77.3% [56] | Direct and Indirect | Wet chamber/Natural diet |
| M. brunneum Met52®, I. fumosorosea PFR-97®, B. bassiana GHA(BotaniGard®) | Xyleborus bispinatus X. crassiusculus Xyleborus volvulus | Unspecified [57] | Indirect | Wet chamber |
| Purpureocillium lilacinum TR1 | X. germanus Xyleborus dispar | 94.7–100% [58] | Direct | Natural diet |
| I. fumosorosea TR-78-3 | Anisandrus dispar X. germanus | 90–100% [59] | Direct and Indirect | Wet chamber |
| M. anisopliae TR-106, B. bassiana TR-217 | X. germanus | 64–100% [60] | Direct and Indirect | Wet chamber |
| B. bassiana CNRCB-CHE 44, 171, 431 and 485 | Xyleborus affinis | 40–58.7% [61] | Direct | Artificial diet |
| Metarhizium robertsii Xoch 8.1 | X. affinis | 76% [62] | Direct | Artificial diet |
| B. bassiana CNRCB-CHE 44, 171, 431 and 485 | X. affinis | 3.4–50.3% [63] | Direct | Artificial diet |
| M. anisopliae + B. bassiana (Bio-Insek®), B. bassiana (Eco Bb®) | Euwallacea fornicatus | Unspecified [19] | Direct and Indirect | Wet chamber/ Natural diet |
| B. bassiana CNRCB-CHE 44, and 485 strains | X. affinis | 10–20% [64] | Indirect | Artificial diet |
| B. bassiana B-BB1 | Euwallacea interjectus | 64–100% [65] | Direct and Indirect | Wet chamber/ Artificial diet/ Natural diet |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castrejón-Antonio, J.E.; Tamez-Guerra, P. Overview and Recent Advances in Bioassays to Evaluate the Potential of Entomopathogenic Fungi Against Ambrosia Beetles. Insects 2025, 16, 615. https://doi.org/10.3390/insects16060615
Castrejón-Antonio JE, Tamez-Guerra P. Overview and Recent Advances in Bioassays to Evaluate the Potential of Entomopathogenic Fungi Against Ambrosia Beetles. Insects. 2025; 16(6):615. https://doi.org/10.3390/insects16060615
Chicago/Turabian StyleCastrejón-Antonio, Jesús Enrique, and Patricia Tamez-Guerra. 2025. "Overview and Recent Advances in Bioassays to Evaluate the Potential of Entomopathogenic Fungi Against Ambrosia Beetles" Insects 16, no. 6: 615. https://doi.org/10.3390/insects16060615
APA StyleCastrejón-Antonio, J. E., & Tamez-Guerra, P. (2025). Overview and Recent Advances in Bioassays to Evaluate the Potential of Entomopathogenic Fungi Against Ambrosia Beetles. Insects, 16(6), 615. https://doi.org/10.3390/insects16060615







