Future Range Shifts in Major Maize Insect Pests Suggest Their Increasing Impacts on Global Maize Production
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Area
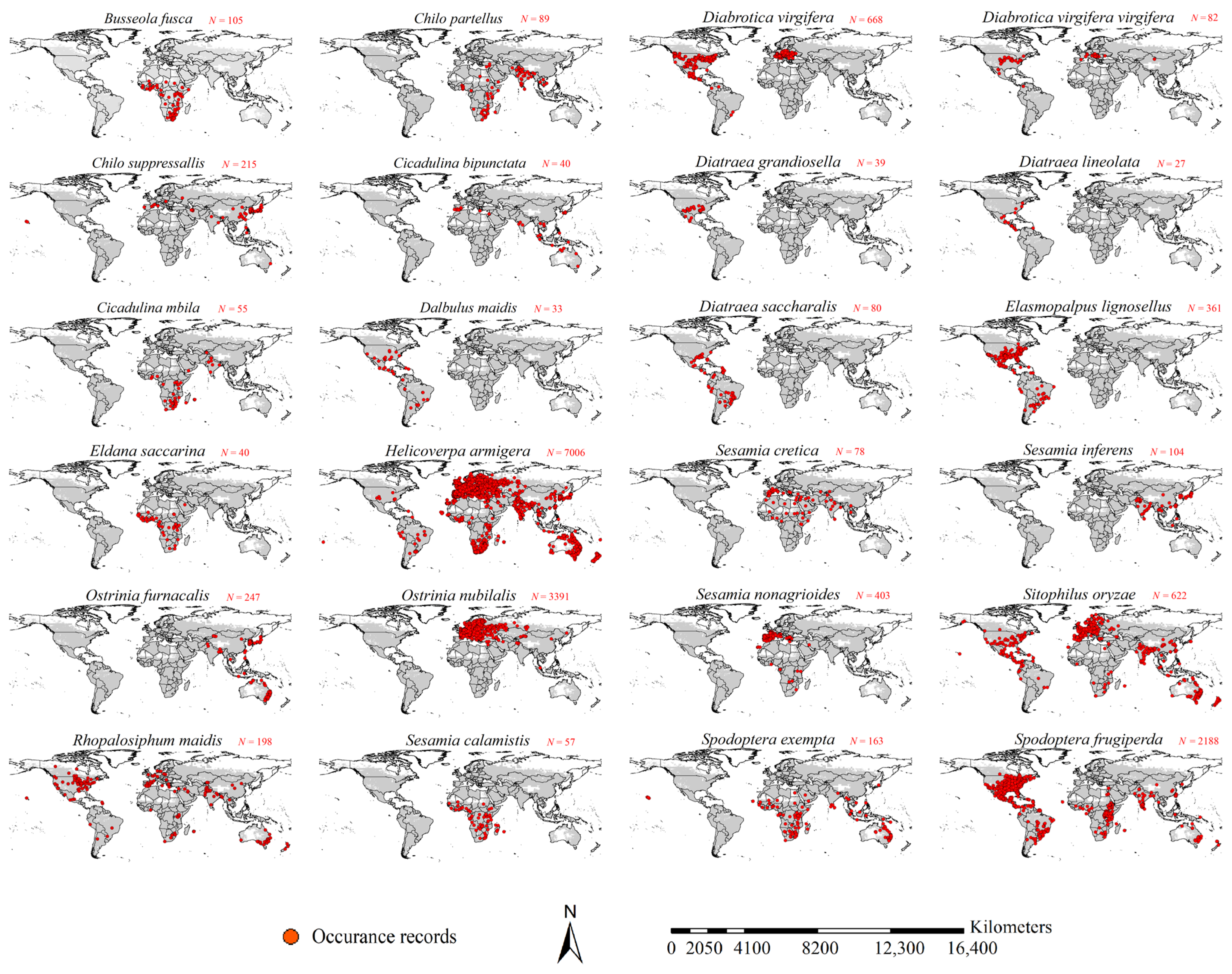
2.2. Occurrence Records
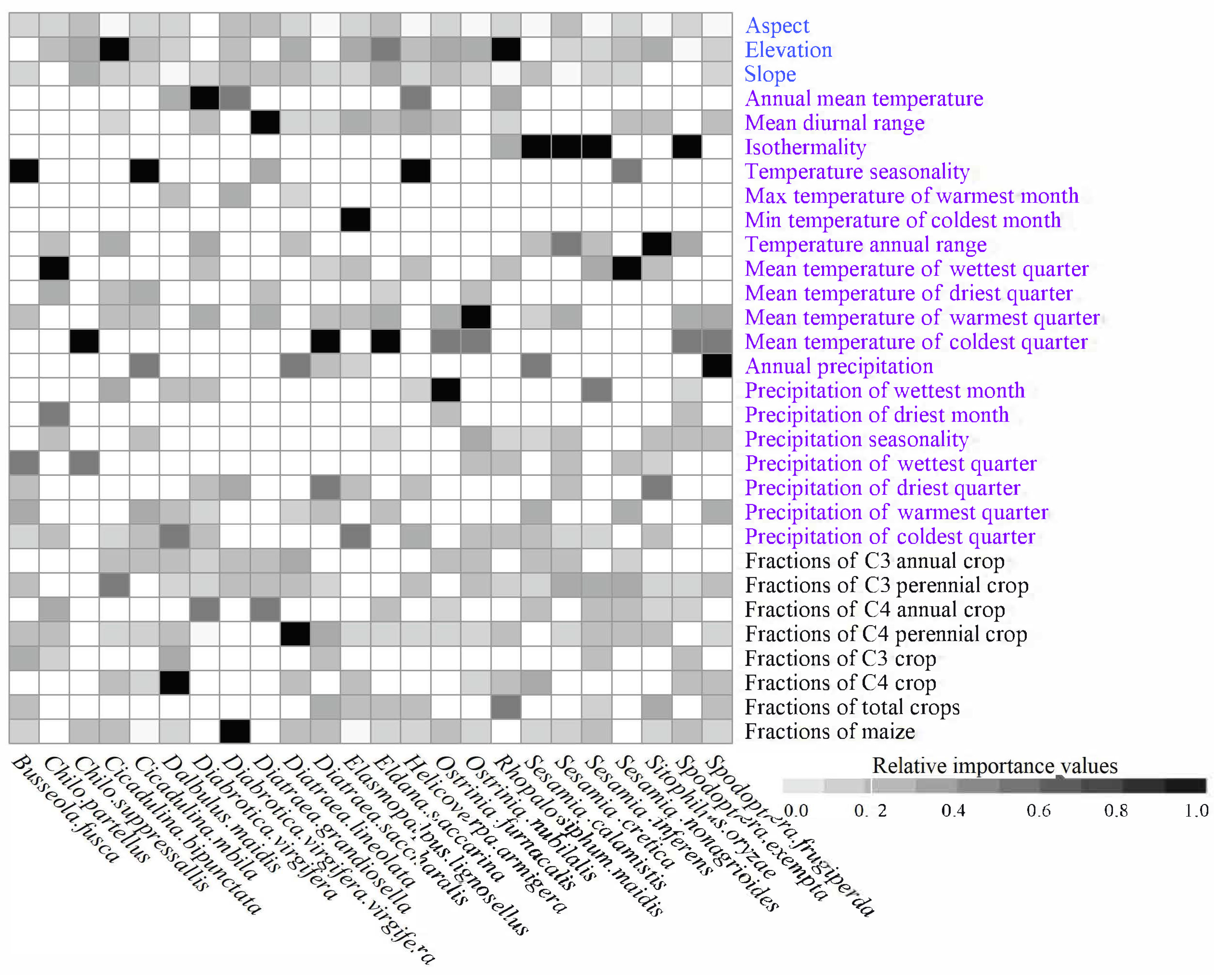
2.3. Selecting Predictors
2.4. Building Models
2.5. Calibrating Habitat Suitability and Ranges
2.6. Range Dynamic Investigation
3. Results
3.1. Reliability of the Models
3.2. Top Predictors in the Models
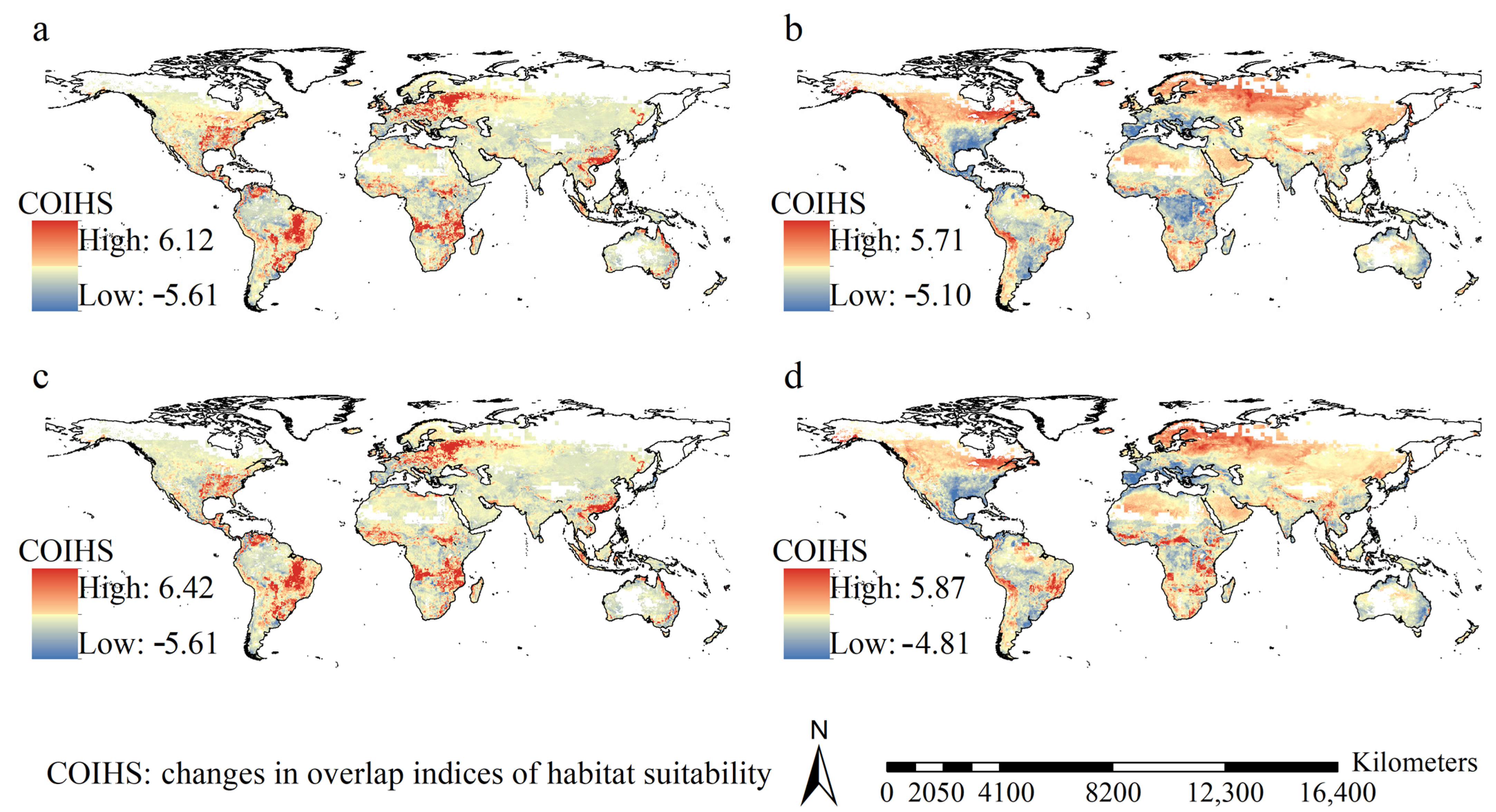
3.3. Dynamics of the Habitat Suitability
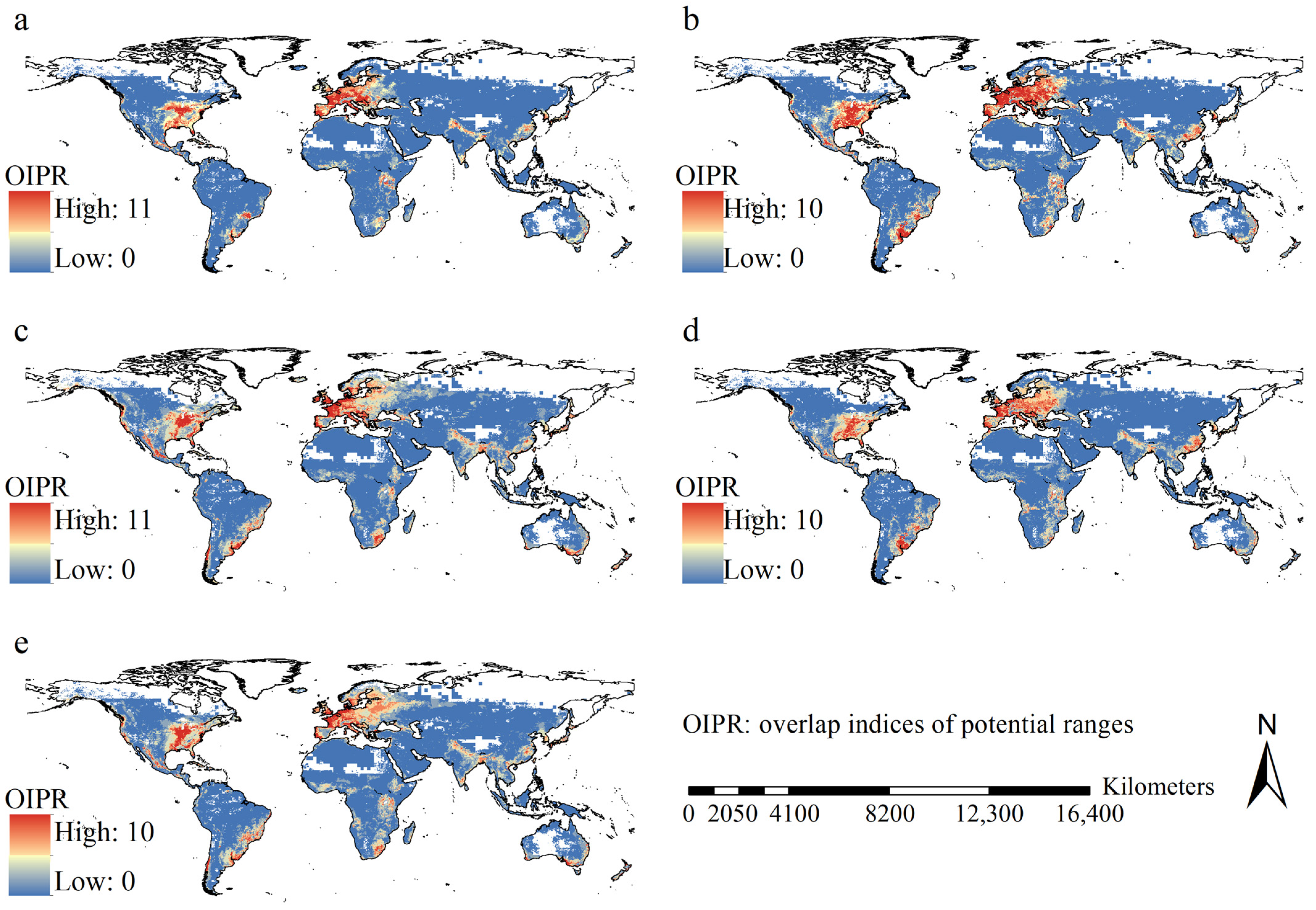
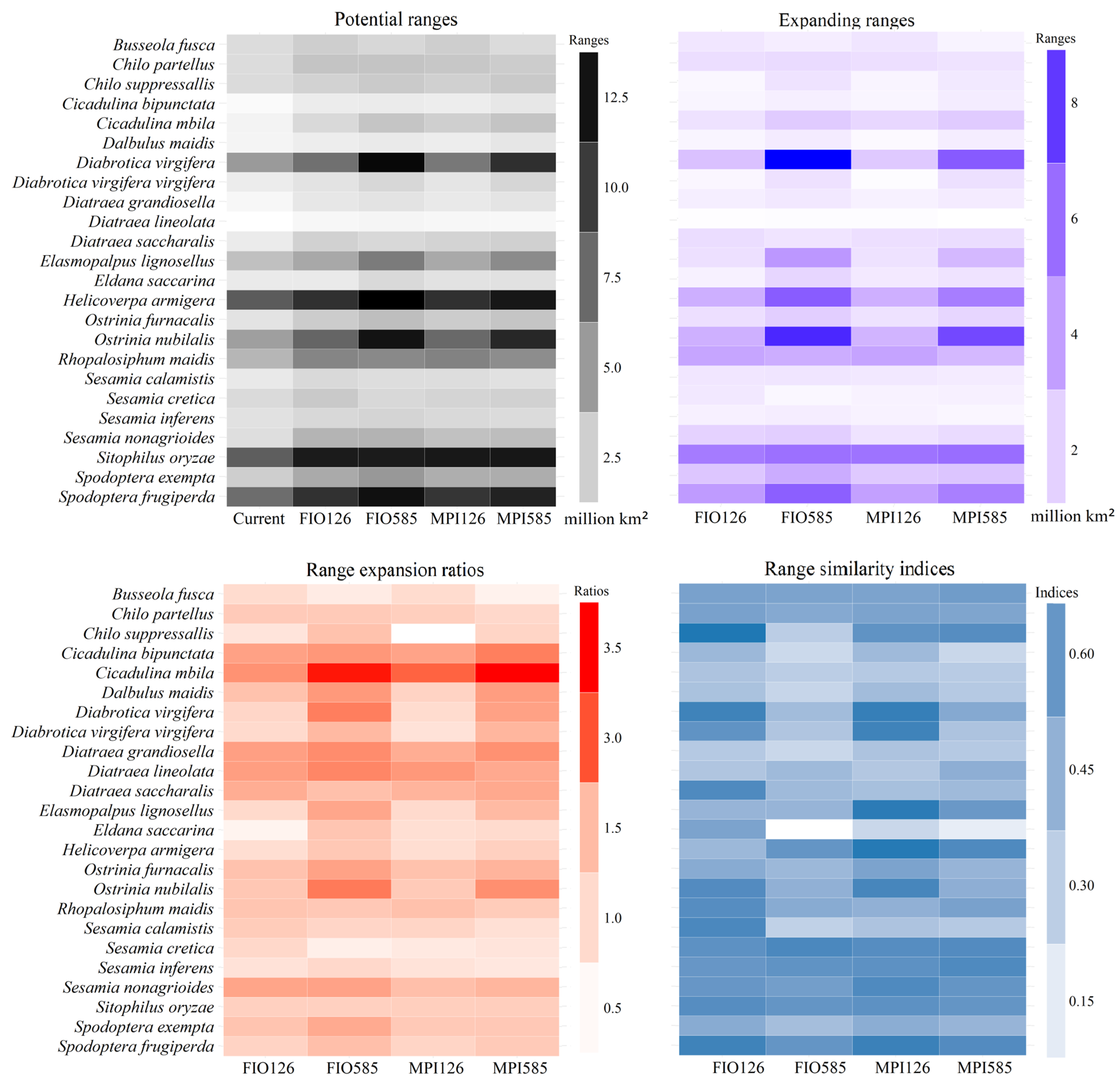
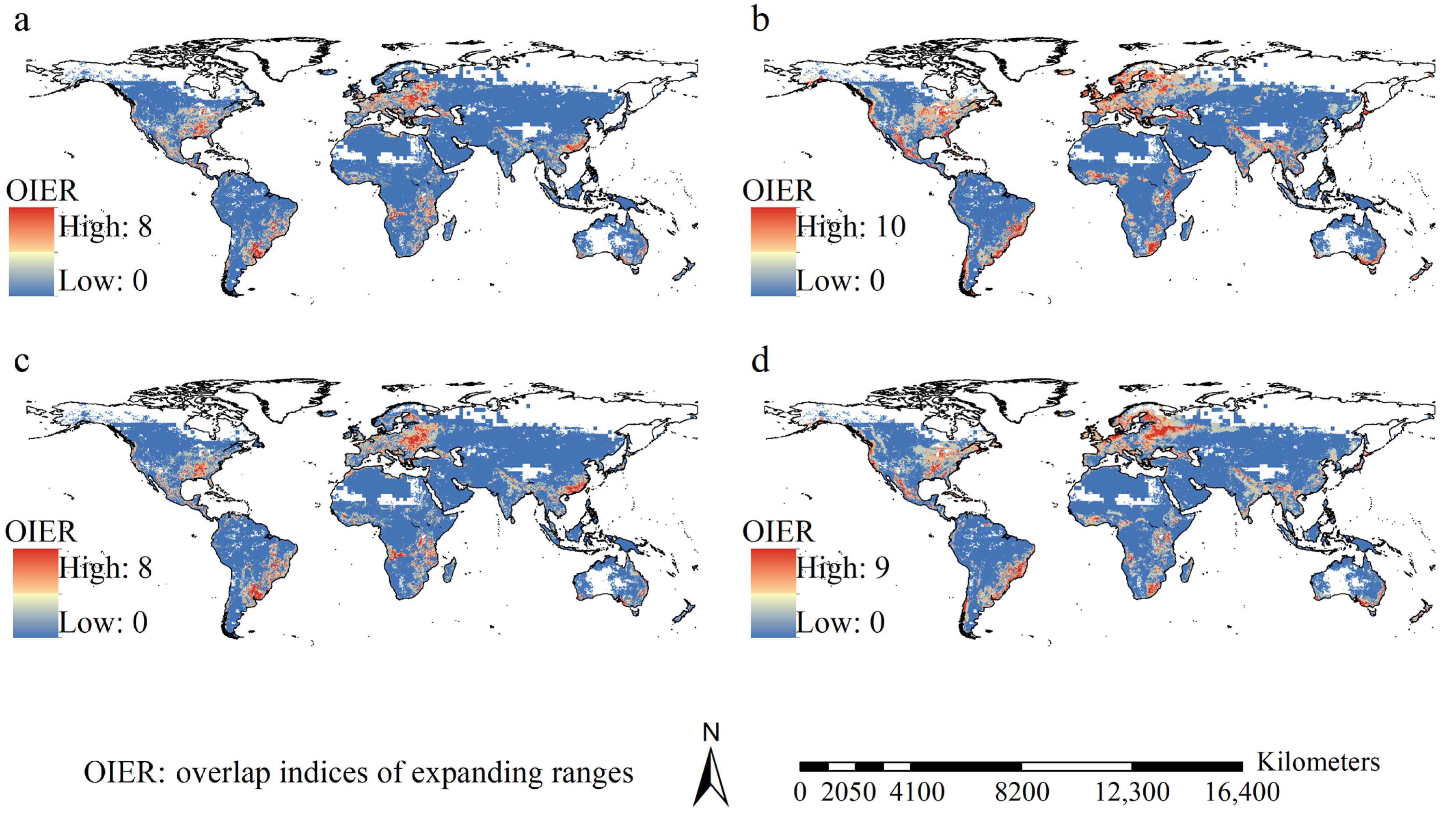
3.4. Potential Ranges and Their Dynamics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ranum, P.; Peña-Rosas, J.P.; Garcia-Casal, M.N. Global maize production, utilization, and consumption. Ann. N. Y. Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Paasche, C. The many, many uses for corn: How a simple crop became a cornerstone of the global economy. Fortune Mag. 2012, 15, 13–19. [Google Scholar]
- Diffenbaugh, N.S.; Hertel, T.W.; Scherer, M.; Verma, M. Response of corn markets to climate volatility and alternative energy futures. Nat. Clim. Chang. 2012, 2, 514–518. [Google Scholar] [CrossRef]
- Gustafson, D.I.; Jones, J.W.; Porter, C.H.; Hyman, G.; Edgerton, M.D.; Gocken, T.; Shryock, J.; Doane, M.; Budreski, K.; Stone, C.; et al. Climate adaptation imperatives: Untapped global maize yield opportunities. Int. J. Agric. Sustain. 2014, 12, 471–486. [Google Scholar] [CrossRef]
- Oerke, E.C.; Dehne, H.W.; Schönbeck, F.; Weber, A. Crop Production and Crop Protection; Elsevier: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Li, M.; Jin, Z.; Qi, Y.; Zhao, H.; Yang, N.; Guo, J.; Chen, B.; Xian, X.; Liu, W. Risk assessment of Spodoptera exempta against food security: Estimating the potential global overlapping areas of wheat, maize, and rice under climate change. Insects 2024, 15, 348. [Google Scholar] [CrossRef]
- Rajashekhar, M.; Rajashekar, B.; Reddy, T.P.; Manikyanahalli Chandrashekara, K.; Vanisree, K.; Ramakrishna, K.; Sunitha, V.; Shaila, O.; Sathyanarayana, E.; Shahanaz; et al. Evaluation of farmers-friendly IPM modules for the management of fall armyworm, Spodoptera frugiperda (J.E. Smith), in maize in the hot semiarid region of India. Sci. Rep. 2024, 14, 7118. [Google Scholar] [CrossRef]
- Diffenbaugh, N.S.; Krupke, C.H.; White, M.A.; Alexander, C.E. Global warming presents new challenges for maize pest management. Environ. Res. Lett. 2008, 3, 044007. [Google Scholar] [CrossRef]
- He, S.; Gong, Y.; Zheng, Z.; Luo, Z.; Tan, B.; Zhang, Y. Effects of rainfall intensities and slope gradients on nitrogen loss at the seedling stage of maize (Zea mays L.) in the purple soil regions of China. Int. J. Agric. Biol. Eng. 2022, 15, 142–148. [Google Scholar] [CrossRef]
- Chiriloaie Palade, A.; Gîdea, M.; Ciontu, V.M.; Georgescu, R.G. Maize insect pest management in a changing climate: A review. Sci. Pap. Ser. A Agron. 2024, 1, 67–74. [Google Scholar]
- Matsukura, K.; Yoshida, K.; Matsumura, M. Estimation of climatic factors relating to occurrence of the maize orange leafhopper, Cicadulina bipunctata. Popul. Ecol. 2012, 54, 397–403. [Google Scholar] [CrossRef]
- Senay, S.D.; Pardey, P.G.; Chai, Y.; Doughty, L.; Day, R. Fall armyworm from a maize multi-peril pest risk perspective. Front. Insect Sci. 2022, 2, 971396. [Google Scholar] [CrossRef] [PubMed]
- Santana, P.A.; Kumar, L.; Da Silva, R.S.; Picanço, M.C. Global geographic distribution of Tuta absoluta as affected by climate change. J. Pest Sci. 2019, 92, 1373–1385. [Google Scholar] [CrossRef]
- Mengesha, G.G.; Salo, S.K.; Fantaye, F.Y.; Mohammed, K.B. Geographic distribution, relative importance, and association of factors influencing fall armyworm [Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae)] on maize (Zea mays L.) in Southern Ethiopia. Int. J. Pest Manag. 2023, 69, 255–269. [Google Scholar] [CrossRef]
- Liu, T.; Wang, J.M.; Hu, X.K.; Feng, J.M. Land-use change drives present and future distributions of fall armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Sci. Total Environ. 2019, 706, 135872. [Google Scholar] [CrossRef]
- Da Cunha, T.G.; Veloso, R.V.S.; De Araújo, M.M.M.; Tavares, L.G.; Ribeiro, L.F.B.; Tormen, G.P.; Campos, D.S.; Picanço, M.C.; Lopes, E.A.; Pereira, R.R.; et al. The distribution of Dalbulus maidis (DeLong) (Hemiptera: Cicadellidae) and the incidence of Maize Rayado Fino Virus and Candidatus Phytoplasma asteris in corn succession planting systems. Hum. Exp. Toxicol. 2023, 79, 2325–2337. [Google Scholar] [CrossRef]
- Jin, Z.; Xian, X.; Zhao, H.; Li, M.; Guo, J.; Yang, N.; Wan, F.; Toepfer, S.; Liu, W. Better preparedness for invasive crop pests in changing climate: Assessing the risk of western corn rootworm (Coleoptera: Chrysomelidae) colonization in global maize-growing regions and future spread dynamics. New Plant Prot. 2024, 1, e12. [Google Scholar] [CrossRef]
- Chardon, N.I.; Cornwell, W.K.; Flint, L.E.; Flint, A.L.; Ackerly, D.D. Topographic, latitudinal and climatic distribution of Pinus coulteri: Geographic range limits are not at the edge of the climate envelope. Ecography 2015, 38, 590–601. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Sandel, B.; Blank, D.; Liu, Z.; Liu, X.; Yan, S. Climate and topography explain range sizes of terrestrial vertebrates. Nat. Clim. Chang. 2016, 6, 498–502. [Google Scholar] [CrossRef]
- Weintraub, S.R.; Brooks, P.D.; Bowen, G.J. Interactive effects of vegetation type and topographic position on nitrogen availability and loss in a temperate montane ecosystem. Ecosystems 2017, 20, 1073–1088. [Google Scholar] [CrossRef]
- Michalak, J.L.; Lawler, J.J.; Roberts, D.R.; Carroll, C. Distribution and protection of climatic refugia in North America. Conserv. Biol. 2018, 32, 1414–1425. [Google Scholar] [CrossRef]
- He, Y.; Wang, K.; Du, G.; Zhang, Q.; Li, B.; Zhao, L.; He, P.; Chen, B. Temporal and spatial distribution patterns of Spodoptera frugiperda in mountain maize fields in China. Insects 2022, 13, 938. [Google Scholar] [CrossRef] [PubMed]
- Erenstein, O.; Chamberlin, J.; Sonder, K. Estimating the global number and distribution of maize and wheat farms. Glob. Food Secur. 2021, 30, 100558. [Google Scholar] [CrossRef]
- Li, K.; Pan, J.; Xiong, W.; Ali, T. The impact of 1.5 °C and 2.0 °C global warming on global maize production and trade. Sci. Rep. 2022, 12, 17268. [Google Scholar] [CrossRef]
- Paudel Timilsena, B.; Niassy, S.; Kimathi, E.; Abdel-Rahman, E.M.; Seidl-Adams, I.; Wamalwa, M.; Tonnang, H.E.; Ekesi, S.; Hughes, D.P.; Rajotte, E.G.; et al. Potential distribution of fall armyworm in Africa and beyond, considering climate change and irrigation patterns. Sci. Rep. 2022, 12, 539. [Google Scholar] [CrossRef] [PubMed]
- Azerefegne, F.; Kitaw, D.; Asayehegne, B. Major insect pests of maize and their management: A review. In Enhancing the Contribution of Maize to Food Security in Ethiopia: Proceedings of the Second National Maize Workshop of Ethiopia, Addis Ababa, Ethiopia, 12–16 November 2001; Central Printing Press PLC: Addis Ababa, Ethiopia, 2002; pp. 89–96. [Google Scholar]
- Naz, F.; Hussain, M.; Din, M. Insect pests of maize and their losses. Asian J. Plant Sci. 2003, 2, 412–414. [Google Scholar] [CrossRef]
- Dhillon, M.K.; Kalia, V.K.; Gujar, G.T. Insect-pests and their management: Current status and future need of research in quality maize. In Maize: Nutrition Dynamics and Novel Uses; Springer India: New Delhi, India, 2013; pp. 95–103. [Google Scholar]
- Blanco, C.A.; Pellegaud, J.G.; Nava-Camberos, U.; Lugo-Barrera, D.; Vega-Aquino, P.; Coello, J.; Terán-Vargas, A.P.; Vargas-Camplis, J. Maize pests in Mexico and challenges for the adoption of integrated pest management programs. J. Integr. Pest Manag. 2014, 5, E1–E9. [Google Scholar] [CrossRef]
- Kumar, P.; Kaur, J.; Suby, S.B.; Sekhar, J.C.; Lakshmi, S.P. Pests of maize. In Pests and Their Management; Springer: Singapore, 2018; pp. 51–79. [Google Scholar]
- Lodos, N. Maize pests and their importance in Turkey. EPPO Bull. 1981, 11, 87–89. [Google Scholar] [CrossRef]
- Hutchison, W.D.; Cira, T.M. Economically important insect pests of maize. In Achieving Sustainable Cultivation of Maize; Burleigh Dodds Science Publishing: Cambridge, UK, 2017; Volume 2, pp. 263–292. [Google Scholar]
- Steffey, K.L.; Rice, M.E.; All, J.; Andow, D.A.; Gray, M.E.; Van Duy, J.W. Handbook of Corn Insects; Entomological Society of America: Lanham, MD, USA, 1999. [Google Scholar]
- Nie, P.; He, C.; Feng, J. Range dynamics of Anopheles mosquitoes in Africa suggest a significant increase in the malaria transmission risk. Ecol. Evol. 2024, 14, e70059. [Google Scholar] [CrossRef]
- Beck, J.; Böller, M.; Erhardt, A.; Schwanghart, W. Spatial bias in the GBIF database and its effect on modeling species’ geographic distributions. Ecol. Inform. 2014, 19, 10–15. [Google Scholar] [CrossRef]
- Cao, R.; Feng, J. Future range shifts suggest that the six-spined spruce bark beetle might pose a greater threat to Norway spruce in Europe than the eight-spined spruce bark beetle. Forests 2023, 14, 2048. [Google Scholar] [CrossRef]
- Brown, J.L. SDM toolbox: A python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Xu, Z.; Han, Y.; Guo, W. Evaluation of CMIP6 models toward dynamical downscaling over 14 CORDEX domains. Clim. Dyn. 2022, 62, 4475–4489. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, C.; Nie, P.; Feng, J.; Hu, X. Invasive pest and invasive host: Where might spotted-wing drosophila (Drosophila suzukii) and American black cherry (Prunus serotina) cross paths in Europe? Forests 2024, 15, 206. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Nie, P.; Feng, J. Niche and range shifts of Aedes aegypti and Ae. albopictus suggest that the latecomer shows a greater invasiveness. Insects 2023, 14, 810. [Google Scholar] [CrossRef]
- Liu, C.; Newell, G.; White, M. On the selection of thresholds for predicting species occurrence with presence-only data. Ecol. Evol. 2016, 6, 337–348. [Google Scholar] [CrossRef]
- Trnka, M.; Muška, F.; Semerádová, D.; Dubrovský, M.; Kocmánková, E.; Žalud, Z. European corn borer life stage model: Regional estimates of pest development and spatial distribution under present and future climate. Ecol. Model. 2007, 207, 61–84. [Google Scholar] [CrossRef]
- Kocmánková, E.; Trnka, M.; Žalud, Z.; Semerádová, D.; Dubrovský, M.; Muška, F.; Možný, M. Comparison of two mapping methods of potential distribution of pests under present and changed climate. Plant Prot. Sci. 2008, 44, 49–56. [Google Scholar] [CrossRef]
- Zacarias, D.A. Global bioclimatic suitability for the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), and potential co-occurrence with major host crops under climate change scenarios. Clim. Change 2020, 161, 555–566. [Google Scholar] [CrossRef]
- Leff, B.; Ramankutty, N.; Foley, J.A. Geographic distribution of major crops across the world. Glob. Biogeochem. Cycles 2004, 18, GB1009. [Google Scholar] [CrossRef]
- You, L.; Wood, S.; Wood-Sichra, U.; Wu, W. Generating global crop distribution maps: From census to grid. Agric. Syst. 2014, 127, 53–60. [Google Scholar] [CrossRef]
- Assefa, Y.; Conlong, D.E.; Van den Berg, J.; Moolman, J. The wider distribution of Eldana saccharina (Lepidoptera: Pyralidae) in South Africa and its potential risk to maize production. Proc. S. Afr. Sug. Technol. Ass. 2008, 81, 290–297. [Google Scholar]
- Feusthuber, E.; Mitter, H.; Schönhart, M.; Schmid, E. Integrated modelling of efficient crop management strategies in response to economic damage potentials of the western corn rootworm in Austria. Agric. Syst. 2017, 157, 93–106. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Miedaner, T.; Juroszek, P. Global warming and increasing maize cultivation demand comprehensive efforts in disease and insect resistance breeding in north-western Europe. Plant Pathol. 2021, 70, 1032–1046. [Google Scholar] [CrossRef]
- Overton, K.; Maino, J.L.; Day, R.; Umina, P.A.; Bett, B.; Carnovale, D.; Ekesi, S.; Meagher, R.; Reynolds, O.L. Global crop impacts, yield losses and action thresholds for fall armyworm (Spodoptera frugiperda): A review. Crop Prot. 2021, 145, 105641. [Google Scholar] [CrossRef]
- Kocmánková, E.; Trnka, M.; Eitzinger, J.; Formayer, H.; Dubrovský, M.; Semerádová, D.; Žalud, Z.; Juroch, J.; Možný, M. Estimating the impact of climate change on the occurrence of selected pests in the Central European region. Clim. Res. 2010, 44, 95–105. [Google Scholar] [CrossRef]
- Li, B.; Dopman, E.B.; Dong, Y.; Yang, Z. Forecasting habitat suitability and niche shifts of two global maize pests: Ostrinia furnacalis and Ostrinia nubilalis (Lepidoptera: Crambidae). Pest Manag. Sci. 2024, 80, 5286–5298. [Google Scholar] [CrossRef]
- Gillyboeuf, N.; Anglade, P.; Lavenseau, L.; Peypelut, L. Cold hardiness and overwintering strategy of the pink maize stalk borer, Sesamia nonagrioides Lef (Lepidoptera, Noctuidae). Oecologia 1994, 99, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Matsukura, K.; Izumi, Y.; Kumashiro, S.; Matsumura, M. Cold tolerance of the maize orange leafhopper, Cicadulina bipunctata. J. Insect Physiol. 2014, 67, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Régnier, B.; Legrand, J.; Calatayud, P.A.; Rebaudo, F. Developmental differentiations of major maize stemborers due to global warming in temperate and tropical climates. Insects 2023, 14, 51. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.W.; Hong, L.; Chen, J.; Liang, Y.Y. Fitness and cold tolerance of Spodoptera frugiperda fed on corn and two winter crops. J. Appl. Entomol. 2024, 148, 49–56. [Google Scholar] [CrossRef]
- Matsukura, K.; Matsumura, M. Ecology of the maize orange leafhopper, Cicadulina bipunctata (Melichar) (Hemiptera: Cicadellidae). Jpn. Agricul. Res. Quart. 2013, 47, 365–369. [Google Scholar] [CrossRef][Green Version]
- Hudon, M.; LeRoux, E.J.; Harcourt, D.G. Seventy years of European corn borer (Ostrinia nubilalis) research in North America. Agric. Zool. Rev. 1989, 3, 53–96. [Google Scholar]
- Nault, L.R. Dalbulus maidis identification, biology, ecology and pest status. In Diagnosing Maize Diseases in Latin America; Casela, C., Renfro, R.B., Krattiger, A.F., Eds.; ISAAA: New York, NY, USA, 1998; pp. 18–21. [Google Scholar]
- Myers, J.G. The ecological distribution of some South American grass and sugar cane borers (Diatraee spp. Lep., Pyralidae). Bull. Ent. Res. 1935, 26, 335–342. [Google Scholar] [CrossRef]
- Box, H.E. New species and records of Diatraea Guild. from northern Venezuela (Lepid., Pyral.). Bull. Ent. Res. 1951, 42, 379–398. [Google Scholar] [CrossRef]
- Rodríguez-del-Bosque, L.A.; Smith, J.W., Jr.; Browning, H.W. Bibliography of the neotropical cornstalk borer, Diatraea lineolata (Lepidoptera: Pyralidae). Fla. Entomol. 1988, 71, 176–186. [Google Scholar] [CrossRef]
- Zhang, X.; Nie, P.; Hu, X.; Feng, J. A host tree and its specialist insects: Black locust (Robinia pseudoacacia) availability largely determines the future range dynamics of its specialist insects in Europe. Insects 2024, 15, 765. [Google Scholar] [CrossRef]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The impact of climate change on agricultural insect pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wolter, C.; Xian, W.; Jeschke, J.M. Most invasive species largely conserve their climatic niche. Proc. Natl. Acad. Sci. USA 2020, 117, 23643–23651. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Q.; Zhang, X.; Yang, F.; Duan, S.; Fan, Z.; Nie, P.; Chen, Z.; Feng, J. Future Range Shifts in Major Maize Insect Pests Suggest Their Increasing Impacts on Global Maize Production. Insects 2025, 16, 568. https://doi.org/10.3390/insects16060568
Wei Q, Zhang X, Yang F, Duan S, Fan Z, Nie P, Chen Z, Feng J. Future Range Shifts in Major Maize Insect Pests Suggest Their Increasing Impacts on Global Maize Production. Insects. 2025; 16(6):568. https://doi.org/10.3390/insects16060568
Chicago/Turabian StyleWei, Qiance, Xueyou Zhang, Fang Yang, Sixi Duan, Zejian Fan, Peixiao Nie, Zhihong Chen, and Jianmeng Feng. 2025. "Future Range Shifts in Major Maize Insect Pests Suggest Their Increasing Impacts on Global Maize Production" Insects 16, no. 6: 568. https://doi.org/10.3390/insects16060568
APA StyleWei, Q., Zhang, X., Yang, F., Duan, S., Fan, Z., Nie, P., Chen, Z., & Feng, J. (2025). Future Range Shifts in Major Maize Insect Pests Suggest Their Increasing Impacts on Global Maize Production. Insects, 16(6), 568. https://doi.org/10.3390/insects16060568





