Reproductive Senescence in the Pollinator, Megachile rotundata
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Rearing and Marking Bees
2.2. Ovarian Development
2.3. Foraging Behavior
2.4. Offspring Quantity and Quality
2.5. Statistical Analyses
3. Results
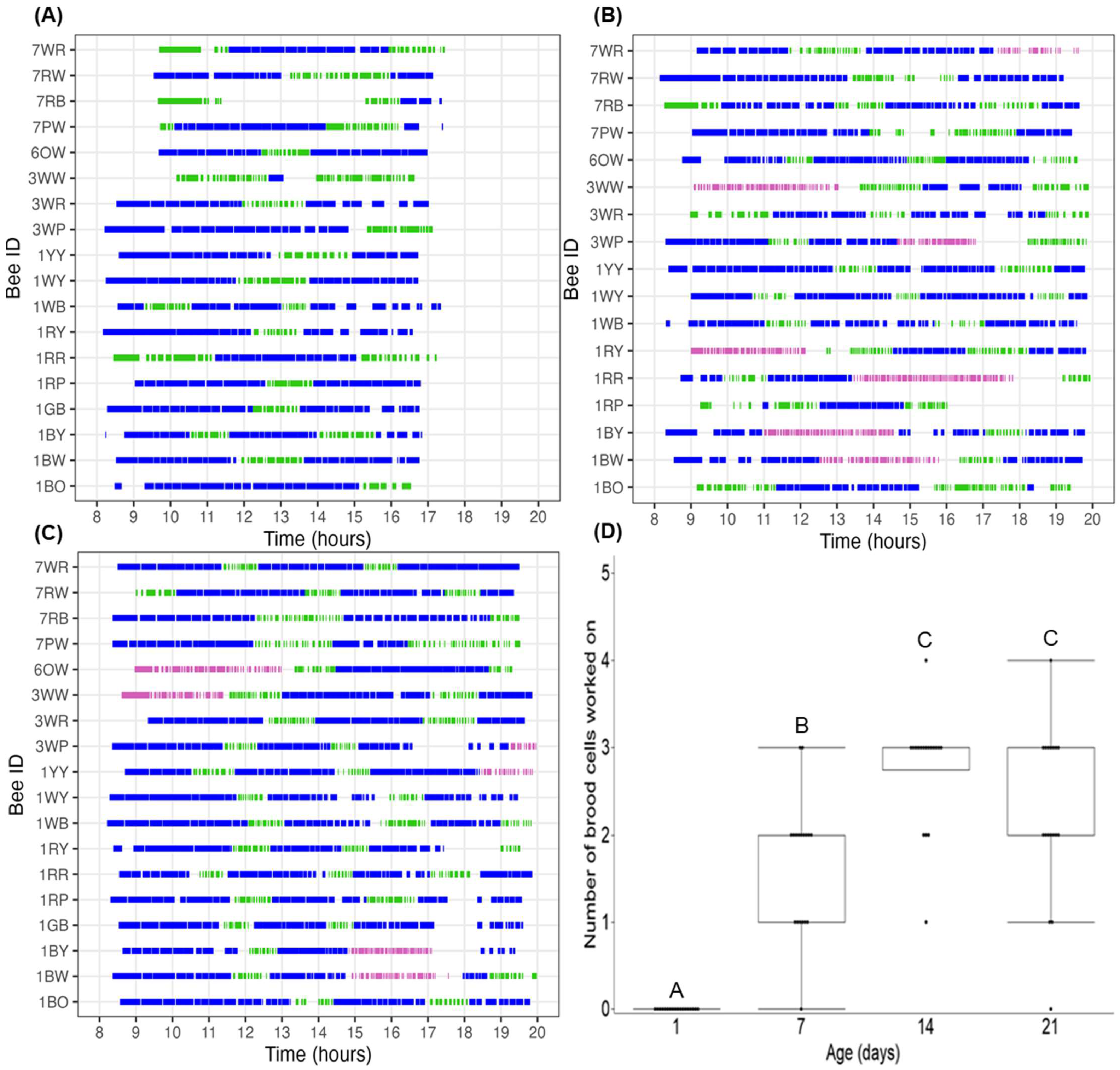
3.1. Ovarian Development
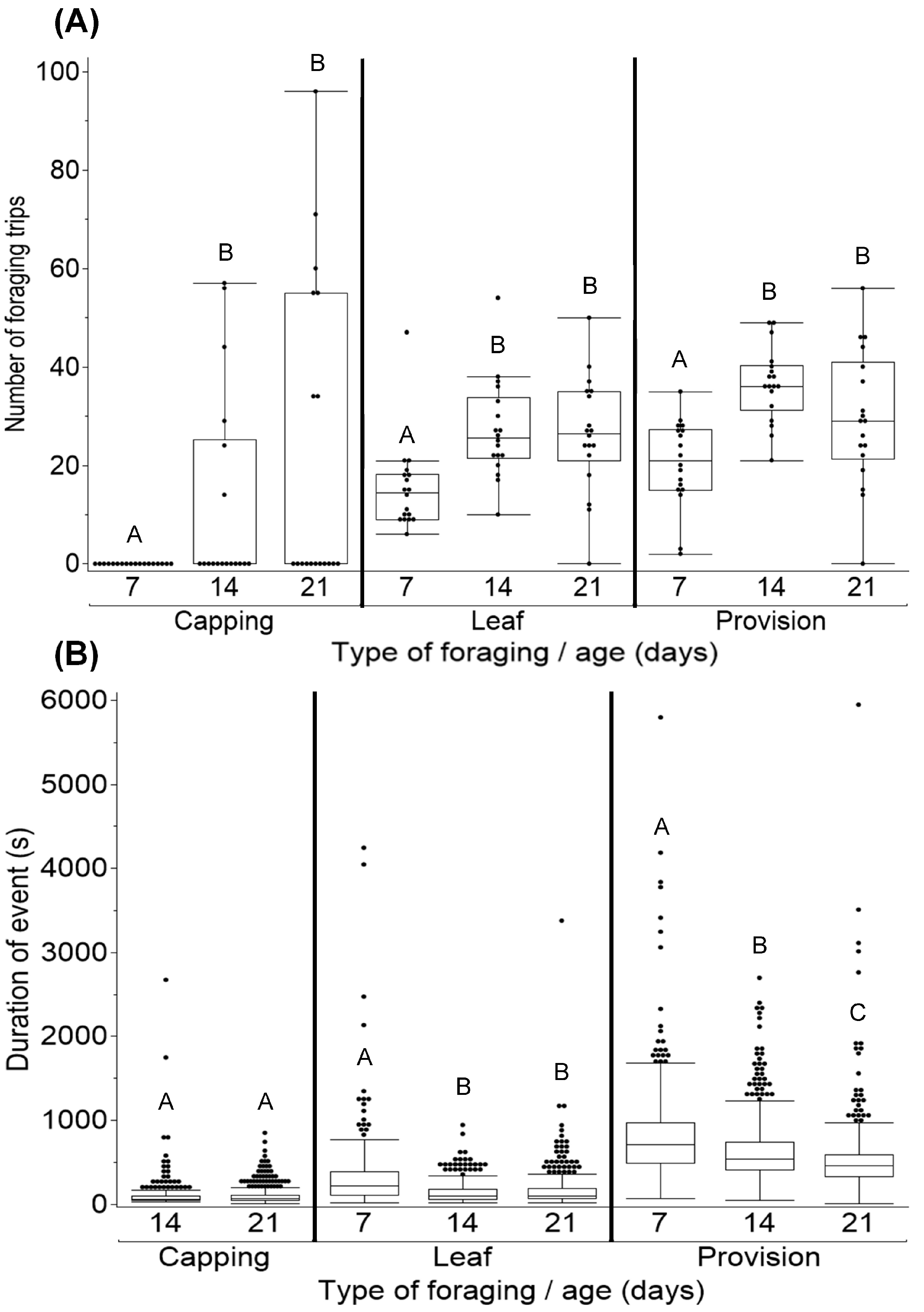
3.2. Foraging Behavior
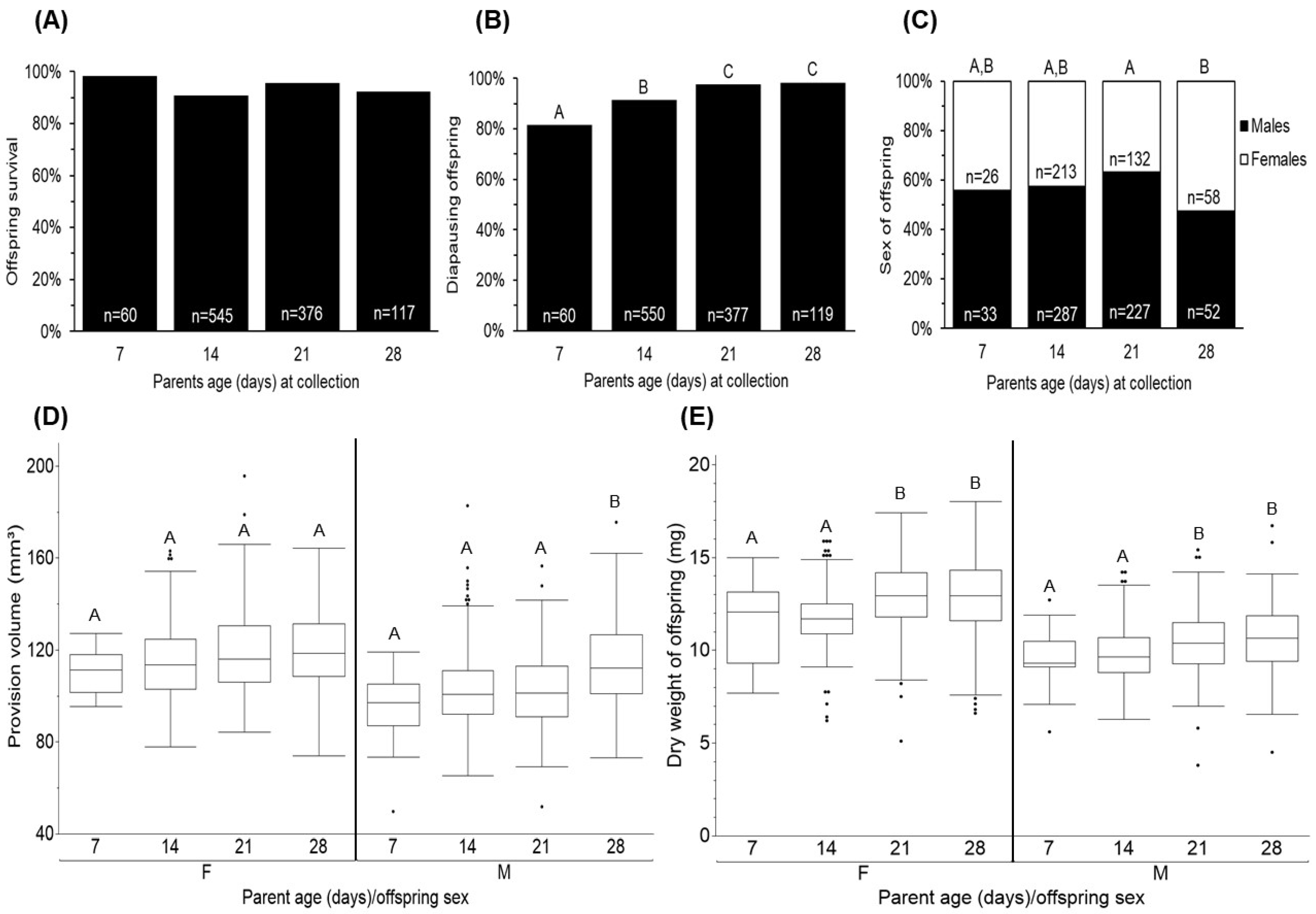
3.3. Offspring Quantity and Quality
4. Discussion
4.1. Ovarian Development
4.2. Foraging Behavior
4.3. Offspring Quantity and Quality
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirkwood, T.B. The disposable soma theory. Evol. Senescence Tree Life 2017, 552, 23–39. [Google Scholar]
- Constant, N.; Santorelli, L.A.; Lopes, J.F.; Hughes, W.O. The effects of genotype, caste, and age on foraging performance in leaf-cutting ants. Behav. Ecol. 2012, 23, 1284–1288. [Google Scholar] [CrossRef]
- Giejdasz, K.; Fliszkiewicz, M.; Bednárová, A.; Krishnan, N. Reproductive potential and nesting effects of Osmia rufa (syn. bicornis) female (Hymenoptera: Megachilidae). J. Apic. Sci. 2016, 60, 75–86. [Google Scholar] [CrossRef]
- Sohal, R.S. The free radical hypothesis of aging: An appraisal of the current status. Aging Clin. Exp. Res. 1993, 5, 3–17. [Google Scholar] [CrossRef]
- De Loof, A. Longevity and aging in insects: Is reproduction costly; cheap; beneficial or irrelevant? A critical evaluation of the “trade-off” concept. J. Insect Physiol. 2011, 57, 1–11. [Google Scholar] [CrossRef]
- Lemaître, J.F.; Gaillard, J.M. Reproductive senescence: New perspectives in the wild. Biol. Rev. 2017, 92, 2182–2199. [Google Scholar] [CrossRef]
- Guo, S.; Wang, X.; Kang, L. Special significance of non-Drosophila insects in aging. Front. Cell Dev. Biol. 2020, 8, 576571. [Google Scholar] [CrossRef]
- Heinze, J.; Schrempf, A. Aging and reproduction in social insects–a mini-review. Gerontology 2008, 54, 160–167. [Google Scholar] [CrossRef]
- Korb, J.; Heinze, J. Ageing and sociality: Why, when and how does sociality change ageing patterns? Philos. Trans. R. Soc. B 2021, 376, 20190727. [Google Scholar] [CrossRef]
- Promislow, D.E.; Flatt, T.; Bonduriansky, R. The biology of aging in insects: From Drosophila to other insects and back. Annu. Rev. Entomol. 2022, 67, 83–103. [Google Scholar] [CrossRef]
- Fredriksson, Å.; Johansson Krogh, E.; Hernebring, M.; Pettersson, E.; Javadi, A.; Almstedt, A.; Nyström, T. Effects of aging and reproduction on protein quality control in soma and gametes of Drosophila melanogaster. Aging Cell 2012, 11, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Al-Lawati, H.; Bienefeld, K. Maternal age effects on embryo mortality and juvenile development of offspring in the honey bee (Hymenoptera: Apidae). Ann. Entomol. Soc. Am. 2009, 102, 881–888. [Google Scholar] [CrossRef]
- Nikola, T.; Darka, Š.; Vesna, S. The short-term and long-term effects of parental age in the bean weevil (Acanthoscelides obtectus). Evol. Ecol. 2004, 18, 187–201. [Google Scholar] [CrossRef]
- Mohaghegh, J.; DeClercq, P.; Tirry, L. Effects of maternal age and egg weight on developmental time and body weight of offspring of Podisus maculiventris (Heteroptera: Pentatomidae). Ann. Entomol. Soc. Am. 1998, 91, 315–322. [Google Scholar] [CrossRef]
- Mandal, S.; Brahma, A. Getting older, getting smarter: Ontogeny of foraging behaviour in the tropical social wasp Ropalidia marginata. J. Exp. Biol. 2019, 222, jeb199844. [Google Scholar] [CrossRef]
- Vance, J.T.; Williams, J.B.; Elekonich, M.M.; Roberts, S.P. The effects of age and behavioral development on honey bee (Apis mellifera) flight performance. J. Exp. Biol. 2009, 212, 2604–2611. [Google Scholar] [CrossRef]
- David, J.; Cohet, Y.; Fouillet, P. The variability between individuals as a measure of senescence: A study of the number of eggs laid and the percentage of hatched eggs in the case of Drosophila melanogaster. Exp. Gerontol. 1975, 10, 17–25. [Google Scholar] [CrossRef]
- Novoseltsev, V.N.; Novoseltseva, J.A.; Yashin, A.I. What does a fly’s individual fecundity pattern look like? The dynamics of resource allocation in reproduction and ageing. Mech. Ageing Dev. 2003, 124, 605–617. [Google Scholar] [CrossRef]
- Torchio, P.F.; Tepedino, V.J. Sex ratio, body size and seasonality in a solitary bee, Osmia lignaria propinqua Cresson (Hymenoptera: Megachilidae). Evolution 1980, 34, 993–1003. [Google Scholar] [CrossRef]
- Bérubé, C.H.; Festa-Bianchet, M.; Jorgenson, J.T. Individual differences, longevity, and reproductive senescence in bighorn ewes. Ecology 1999, 80, 2555–2565. [Google Scholar] [CrossRef]
- Churchill, E.R.; Dytham, C.; Thom, M.D. Differing effects of age and starvation on reproductive performance in Drosophila melanogaster. Sci. Rep. 2019, 9, 2167. [Google Scholar] [CrossRef]
- Dixon, A.F.G.; Agarwala, B.K. Triangular fecundity function and ageing in ladybird beetles. Ecol. Entomol. 2002, 27, 433–440. [Google Scholar] [CrossRef]
- Ericsson, G.; Wallin, K.; Ball, J.P.; Broberg, M. Age-related reproductive effort and senescence in free-ranging moose, Alces alces. Ecology 2001, 82, 1613–1620. [Google Scholar] [CrossRef]
- Foster, S.P.; Howard, A.J. The effects of mating, age at mating, and plant stimuli, on the lifetime fecundity and fertility of the generalist herbivore Epiphyas postvittana. Entomol. Exp. Applicata 1999, 91, 287–295. [Google Scholar] [CrossRef]
- Langley, P.A.; Clutton-Brock, T.H. Does reproductive investment change with age in tsetse flies, Glossina morsitans morsitans (Diptera: Glossinidae)? Funct. Ecol. 1998, 12, 866–870. [Google Scholar] [CrossRef]
- Lee, P.C.; Fishlock, V.; Webber, C.E.; Moss, C.J. The reproductive advantages of a long life: Longevity and senescence in wild female African elephants. Behav. Ecol. Sociobiol. 2016, 70, 337–345. [Google Scholar] [CrossRef]
- Martin, J.G.; Festa-Bianchet, M. Age-independent and age-dependent decreases in reproduction of females. Ecol. Lett. 2011, 14, 576–581. [Google Scholar] [CrossRef]
- Moya-Larano, J. Senescence and food limitation in a slowly ageing spider. Funct. Ecol. 2002, 16, 734–741. [Google Scholar] [CrossRef]
- Nussey, D.H.; Kruuk, L.E.; Morris, A.; Clutton-Brock, T.H. Environmental conditions in early life influence ageing rates in a wild population of red deer. Curr. Biol. 2007, 17, R1000–R1001. [Google Scholar] [CrossRef]
- Rauser, C.L.; Tierney, J.J.; Gunion, S.M.; Covarrubias, G.M.; Mueller, L.D.; Rose, M.R. Evolution of late-life fecundity in Drosophila melanogaster. J. Evol. Biol. 2006, 19, 289–301. [Google Scholar] [CrossRef]
- Robertson, R.J.; Rendell, W.B. A long-term study of reproductive performance in tree swallows: The influence of age and senescence on output. J. Anim. Ecol. 2001, 70, 1014–1031. [Google Scholar] [CrossRef]
- Sparkman, A.M.; Blois, M.; Adams, J.; Waits, L.; Miller, D.A.; Murray, D.L. Evidence for sex-specific reproductive senescence in monogamous cooperatively breeding red wolves. Behav. Ecol. Sociobiol. 2017, 71, 1–11. [Google Scholar] [CrossRef]
- Tasnin, M.S.; Kay, B.J.; Peek, T.; Merkel, K.; Clarke, A.R. Age-related changes in the reproductive potential of the Queensland fruit fly. J. Insect Physiol. 2021, 131, 104245. [Google Scholar] [CrossRef]
- Zhao, R.; Xuan, Y.; Li, X.; Xi, R. Age-related changes of germline stem cell activity, niche signaling activity and egg production in Drosophila. Aging Cell 2008, 7, 344–354. [Google Scholar] [CrossRef]
- DelGiudice, G.D.; Lenarz, M.S.; Powell, M.C. Age-specific fertility and fecundity in northern free-ranging white-tailed deer: Evidence for reproductive senescence? J. Mammal. 2007, 88, 427–435. [Google Scholar] [CrossRef]
- Hayward, A.D.; Wilson, A.J.; Pilkington, J.G.; Clutton-Brock, T.H.; Pemberton, J.M.; Kruuk, L.E. Reproductive senescence in female Soay sheep: Variation across traits and contributions of individual ageing and selective disappearance. Funct. Ecol. 2013, 27, 184–195. [Google Scholar] [CrossRef]
- Michalkova, V.; Benoit, J.B.; Attardo, G.M.; Medlock, J.; Aksoy, S. Amelioration of reproduction-associated oxidative stress in a viviparous insect is critical to prevent reproductive senescence. PLoS ONE 2014, 9, e87554. [Google Scholar] [CrossRef]
- Parker, H.; Zedrosser, A.; Rosell, F. Age-specific reproduction in relation to body size and condition in female Eurasian beavers. J. Zool. 2017, 302, 236–243. [Google Scholar] [CrossRef]
- Sparkman, A.M.; Arnold, S.J.; Bronikowski, A.M. An empirical test of evolutionary theories for reproductive senescence and reproductive effort in the garter snake Thamnophis elegans. Proc. R. Soc. B Biol. Sci. 2007, 274, 943–950. [Google Scholar] [CrossRef]
- Descamps, S.; Boutin, S.; Berteaux, D.; Gaillard, J.M. Age-specific variation in survival, reproductive success and offspring quality in red squirrels: Evidence of senescence. Oikos 2008, 117, 1406–1416. [Google Scholar] [CrossRef]
- Fox, C.W.; Bush, M.L.; Wallin, W.G. Maternal age affects offspring lifespan of the seed beetle, Callosobruchus maculatus. Funct. Ecol. 2003, 17, 811–820. [Google Scholar] [CrossRef]
- Halle, S.; Nowizki, A.; Scharf, I. The consequences of parental age for development, body mass and resistance to stress in the red flour beetle. Biol. J. Linn. Soc. 2015, 115, 305–314. [Google Scholar] [CrossRef]
- Ivimey-Cook, E.; Moorad, J. Disentangling pre-and postnatal maternal age effects on offspring performance in an insect with elaborate maternal care. Am. Nat. 2018, 192, 564–576. [Google Scholar] [CrossRef]
- Karniski, C.; Krzyszczyk, E.; Mann, J. Senescence impacts reproduction and maternal investment in bottlenose dolphins. Proc. R. Soc. B Biol. Sci. 2018, 285, 20181123. [Google Scholar] [CrossRef]
- Kern, S.; Ackermann, M.; Stearns, S.C.; Kawecki, T.J. Decline in offspring viability as a manifestation of aging in Drosophila melanogaster. Evolution 2001, 55, 1822–1831. [Google Scholar]
- Newton, I.; Rothery, P. Senescence and reproductive value in sparrowhawks. Ecology 1997, 78, 1000–1008. [Google Scholar] [CrossRef]
- Srivastava, S. Age-specific mating and reproductive senescence in the seven-spotted ladybird, Coccinella septempunctata. J. Appl. Entomol. 2004, 128, 452–458. [Google Scholar] [CrossRef]
- Lord, J.S.; Leyland, R.; Haines, L.R.; Barreaux, A.M.; Bonsall, M.B.; Torr, S.J.; English, S. Effects of maternal age and stress on offspring quality in a viviparous fly. Ecol. Lett. 2021, 24, 2113–2122. [Google Scholar] [CrossRef]
- Berman, M.; Gaillard, J.M.; Weimerskirch, H. Contrasted patterns of age-specific reproduction in long-lived seabirds. Proc. R. Soc. B Biol. Sci. 2009, 276, 375–382. [Google Scholar] [CrossRef]
- Fay, R.; Schaub, M.; Border, J.A.; Henderson, I.G.; Fahl, G.; Feulner, J.; Horch, P.; Müller, M.; Rebstock, H.; Shitikov, D.; et al. Evidence for senescence in survival but not in reproduction in a short-lived passerine. Ecol. Evol. 2020, 10, 5383–5390. [Google Scholar] [CrossRef]
- Richards, K.W. Ovarian development in the alfalfa leafcutter bee, Megachile rotundata. J. Apic. Res. 1994, 33, 199–203. [Google Scholar] [CrossRef]
- Andux, S.; Ellis, R.E. Apoptosis maintains oocyte quality in aging Caenorhabditis elegans females. PLoS Genet. 2008, 4, e1000295. [Google Scholar] [CrossRef] [PubMed]
- Takeo, S.; Kimura, K.; Shirasuna, K.; Kuwayama, T.; Iwata, H. Age-associated deterioration in follicular fluid induces a decline in bovine oocyte quality. Reprod. Fertil. Dev. 2017, 29, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Crosier, A.E.; Comizzoli, P.; Baker, T.; Davidson, A.; Munson, L.; Howard, J.; Marker, L.L.; Wildt, D.E. Increasing age influences uterine integrity, but not ovarian function or oocyte quality, in the cheetah (Acinonyx jubatus). Biol. Reprod. 2011, 85, 243–253. [Google Scholar] [CrossRef]
- Bosch, J.; Vicens, N. Sex allocation in the solitary bee Osmia cornuta: Do females behave in agreement with Fisher’s theory? Behav. Ecol. Sociobiol. 2005, 59, 124–132. [Google Scholar] [CrossRef]
- Sugiura, N.; Maeta, Y. Parental investment and offspring sex ratio in a solitary mason bee, Osmia cornifrons (Radoszkowski) (Hymenoptera, Megachilidae). Jpn. J. Ent. 1989, 57, 861–875. [Google Scholar]
- Detoni, M.; Johnson, S.L.; Adams, C.I.; Bengston, S.; Jandt, J.M. Older, but not wiser: Social wasp colony defensive behavior decreases with time, not experience. Insectes Sociaux 2023, 70, 81–96. [Google Scholar] [CrossRef]
- Sugiura, N. Parental investment and offspring sex ratio in a solitary bee, Anthidium septemspinosum Lepeletier (Hymenoptera: Megachilidae). J. Ethol. 1994, 12, 131–139. [Google Scholar] [CrossRef]
- Frohlich, D.R.; Tepedino, V.J. Sex ratio, parental investment, and interparent variability in nesting success in a solitary bee. Evolution 1986, 40, 142–151. [Google Scholar] [CrossRef]
- Cotter, S.C.; Ward, R.J.; Kilner, R.M. Age-specific reproductive investment in female burying beetles: Independent effects of state and risk of death. Funct. Ecol. 2011, 25, 652–660. [Google Scholar] [CrossRef]
- Ortega, S.; Sánchez-Macouzet, O.; Urrutia, A.; Rodríguez, C.; Drummond, H. Age-related parental care in a long-lived bird: Implications for offspring development. Behav. Ecol. Sociobiol. 2017, 71, 132. [Google Scholar] [CrossRef]
- Zöttl, M.; Vullioud, P.; Goddard, K.; Torrents-Ticó, M.; Gaynor, D.; Bennett, N.C.; Clutton-Brock, T. Allo-parental care in Damaraland mole-rats is female biased and age dependent, though independent of testosterone levels. Physiol. Behav. 2018, 193, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Frias, B.E.D.; Barbosa, C.D.; Lourenço, A.P. Pollen nutrition in honey bees (Apis mellifera): Impact on adult health. Apidologie 2016, 47, 15–25. [Google Scholar] [CrossRef]
- Redmond, L.J.; Murphy, M.T.; Dolan, A.C.; Sexton, K. Parental investment theory and nest defense by Eastern Kingbirds. Wilson J. Ornithol. 2009, 121, 1–11. [Google Scholar] [CrossRef]
- Broussard, D.R.; Risch, T.S.; Dobson, F.S.; Murie, J.O. Senescence and age-related reproduction of female Columbian ground squirrels. J. Anim. Ecol. 2003, 72, 212–219. [Google Scholar] [CrossRef]
- Maeta, Y.; Kitamura, T. On the number of eggs laid by one individual of females in the alfalfa leaf-cutting bee, Megachile (Eutricharaea) rotundata (Fabricius) (Hymenoptera, Megachilidae). Chugoku Kontyu 2005, 19, 39–43. [Google Scholar]
- Hassall, C.; Sherratt, T.N.; Watts, P.C.; Thompson, D.J. Live fast, die old: No evidence of reproductive senescence or costs of mating in a damselfly (O donata: Zygoptera). J. Anim. Ecol. 2015, 84, 1542–1554. [Google Scholar] [CrossRef]
- Jones, T.M.; Balmford, A.; Quinnell, R.J. Adaptive female choice for middle–aged mates in a lekking sandfly. Proceedings of the Royal Society of London. Ser. B Biol. Sci. 2000, 267, 681–686. [Google Scholar] [CrossRef]
- O’Hanlon, J.C.; Wignall, A.E.; Herberstein, M.E. Short and fast vs long and slow: Age changes courtship in male orb-web spiders (Argiope keyserlingi). Sci. Nat. 2018, 105, 3. [Google Scholar] [CrossRef]
- Papanastasiou, S.A.; Diamantidis, A.D.; Nakas, C.T.; Carey, J.R.; Papadopoulos, N.T. Dual reproductive cost of aging in male medflies: Dramatic decrease in mating competitiveness and gradual reduction in mating performance. J. Insect Physiol. 2011, 57, 1368–1374. [Google Scholar] [CrossRef]
- Rivera-Gutierrez, H.F.; Pinxten, R.; Eens, M. Tuning and fading voices in songbirds: Age-dependent changes in two acoustic traits across the life span. Anim. Behav. 2012, 83, 1279–1283. [Google Scholar] [CrossRef]
- Rodríguez-Muñoz, R.; Hopwood, P.; Fisher, D.; Skicko, I.; Tucker, R.; Woodcock, K.; Slate, J.; Walling, C.; Tregenza, T. Older males attract more females but get fewer matings in a wild field cricket. Anim. Behav. 2019, 153, 1–14. [Google Scholar] [CrossRef]
- Gasparini, C.; Marino, I.A.M.; Boschetto, C.; Pilastro, A. Effect of male age on sperm traits and sperm competition success in the guppy (Poecilia reticulata). J. Evol. Biol. 2010, 23, 124–135. [Google Scholar] [CrossRef]
- Lucio, R.A.; Tlachi-López, J.L.; Eguibar, J.R.; Ågmo, A. Sperm count and sperm motility decrease in old rats. Physiol. Behav. 2013, 110, 73–79. [Google Scholar] [CrossRef]
- Møller, A.P.; Mousseau, T.A.; Rudolfsen, G.; Balbontin, J.; Marzal, A.; Hermosell, I.; De Lope, F. Senescent sperm performance in old male birds. J. Evol. Biol. 2009, 22, 334–344. [Google Scholar] [CrossRef]
- Vega-Trejo, R.; Fox, R.J.; Iglesias-Carrasco, M.; Head, M.L.; Jennions, M.D. The effects of male age, sperm age and mating history on ejaculate senescence. Funct. Ecol. 2019, 33, 1267–1279. [Google Scholar] [CrossRef]
- Kanuga, M.K.; Benner, M.J.; Doble, J.A.; Wilson-Leedy, J.G.; Robison, B.D.; Ingermann, R.L. Effect of aging on male reproduction in zebrafish (Danio rerio). J. Exp. Zool. Part A Ecol. Genet. Physiol. 2011, 315, 156–161. [Google Scholar] [CrossRef]
- Kervinen, M.; Lebigre, C.; Alatalo, R.V.; Siitari, H.; Soulsbury, C.D. Life-history differences in age-dependent expressions of multiple ornaments and behaviors in a lekking bird. Am. Nat. 2015, 185, 13–27. [Google Scholar] [CrossRef]
- Sasson, D.A.; Johnson, S.L.; Brockmann, H.J. The role of age on sperm traits in the American horseshoe crab, Limulus polyphemus. Anim. Behav. 2012, 84, 975–981. [Google Scholar] [CrossRef]
- Casselman, S.J.; Montgomerie, R. Sperm traits in relation to male quality in colonial spawning bluegill. J. Fish Biol. 2004, 64, 1700–1711. [Google Scholar] [CrossRef]
- Liley, N.R.; Tamkee, P.; Tsai, R.; Hoysak, D.J. Fertilization dynamics in rainbow trout (Oncorhynchus mykiss): Effect of male age, social experience, and sperm concentration and motility on in vitro fertilization. Can. J. Fish. Aquat. Sci. 2002, 59, 144–152. [Google Scholar] [CrossRef]
- Malawey, A.S.; Zhang, H.; McGuane, A.S.; Walsh, E.M.; Rusch, T.W.; Hjelmen, C.E.; Delclos, P.J.; Rangel, J.; Zheng, L.; Cai, M.; et al. Interaction of age and temperature on heat shock protein expression, sperm count, and sperm viability of the adult black soldier fly (Diptera: Stratiomyidae). J. Insects Food Feed. 2021, 7, 21–33. [Google Scholar] [CrossRef]
- Metz, B.N.; Tarpy, D.R. Reproductive senescence in drones of the honey bee (Apis mellifera). Insects 2019, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Vazquez, B.P.; Rosas, C.; Racotta, I.S. Sperm quality in relation to age and weight of white shrimp Litopenaeus vannamei. Aquaculture 2003, 228, 141–151. [Google Scholar] [CrossRef]
- Velando, A.; Noguera, J.C.; Drummond, H.; Torres, R. Senescent males carry premutagenic lesions in sperm. J. Evol. Biol. 2011, 24, 693–697. [Google Scholar] [CrossRef]
- Locke, S.J.; Peng, Y.S. The effects of drone age, semen storage and contamination on semen quality in the honey bee (Apis mellifera). Physiol. Entomol. 1993, 18, 144–148. [Google Scholar] [CrossRef]
- Stürup, M.; Baer-Imhoof, B.; Nash, D.R.; Boomsma, J.J.; Baer, B. When every sperm counts: Factors affecting male fertility in the honeybee Apis mellifera. Behav. Ecol. 2013, 24, 1192–1198. [Google Scholar] [CrossRef]
- Curren, L.J.; Wedele, M.L.; Holekamp, K.E. Ejaculate quality in spotted hyenas: Intraspecific variation in relation to life-history traits. J. Mammal. 2013, 94, 90–99. [Google Scholar] [CrossRef]
- Bonduriansky, R.; Brassil, C.E. Rapid and costly ageing in wild male flies. Nature 2002, 420, 377. [Google Scholar] [CrossRef]
- De Luca, P.A.; Cocroft, R.B. The influence of age on male mate-searching behaviour in thornbug treehoppers. Ethology 2011, 117, 440–450. [Google Scholar] [CrossRef]
- Mayer, M.S.; Brazzel, J.R. The mating behavior of the boll weevil, Anthonomus grandis. J. Econ. Entomol. 1963, 56, 605–609. [Google Scholar] [CrossRef]
- Pitts-Singer, T.L.; James, R.R. Past and present management of alfalfa bees. Bee Pollinat. Agric. Ecosyst. 2008, 9, 105–123. [Google Scholar]
- Royauté, R.; Wilson, E.S.; Helm, B.R.; Mallinger, R.E.; Prasifka, J.; Greenlee, K.J.; Bowsher, J.H. Phenotypic integration in an extended phenotype: Among-individual variation in nest-building traits of the alfalfa leafcutting bee (Megachile rotundata). J. Evol. Biol. 2018, 31, 944–956. [Google Scholar] [CrossRef]
- Pitts-Singer, T.L.; Cane, J.H. The alfalfa leafcutting bee, Megachile rotundata: The world’s most intensively managed solitary bee. Annu. Rev. Entomol. 2011, 56, 221–237. [Google Scholar] [CrossRef]
- Klostermeyer, E.C.; Gerber, H.S. Nesting behavior of Megachile rotundata (Hymenoptera: Megachilidae) monitored with an event recorder. Ann. Entomol. Soc. Am. 1969, 62, 1321–1325. [Google Scholar] [CrossRef]
- Klostermeyer, E.C.; Mech, S.J., Jr.; Rasmussen, W.B. Sex and weight of Megachile rotundata (Hymenoptera: Megachilidae) progeny associated with provision weights. J. Kans. Entomol. Soc. 1973, 46, 536–548. [Google Scholar]
- Stephen, W.P.; Toechio, P.F. Biological notes on the leaf-cutter bee, Megachile (Eutricharaea) rotundata (Fabricius) (Hymenoptera: Mega-chilidae). Pan-Pac. Entomol. 1961, 37, 85–94. [Google Scholar]
- Rossi, B.H.; Nonacs, P.; Pitts-Singer, T.L. Sexual harassment by males reduces female fecundity in the alfalfa leafcutting bee, Megachile rotundata. Anim. Behav. 2010, 79, 165–171. [Google Scholar] [CrossRef]
- Peterson, J.H.; Roitberg, B.D.; Peterson, J.H. Impacts of flight distance on sex ratio and resource allocation to offspring in the leafcutter bee, Megachile rotundata. Behav. Ecol. Sociobiol. 2006, 59, 589–596. [Google Scholar] [CrossRef]
- Pithan, J.B.; Rinehart, J.; Greenlee, K.; López-Martínez, G. Effects of age on oxidative stress and locomotion in the pollinator, Megachile rotundata. J. Insect Physiol. 2024, 157, 104666. [Google Scholar] [CrossRef]
- Rosenheim, J.A.; Nonacs, P.; Mangel, M. Sex ratios and multifaceted parental investment. Am. Nat. 1996, 148, 501–535. [Google Scholar] [CrossRef]
- Zajitschek, F.; Zajitschek, S.; Bonduriansky, R. Senescence in wild insects: Key questions and challenges. Funct. Ecol. 2020, 34, 26–37. [Google Scholar] [CrossRef]
- Fisher, D.N.; David, M.; Tregenza, T.; Rodríguez-Muñoz, R. Dynamics of among-individual behavioral variation over adult lifespan in a wild insect. Behav. Ecol. 2015, 26, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Muñoz, R.; Boonekamp, J.J.; Liu, X.P.; Skicko, I.; Haugland Pedersen, S.; Fisher, D.N.; Hopwood, P.; Tregenza, T. Comparing individual and population measures of senescence across 10 years in a wild insect population. Evolution 2019, 73, 293–302. [Google Scholar] [CrossRef]
- Harshman, L.G.; Zera, A.J. The cost of reproduction: The devil in the details. Trends Ecol. Evol. 2007, 22, 80–86. [Google Scholar] [CrossRef]
- Müller, A.; Diener, S.; Schnyder, S.; Stutz, K.; Sedivy, C.; Dorn, S. Quantitative pollen requirements of solitary bees: Implications for bee conservation and the evolution of bee–flower relationships. Biol. Conserv. 2006, 130, 604–615. [Google Scholar] [CrossRef]
- Romero, G.Q.; Antiqueira, P.A.; Koricheva, J. A meta-analysis of predation risk effects on pollinator behaviour. PLoS ONE 2011, 6, e20689. [Google Scholar] [CrossRef]
- Saleh, N.W.; Ramírez, S.R. Sociality emerges from solitary behaviours and reproductive plasticity in the orchid bee Euglossa dilemma. Proc. R. Soc. B 2019, 286, 20190588. [Google Scholar] [CrossRef]
- Strohm, E.; Daniels, H.; Warmers, C.; Stoll, C. Nest provisioning and a possible cost of reproduction in the megachilid bee Osmia rufa studied by a new observation method. Ethol. Ecol. Evol. 2002, 14, 255–268. [Google Scholar] [CrossRef]
- O’Neill, K.M.; Delphia, C.M.; O’Neill, R.P. Oocyte size, egg index, and body lipid content in relation to body size in the solitary bee Megachile rotundata. PeerJ 2014, 2, e314. [Google Scholar] [CrossRef]
- Michener, C.D. The Bees of the World; Johns Hopkins University Press: Baltimore, MD, USA, 2000. [Google Scholar]
- Roulston, T.A.H.; Cane, J.H. Pollen nutritional content and digestibility for animals. Plant Syst. Evol. 2000, 222, 187–209. [Google Scholar] [CrossRef]
- Vaudo, A.D.; Tooker, J.F.; Grozinger, C.M.; Patch, H.M. Bee nutrition and floral resource restoration. Curr. Opin. Insect science 2015, 10, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Cane, J.H. Adult pollen diet essential for egg maturation by a solitary Osmia bee. J. Insect Physiol. 2016, 95, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, C.S.; Ikemoto, M.; Nikkeshi, A.; Kanbe, Y.; Mitsuhata, M.; Yokoi, T. Ovarian development related to pollen feeding in workers of the bumblebee Bombus ignitus (Hymenoptera: Apidae). Appl. Entomol. Zool. 2019, 54, 85–89. [Google Scholar] [CrossRef]
- Korman, A.K.; Oseto, C.Y. Structure of the female reproductive system and maturation of oöcytes in Smicronyx fulvus (Coleoptera: Curculionidae). Ann. Entomol. Soc. Am. 1989, 82, 94–100. [Google Scholar] [CrossRef]
- Maeta, Y.; Kurihara, M. Anatomical and histological studies on the oogenesis and oosorption of terminal oocytes within the genus Osmia. Kontyu 1971, 39, 138–158. [Google Scholar]
- Sihag, R.C. Why does the alfalfa pollinating sub-tropical bee Megachile flavipes make false nesting? Zool. Anz. 1986, 217, 228–233. [Google Scholar]
- Osgood, C.E. Foraging and nesting behavior of the leaf-cutter bee Megachile rotundata (Fabricius). Master’s Thesis, Oregon State University, Corvallis, OR, USA, 1964. [Google Scholar]
- Bosch, J.; Vicens, N. Relationship between body size, provisioning rate, longevity and reproductive success in females of the solitary bee Osmia cornuta. Behav. Ecol. Sociobiol. 2006, 60, 26–33. [Google Scholar] [CrossRef]
- Danforth, B.N.; Minckley, R.L.; Neff, J.L. The Solitary Bees: Biology, Evolution, Conservation; Princeton University Press: Princeton, NJ, USA, 2019. [Google Scholar]
- Chaturvedi, D.; Prabhakar, S.; Aggarwal, A.; Atreya, K.B.; VijayRaghavan, K. Adult Drosophila muscle morphometry through microCT reveals dynamics during ageing. Open Biol. 2019, 9, 190087. [Google Scholar] [CrossRef]
- Johnson, B.G., Jr.; Rowley, W.A. Age-related ultrastructural changes in the flight muscle of the mosquito, Culex tarsalis. J. Insect Physiol. 1972, 18, 2375–2389. [Google Scholar] [CrossRef]
- Anholt, B.R.; Marden, J.H.; Jenkins, D.M. Patterns of mass gain and sexual dimorphism in adult dragonflies (Insecta: Odonata). Can. J. Zool. 1991, 69, 1156–1163. [Google Scholar] [CrossRef]
- Marden, J.H. Bodybuilding dragonflies: Costs and benefits of maximizing flight muscle. Physiol. Zool. 1989, 62, 505–521. [Google Scholar] [CrossRef]
- Wone, B.W.; Pathak, J.; Davidowitz, G. Flight duration and flight muscle ultrastructure of unfed hawk moths. Arthropod Struct. Dev. 2018, 47, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Schippers, M.P.; Dukas, R.; Smith, R.W.; Wang, J.; Smolen, K.; McClelland, G.B. Lifetime performance in foraging honeybees: Behaviour and physiology. J. Exp. Biol. 2006, 209, 3828–3836. [Google Scholar] [CrossRef]
- Spendal, R.C.; Cane, J.H. Multiple daily brood cells define the fecundity of Osmia lignaria bees in a semi-natural setting. Apidologie 2022, 53, 54. [Google Scholar] [CrossRef]
- Torchio, P.F. In-nest biologies and development of immature stages of three Osmia species (Hymenoptera: Megachilidae). Ann. Entomol. Soc. Am. 1989, 82, 599–615. [Google Scholar] [CrossRef]
- Maeta, Y. Comparative studies on the biology of the bees of the genus Osmia of Japan, with special reference to their managements for pollination of crops (Hymenoptera: Megachilidae). Bull. Tohoku Natl. Agric. Exp. Stn. 1978, 57, 1–221. [Google Scholar]
- Neff, J.L. Components of nest provisioning behavior in solitary bees (Hymenoptera: Apoidea). Apidologie 2008, 39, 30–45. [Google Scholar] [CrossRef]
- Dukas, R. Life history of learning: Performance curves of honeybees in settings that minimize the role of learning. Anim. Behav. 2008, 75, 1125–1130. [Google Scholar] [CrossRef]
- Dukas, R. Life history of learning: Performance curves of honeybees in the wild. Ethology 2008, 114, 1195–1200. [Google Scholar] [CrossRef]
- Dukas, R.; Real, L.A. Learning foraging tasks by bees: A comparison between social and solitary species. Anim. Behav. 1991, 42, 269–276. [Google Scholar] [CrossRef]
- Peat, J.; Goulson, D. Effects of experience and weather on foraging rate and pollen versus nectar collection in the bumblebee, Bombus terrestris. Behav. Ecol. Sociobiol. 2005, 58, 152–156. [Google Scholar] [CrossRef]
- Dukas, R. Evolutionary biology of insect learning. Annu. Rev. Entomol. 2008, 53, 145–160. [Google Scholar] [CrossRef]
- Gathmann, A.; Tscharntke, T. Foraging ranges of solitary bees. J. Anim. Ecol. 2002, 71, 757–764. [Google Scholar] [CrossRef]
- Free, J.B. Insect Pollination of Crops; Academic Press: London, UK; New York, NY, USA, 1970. [Google Scholar]
- Polatto, L.P.; Chaud-Netto, J.; Alves-Junior, V.V. Influence of abiotic factors and floral resource availability on daily foraging activity of bees: Influence of abiotic and biotic factors on bees. J. Insect Behav. 2014, 27, 593–612. [Google Scholar] [CrossRef]
- Torchio, P.F. Field experiments with the pollinator species, Osmia lignaria propinqua Cresson, in apple orchards: V (1979-1980), methods of introducing bees, nesting success, seed counts, fruit yields (Hymenoptera: Megachilidae). J. Kans. Entomol. Soc. 1985, 58, 448–464. [Google Scholar]
- Pitts-Singer, T.L.; Bosch, J. Nest establishment, pollination efficiency, and reproductive success of Megachile rotundata (Hymenoptera: Megachilidae) in relation to resource availability in field enclosures. Environ. Entomol. 2010, 39, 149–158. [Google Scholar] [CrossRef]
- Tepedino, V.J.; Parker, F.D. Alternation of sex ratio in a partially bivoltine bee, Megachile rotundata (Hymenoptera: Megachilidae). Ann. Entomol. Soc. Am. 1988, 81, 467–476. [Google Scholar] [CrossRef]
- Denlinger, D.L. Regulation of diapause. Annu. Rev. Entomol. 2002, 47, 93–122. [Google Scholar] [CrossRef]
- Denlinger, D.L. Insect Diapause; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Pitts-Singer, T.L. Photoperiod effect on Megachile rotundata (Hymenoptera: Megachilidae) female regarding diapause status of progeny: The importance of data scrutiny. Environ. Entomol. 2020, 49, 516–527. [Google Scholar] [CrossRef]
- Wilson, E.S.; Murphy, C.E.; Wong, C.; Rinehart, J.P.; Yocum, G.D.; Bowsher, J.H. Environmental impacts on diapause and survival of the alfalfa leafcutting bee, Megachile rotundata. PLoS ONE 2021, 16, e0254651. [Google Scholar] [CrossRef]




| Female Reproductive Aspects | Decreased | Remain Unchanged | Increased |
|---|---|---|---|
| Fecundity | [3,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34] | [35,36,37,38] | [39] |
| Offspring survival | [21,30,37,39,40,41,42,43,44,45,46,47] | [36,46,48] | |
| Breeding success | [49] | [49,50] | |
| Oocyte quality | [11,51,52,53] | [54] | |
| Parental investment | [23,48,55,56,57,58,59,60,61,62] | [63,64,65] | |
| Mating willingness | [47] | [66,67] | |
| Male Reproductive Aspects | Decreased | Remain Unchanged | Increased |
| Courting performance | [21,68,69,70,71] | [72] | |
| Sperm motility | [73,74,75,76] | [77,78,79] | [80] |
| Sperm count | [74,79,81,82,83] | [77,84] | |
| Sperm viability/quality | [77,85,86,87] | [79,83,88] | [84] |
| Pre-copulatory male competition | [72,78] | ||
| Mating rates | [68,72,89,90] | [67] | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pithan, J.B.; Kohler, B.L.; Rajamohan, A.; Greenlee, K.J. Reproductive Senescence in the Pollinator, Megachile rotundata. Insects 2025, 16, 612. https://doi.org/10.3390/insects16060612
Pithan JB, Kohler BL, Rajamohan A, Greenlee KJ. Reproductive Senescence in the Pollinator, Megachile rotundata. Insects. 2025; 16(6):612. https://doi.org/10.3390/insects16060612
Chicago/Turabian StylePithan, Jacob B., Brooke L. Kohler, Arun Rajamohan, and Kendra J. Greenlee. 2025. "Reproductive Senescence in the Pollinator, Megachile rotundata" Insects 16, no. 6: 612. https://doi.org/10.3390/insects16060612
APA StylePithan, J. B., Kohler, B. L., Rajamohan, A., & Greenlee, K. J. (2025). Reproductive Senescence in the Pollinator, Megachile rotundata. Insects, 16(6), 612. https://doi.org/10.3390/insects16060612






