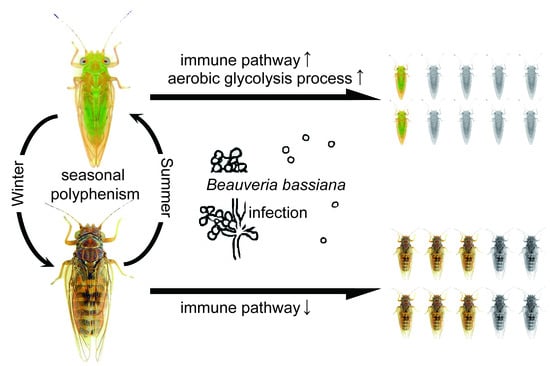
Morphotype-Specific Antifungal Defense in Cacopsylla chinensis Arises from Metabolic and Immune Network Restructuring
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect and Fungi
2.2. Experimental Design
2.3. RNA Extraction and Sequencing
2.4. Data Processing and Analysis
2.5. Gene Functional Annotation
2.6. Differentially Expressed Genes (DEGs) Analysis and Enrichment Analysis
2.7. Quantitative Real-Time PCR Analysis
2.8. Statistical Analysis
3. Results
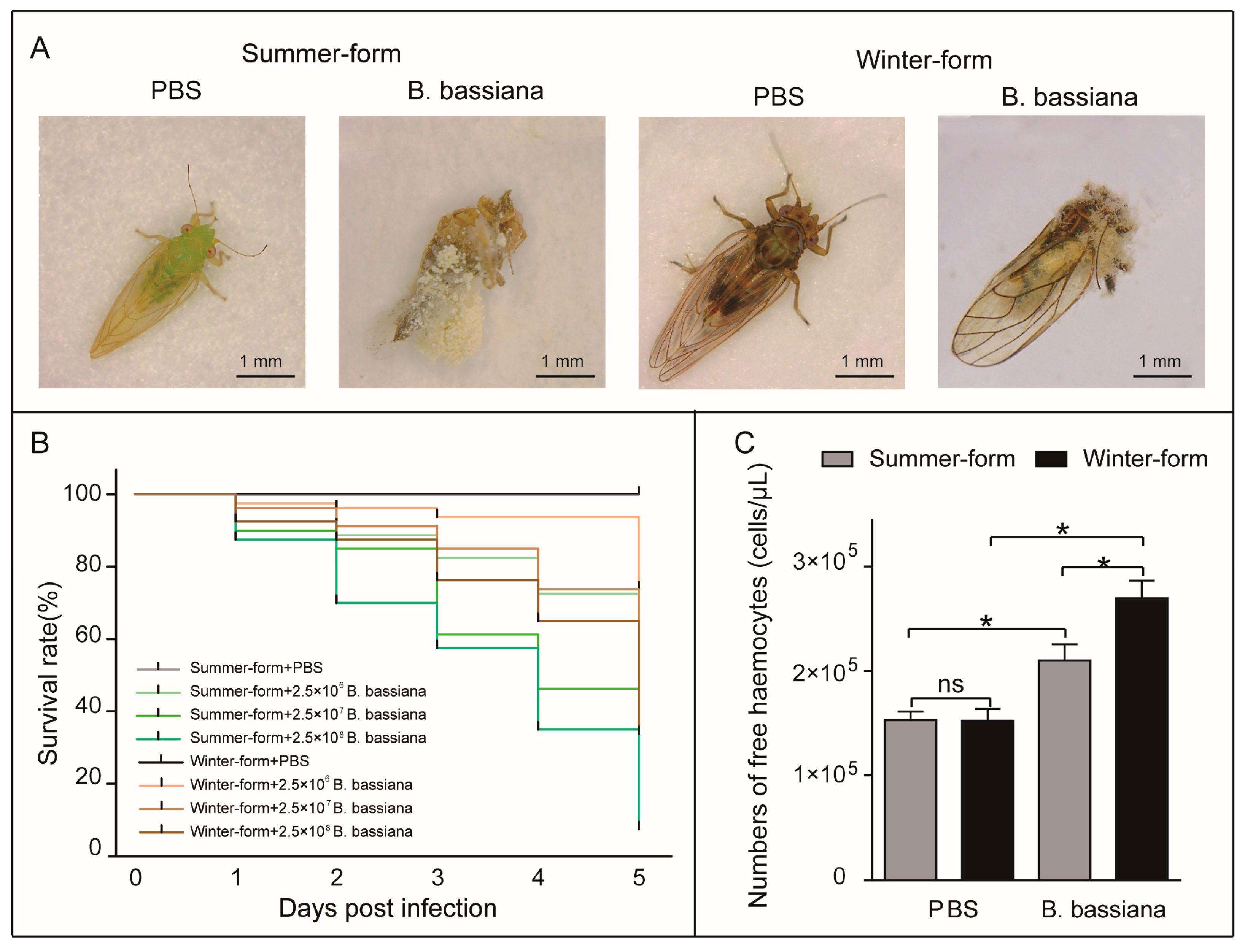
3.1. Summer-Form C. chinensis Was More Sensitive to B. bassiana Than Winter-Form C. chinensis
3.2. Overview of PacBio and Illumina Sequencing
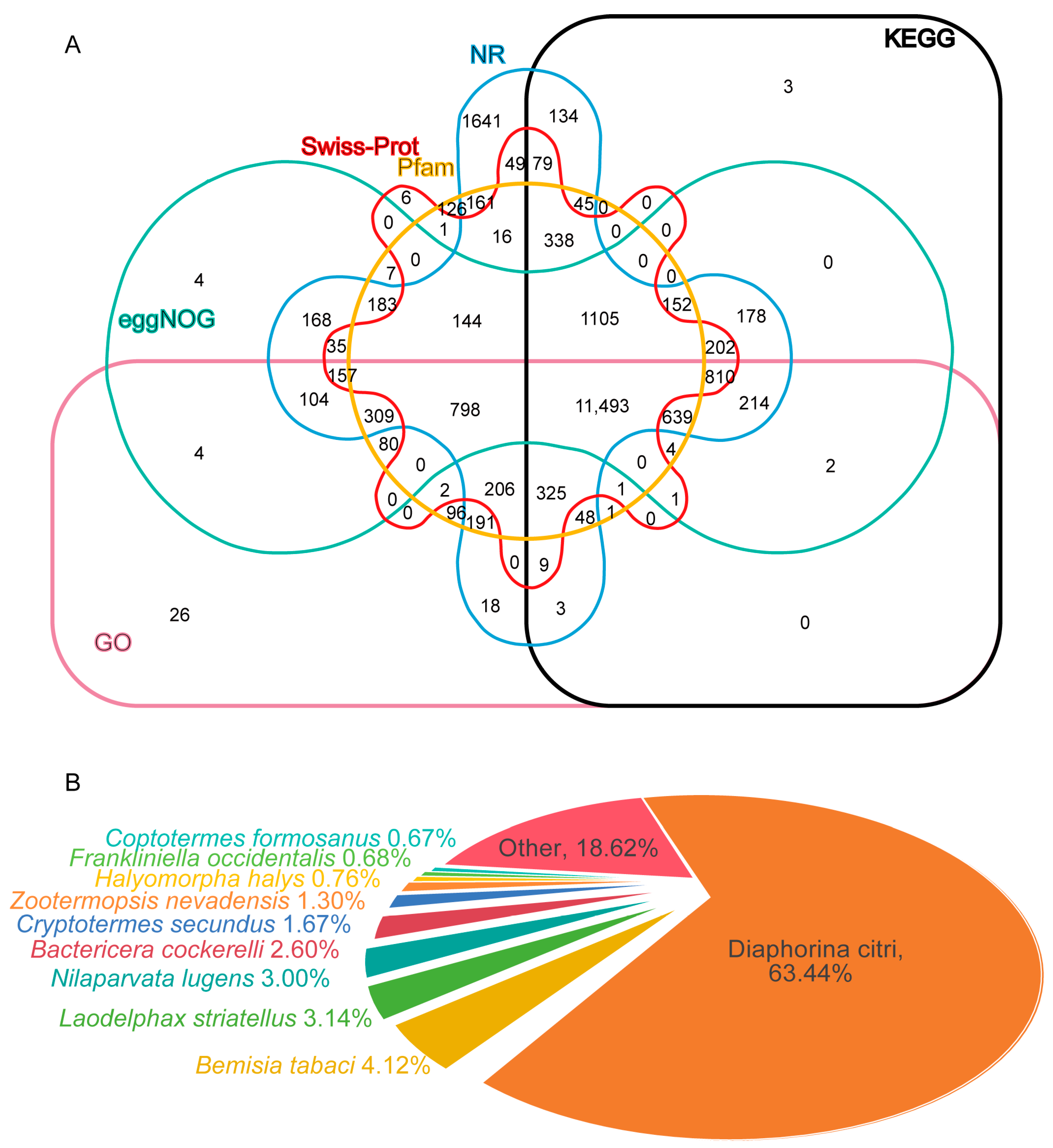
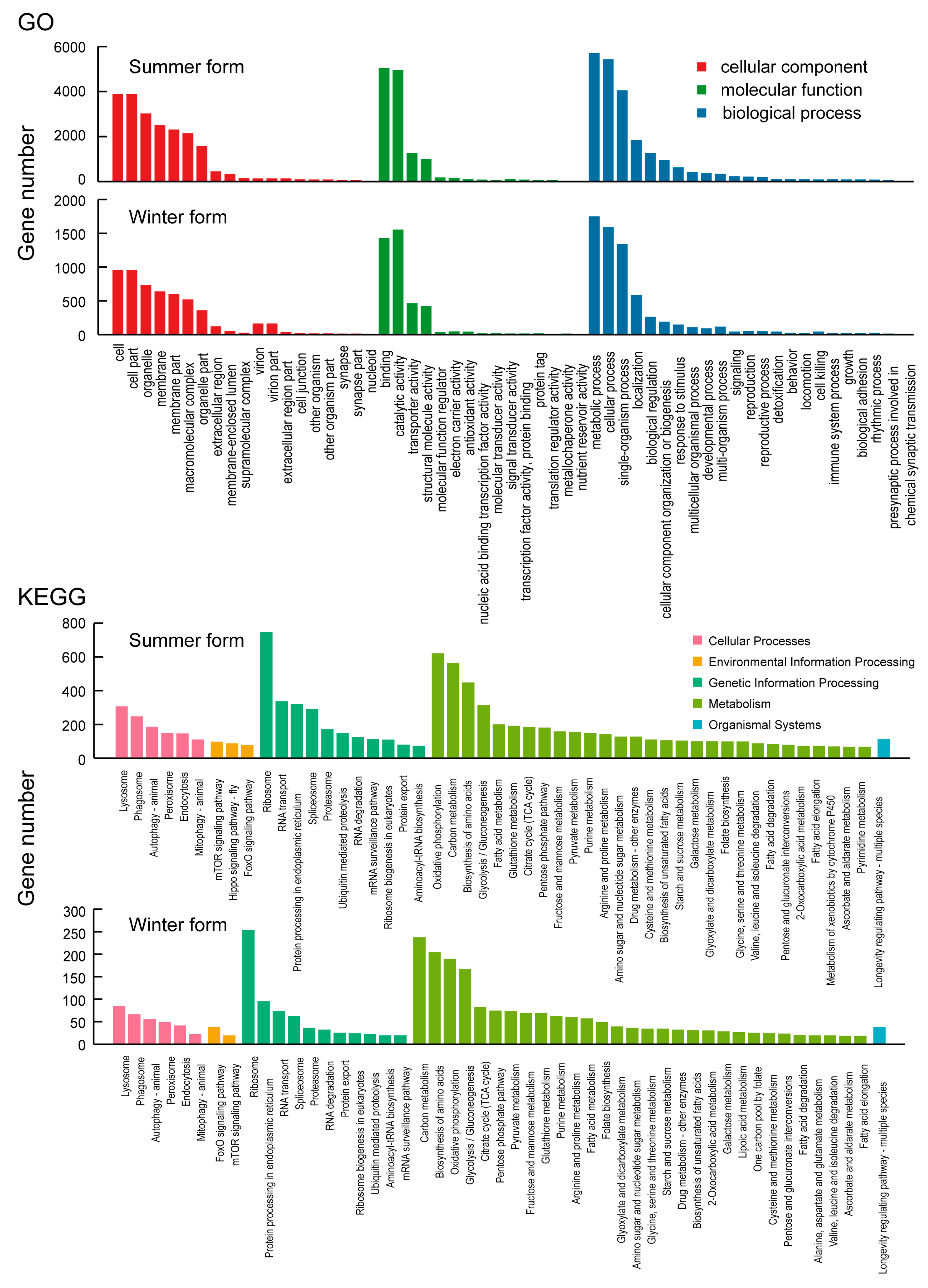
3.3. Functional Annotation and Classification
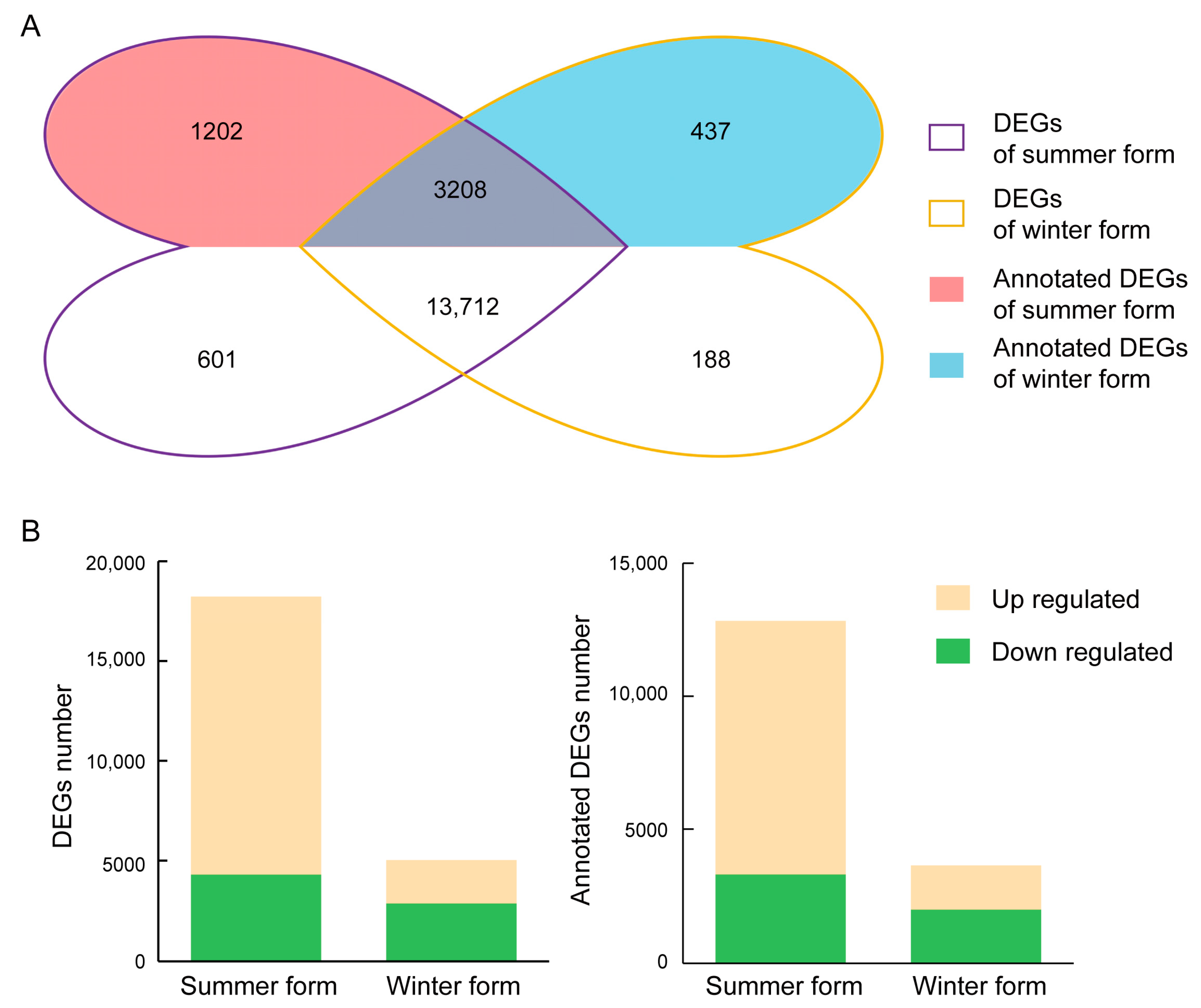
3.4. Identification of DEGs
3.5. DEGs in Response to B. bassiana Infection
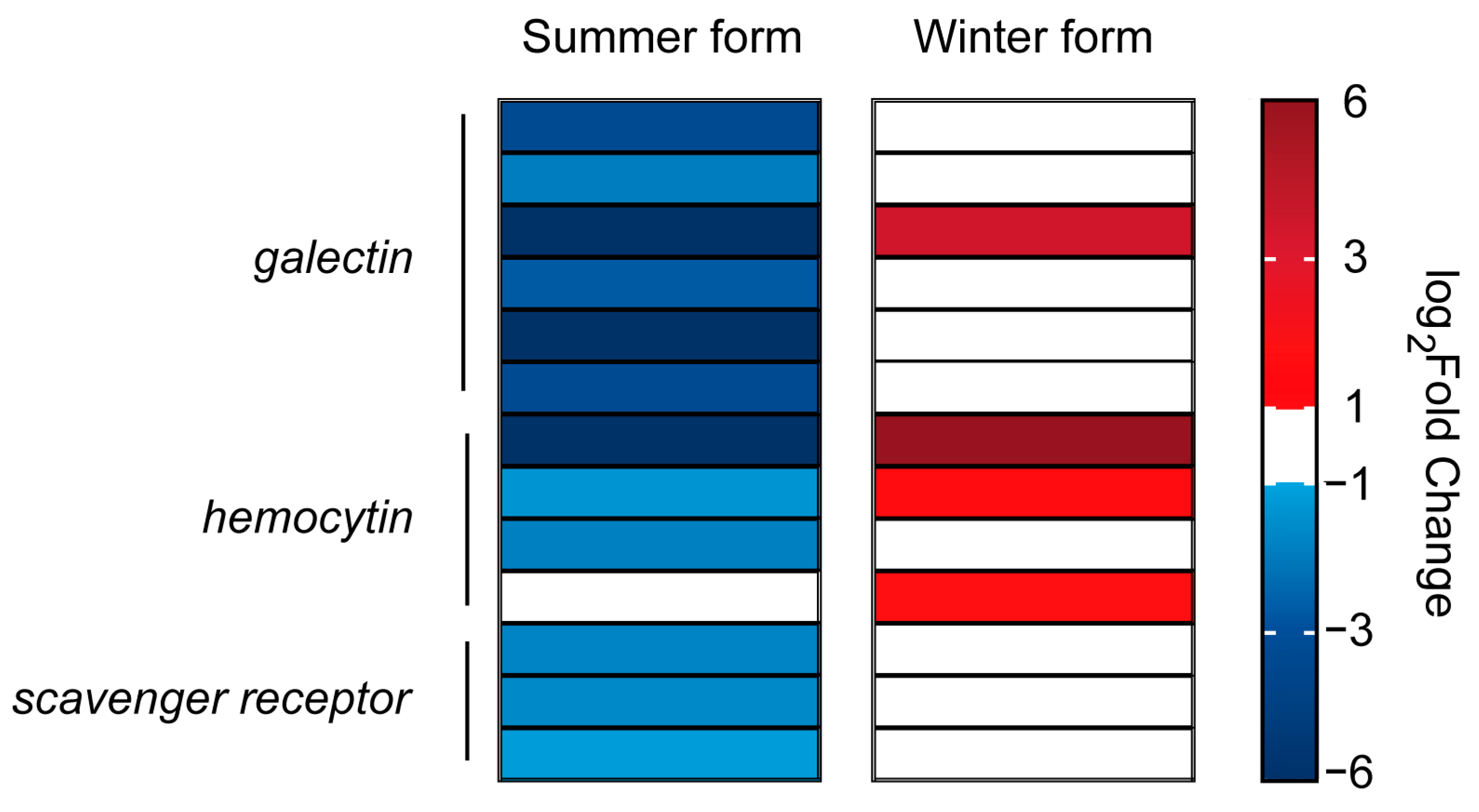
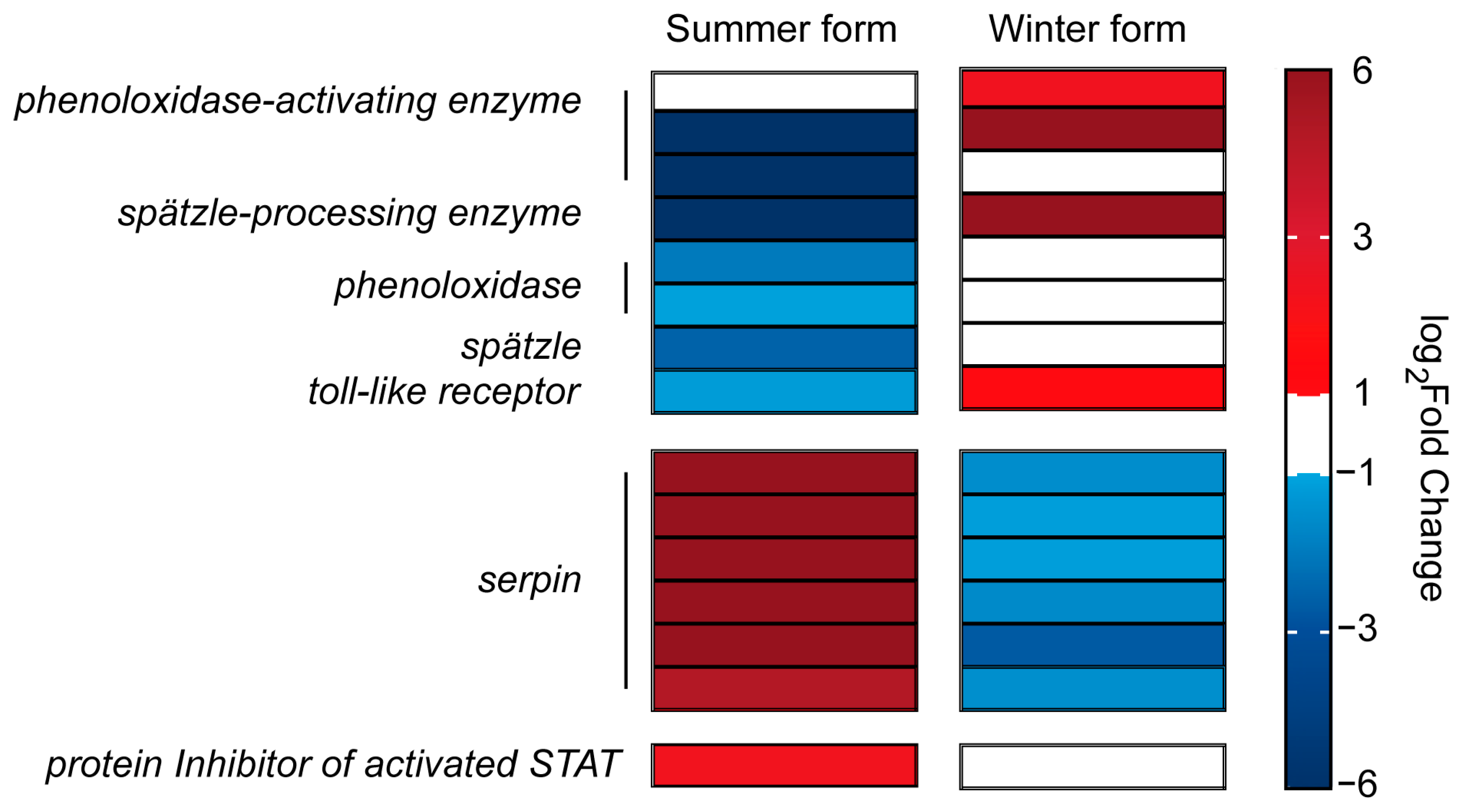
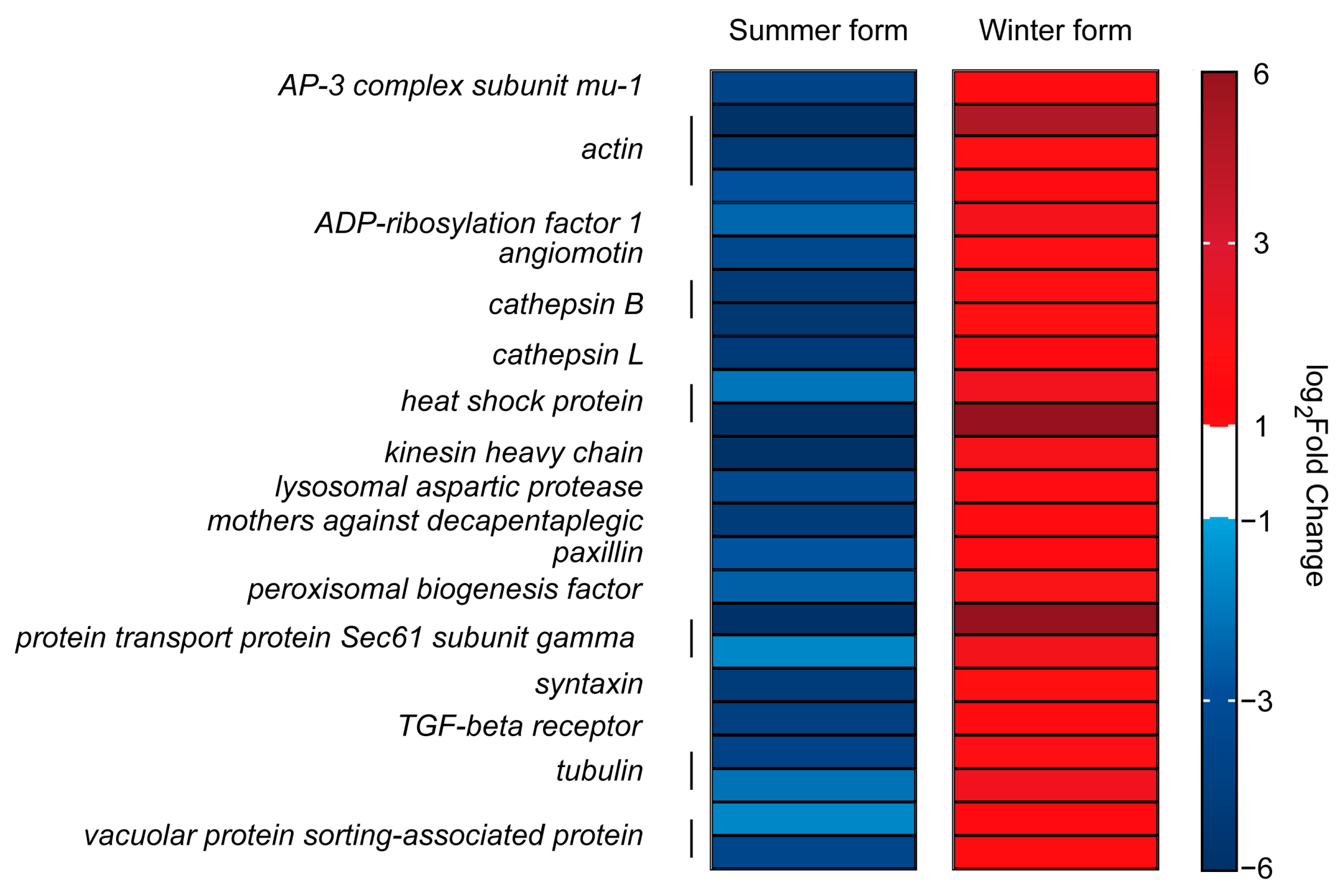
3.6. DEGs in Two Seasonal Phenotypes of C. chinensis Infected by B. bassiana
3.7. Validation of Gene Expression Levels
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, P.; Liu, Q.; Qiao, X.; Wang, J.; Zhang, T. Identification and phylogenetic analysis of pear psyllids (Hemiptera: Psyllidae) in chinese pear orchards. J. Econ. Entomol. 2018, 111, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Liu, Q.; Ma, X.; Liu, J. The impacts of climate change on the potential distribution of Cacopsylla chinensis (Hemiptera: Psyllidae) in China. J. Econ. Entomol. 2025, 118, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Chi, H.; Guo, Y.; Li, X.; Zhao, L.; Ma, R. Demography of Cacopsylla chinensis (Hemiptera: Psyllidae) reared on four cultivars of Pyrus bretschneideri (Rosales: Rosaceae) and P. communis pears with estimations of confidence intervals of specific life table statistics. J. Econ. Entomol. 2020, 113, 2343–2353. [Google Scholar] [CrossRef]
- Ge, Y.; Liu, P.; Zhang, L.; Snyder, W.E.; Smith, O.M.; Shi, W. A sticky situation: Honeydew of the pear psylla disrupts feeding by its predator Orius sauteri. Pest Manag. Sci. 2020, 76, 75–84. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Q.; Luan, X.; Zhou, C. Research progress on the occurrence regularity and integrated control of Cacopsylla chinensis. North. Hortic. 2015, 2015, 180–183. (In Chinese) [Google Scholar]
- Zhang, R.; Zhang, B.; Xu, S.; Wu, Z.; Ma, Y.; Li, Y.; Zhang, D.; Yu, Q. Effects of medium lethal concentration of avermectin on the development of short-term resistance of Psylla chinensis (Hemiptera: Psyllidae) to avermectin. Acta Entomol. Sin. 2023, 66, 1581–1589. (In Chinese) [Google Scholar]
- Tsai, C.; Lee, H.; Cho, G.; Liao, Y.; Yang, M.; Yeh, W. Invasive and quarantine risks of Cacopsylla chinensis (Hemiptera: Psyllidae) in east Asia: Hybridization or gene flow between differentiated lineages. J. Econ. Entomol. 2020, 113, 2890–2899. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, Z.; Zhang, W.; Hu, Z.; Yang, H. Evaluation of natural control effect of Trechnites insidiosus against Cacopsylla chinensis. China Plant Prot. 2020, 40, 72–88. (In Chinese) [Google Scholar]
- Wang, H.; Peng, H.; Li, W.; Cheng, P.; Gong, M. The toxins of Beauveria bassiana and the strategies to improve their virulence to insects. Front. Microbiol. 2021, 12, 705343. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Jaronski, S.T. The production and uses of Beauveria bassiana as a microbial insecticide. World J. Microbiol. Biotechnol. 2016, 32, 177. [Google Scholar] [CrossRef]
- Wang, C.; Fan, M.; Li, Z.; Butt, T.M. Molecular monitoring and evaluation of the application of the insect-pathogenic fungus Beauveria bassiana in southeast China. J. Appl. Microbiol. 2004, 96, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Clifton, E.H.; Hajek, A.E.; Jenkins, N.E.; Roush, R.T.; Rost, J.P.; Biddinger, D.J. Applications of Beauveria bassiana (Hypocreales: Cordycipitaceae) to control populations of spotted lanternfly (Hemiptera: Fulgoridae), in semi-natural landscapes and on grapevines. Environ. Entomol. 2020, 49, 854–864. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, X.; Eleftherianos, I.; Mohamed, A.; Bastin, A.; Keyhani, N.O. Cross-talk between immunity and behavior: Insights from entomopathogenic fungi and their insect hosts. FEMS Microbiol. Rev. 2024, 48, fuae003. [Google Scholar] [CrossRef] [PubMed]
- El Moussawi, L.; Nakhleh, J.; Kamareddine, L.; Osta, M.A. The mosquito melanization response requires hierarchical activation of non-catalytic clip domain serine protease homologs. PLOS Pathog. 2019, 15, e1008194. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Kim, S.; Kan, H.; Kwon, H.; Roh, K.; Jiang, R.; Yang, Y.; Park, J.; Lee, H.; Ha, N.; et al. A three-step proteolytic cascade mediates the activation of the peptidoglycan-induced toll pathway in an insect. J. Biol. Chem. 2008, 283, 7599–7607. [Google Scholar] [CrossRef]
- Takahashi, D.; Garcia, B.L.; Kanost, M.R. Initiating protease with modular domains interacts with β-glucan recognition protein to trigger innate immune response in insects. Proc. Natl. Acad. Sci. USA 2015, 112, 13856–13861. [Google Scholar] [CrossRef]
- Gerardo, N.M.; Altincicek, B.; Anselme, C.; Atamian, H.; Barribeau, S.M.; de Vos, M.; Duncan, E.J.; Evans, J.D.; Gabaldón, T.; Ghanim, M.; et al. Immunity and other defenses in pea aphids, Acyrthosiphon pisum. Genome Biol. 2010, 11, R21. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, F.; Cao, X.; Zou, Z.; Lu, Z.; Kanost, M.R.; Jiang, H. Hemolymph protease-5 links the melanization and Toll immune pathways in the tobacco hornworm. Manduca sexta. Proc. Natl. Acad. Sci. USA 2020, 117, 23581–23587. [Google Scholar] [CrossRef]
- An, C.; Ishibashi, J.; Ragan, E.J.; Jiang, H.; Kanost, M.R. Functions of Manduca sexta hemolymph proteinases HP6 and HP8 in two innate immune pathways. J. Biol. Chem. 2009, 284, 19716–19726. [Google Scholar] [CrossRef]
- Raghavan, V.; Kraft, L.; Mesny, F.; Rigerte, L. A simple guide to de novo transcriptome assembly and annotation. Brief Bioinform. 2022, 23, bbab563. [Google Scholar] [CrossRef]
- Jackson, D.J.; Cerveau, N.; Posnien, N. De novo assembly of transcriptomes and differential gene expression analysis using short-read data from emerging model organisms—A brief guide. Front Zool. 2024, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.S.N.; Huang, J.; Syed, N.H.; Ben-Hur, A.; Dong, S.; Gu, L. Decoding co-/post-transcriptional complexities of plant transcriptomes and epitranscriptome using next-generation sequencing technologies. Biochem. Soc. Trans. 2020, 48, 2399–2414. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Peluso, P.; Babayan, P.; Yeadon, P.J.; Yu, C.; Fisher, W.W.; Chin, C.S.; Rapicavoli, N.A.; Rank, D.R.; Li, J.; et al. Long-read, whole-genome shotgun sequence data for five model organisms. Sci. Data 2014, 1, 140045. [Google Scholar] [CrossRef]
- Rhoads, A.; Au, K.F. PacBio sequencing and its applications. Genom. Proteom. Bioinform. 2015, 13, 278–289. [Google Scholar] [CrossRef]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods. 2015, 12, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- Cherry, S.; Silverman, N. Host-pathogen interactions in Drosophila: New tricks from an old friend. Nat. Immunol. 2006, 7, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Vilcinskas, A.; Kanost, M.R. Immunity in lepidopteran insects. Adv. Exp. Med. Biol. 2010, 708, 181–204. [Google Scholar]
- Strand, M.R. The insect cellular immune response. Insect Sci. 2008, 15, 1–14. [Google Scholar] [CrossRef]
- Hillyer, J.F.; Schmidt, S.L.; Christensen, B.M. Hemocyte-mediated phagocytosis and melanization in the mosquito Armigeres subalbatus following immune challenge by bacteria. Cell Tissue Res. 2003, 313, 117–127. [Google Scholar] [CrossRef]
- Ling, E.; Shirai, K.; Kanekatsu, R.; Kiguchi, K. Hemocyte differentiation in the hematopoietic organs of the silkworm, Bombyx mori: Prohemocytes have the function of phagocytosis. Cell Tissue Res. 2005, 320, 535–543. [Google Scholar] [CrossRef]
- Williams, M.J. Drosophila hemopoiesis and cellular immunity. J. Immunol. 2007, 178, 4711–4716. [Google Scholar] [CrossRef]
- Zambon, R.A.; Nandakumar, M.; Vakharia, V.N.; Wu, L.P. The toll pathway is important for an antiviral response in Drosophila. Proc. Natl. Acad. Sci. USA 2005, 102, 7257–7262. [Google Scholar] [CrossRef]
- Rawlings, J.S.; Rosler, K.M.; Harrison, D.A. The JAK/STAT signaling pathway. J. Cell Sci. 2004, 117, 1281–1283. [Google Scholar] [CrossRef]
- Ma, L.; Liu, L.; Zhao, Y.; Yang, L.; Chen, C.; Li, Z.; Lu, Z. JNK pathway plays a key role in the immune system of the pea aphid and is regulated by microRNA-184. PLoS Pathog. 2020, 16, e1008627. [Google Scholar] [CrossRef]
- Ageitos, J.M.; Sánchez-Pérez, A.; Calo-Mata, P.; Villa, T.G. Antimicrobial peptides (AMPs): Ancient compounds that represent novel weapons in the fight against bacteria. Biochem. Pharmacol. 2017, 133, 117–138. [Google Scholar] [CrossRef] [PubMed]
- Arp, A.P.; Hunter, W.B.; Pelz-Stelinski, K.S. Annotation of the asian citrus psyllid genome reveals a reduced innate immune system. Front. Physiol. 2016, 7, 570. [Google Scholar] [CrossRef]
- Xie, W.; Yang, X.; Chen, C.; Yang, Z.; Guo, L.; Wang, D.; Huang, J.; Zhang, H.; Wen, Y.; Zhao, J.; et al. The invasive MED/Q Bemisia tabaci genome: A tale of gene loss and gene gain. BMC Genom. 2018, 19, 68. [Google Scholar] [CrossRef]
- Gillespie, J.P.; Kanost, M.R.; Trenczek, T. Biological mediators of insect immunity. Annu. Rev. Entomol. 1997, 42, 611–643. [Google Scholar] [CrossRef] [PubMed]
- Ferrandon, D.; Imler, J.; Hetru, C.; Hoffmann, J.A. The Drosophila systemic immune response: Sensing and signalling during bacterial and fungal infections. Nat. Rev. Immunol. 2007, 7, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.H.; Kurtz, J. Dscam in immunity: A question of diversity in insects and crustaceans. Dev. Comp. Immunol. 2020, 105, 103539. [Google Scholar] [CrossRef]
- Schmucker, D.; Clemens, J.C.; Shu, H.; Worby, C.A.; Xiao, J.; Muda, M.; Dixon, J.E.; Zipursky, S.L. Drosophila dscam is an axon guidance receptor exhibiting extraordinary molecular diversity. Cell 2000, 101, 671–684. [Google Scholar] [CrossRef]
- Dong, Y.; Taylor, H.E.; Dimopoulos, G. AgDscam, a hypervariable immunoglobulin domain-containing receptor of the Anopheles gambiae innate immune system. PLoS Biol. 2006, 4, e229. [Google Scholar] [CrossRef]
- Cerliani, J.P.; Stowell, S.R.; Mascanfroni, I.D.; Arthur, C.M.; Cummings, R.D.; Rabinovich, G.A. Expanding the universe of cytokines and pattern recognition receptors: Galectins and glycans in innate immunity. J. Clin. Immunol. 2011, 31, 10–21. [Google Scholar] [CrossRef]
- Zhang, L.L.; Hu, X.H.; Wu, S.Q.; Batool, K.; Chowdhury, M.; Lin, Y.; Zhang, J.; Gill, S.S.; Guan, X.; Yu, X.Q. Aedes aegypti galectin competes with Cry11Aa for binding to ALP1 to modulate cry toxicity. J. Agric. Food Chem. 2018, 66, 13435–13443. [Google Scholar] [CrossRef]
- Koyama, H.; Kato, D.; Minakuchi, C.; Tanaka, T.; Yokoi, K.; Miura, K. Peptidoglycan recognition protein genes and their roles in the innate immune pathways of the red flour beetle, Tribolium castaneum. J. Invertebr. Pathol. 2015, 132, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Lin, Z.; Wang, J.M.; Xing, L.S.; Xiong, G.H.; Zou, Z. CTL14, a recognition receptor induced in late stage larvae, modulates anti-fungal immunity in cotton bollworm Helicoverpa armigera. Dev. Comp. Immunol. 2018, 84, 142–152. [Google Scholar] [CrossRef]
- Wang, C.; Leger, R.J.S. A collagenous protective coat enables Metarhizium anisopliae to evade insect immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 6647–6652. [Google Scholar] [CrossRef]
- Xiao, G.; Ying, S.; Zheng, P.; Wang, Z.; Zhang, S.; Xie, X.; Shang, Y.; Leger, R.J.S.; Zhao, G.; Wang, C.; et al. Genomic perspectives on the evolution of fungal entomopathogenicity in Beauveria bassiana. Sci. Rep. 2012, 2, 483. [Google Scholar] [CrossRef]
- Bergin, D.; Brennan, M.; Kavanagh, K. Fluctuations in haemocyte density and microbial load may be used as indicators of fungal pathogenicity in larvae of Galleria mellonella. Microbes Infect. 2003, 5, 1389–1395. [Google Scholar] [CrossRef]
- Hernando-Ortiz, A.; Eraso, E.; Quindos, G.; Mateo, E. Candidiasis by Candida glabrata, Candida nivariensis and Candida bracarensis in Galleria mellonella: Virulence and therapeutic responses to echinocandins. J. Fungi 2021, 7, 998. [Google Scholar] [CrossRef] [PubMed]
- May, R.C.; Machesky, L.M. Phagocytosis and the actin cytoskeleton. J. Cell Sci. 2001, 114, 1061–1077. [Google Scholar] [CrossRef]
- Sandiford, L.S.; Dong, Y.; Pike, A.; Blumberg, B.J.; Bahia, A.C.; Dimopoulos, G. Cytoplasmic actin is an extracellular insect immune factor which is secreted upon immune challenge and mediates phagocytosis and direct killing of bacteria, and is a Plasmodium antagonist. PLoS Pathog. 2015, 11, e1004631. [Google Scholar] [CrossRef] [PubMed]
- Weiss-Sadan, T.; Itzhak, G.; Kaschani, F.; Yu, Z.; Mahameed, M.; Anaki, A.; Ben-Nun, Y.; Merquiol, E.; Tirosh, B.; Kessler, B.; et al. Cathepsin L regulates metabolic networks controlling rapid cell growth and proliferation. Mol. Cell. Proteom. 2019, 18, 1330–1344. [Google Scholar] [CrossRef]
- Chen, C.; Ahmad, M.J.; Ye, T.; Du, C.; Zhang, X.; Liang, A.; Yang, L. Cathepsin B regulates mice granulosa cells’ apoptosis and proliferation in vitro. Int. J. Mol. Sci. 2021, 22, 11827. [Google Scholar] [CrossRef]
- Di, Y.; Han, X.; Kang, X.; Wang, D.; Chen, C.; Wang, J.; Zhao, X. Autophagy triggers CTSD (cathepsin D) maturation and localization inside cells to promote apoptosis. Autophagy 2021, 17, 1170–1192. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, C.; Xu, W.; Abbas, M.N.; Mu, F.; Ding, W.; Zhang, H.; Li, J. Functions of Bombyx mori cathepsin L-like in innate immune response and anti-microbial autophagy. Dev. Comp. Immunol. 2021, 116, 103927. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Chen, X.; Xiao, C.; Lin, D.; Li, Y.; Luo, S.; Zeng, Z.; Sun, B.; Lei, S. Ar-turmerone inhibits the proliferation and mobility of glioma by downregulating cathepsin B. Aging 2023, 15, 9377–9390. [Google Scholar] [CrossRef]
- Kanost, M.R.; Jiang, H. Clip-domain serine proteases as immune factors in insect hemolymph. Curr. Opin. Insect Sci. 2015, 11, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Law, R.H.; Zhang, Q.; Mcgowan, S.; Buckle, A.M.; Silverman, G.A.; Wong, W.; Rosado, C.J.; Langendorf, C.G.; Pike, R.N.; Bird, P.I.; et al. An overview of the serpin superfamily. Genome Biol. 2006, 7, 216. [Google Scholar] [CrossRef][Green Version]
- Silverman, G.A.; Whisstock, J.C.; Bottomley, S.P.; Huntington, J.A.; Kaiserman, D.; Luke, C.J.; Pak, S.C.; Reichhart, J.; Bird, P.I. Serpins flex their muscle: I. Putting the clamps on proteolysis in diverse biological systems. J. Biol. Chem. 2010, 285, 24299–24305. [Google Scholar] [CrossRef]
- Yan, Z.; Fang, Q.; Liu, Y.; Xiao, S.; Yang, L.; Wang, F.; An, C.; Werren, J.H.; Ye, G. A venom serpin splicing isoform of the endoparasitoid wasp Pteromalus puparum suppresses host prophenoloxidase cascade by forming complexes with host hemolymph proteinases. J. Biol. Chem. 2017, 292, 1038–1051. [Google Scholar] [CrossRef]
- Yan, Z.; Fang, Q.; Song, J.; Yang, L.; Xiao, S.; Wang, J.; Ye, G. A serpin gene from a parasitoid wasp disrupts host immunity and exhibits adaptive alternative splicing. PLoS Pathog. 2023, 19, e1011649. [Google Scholar] [CrossRef]
- Yuan, C.; Xing, L.; Wang, M.; Wang, X.; Yin, M.; Wang, Q.; Hu, Z.; Zou, Z. Inhibition of melanization by serpin-5 and serpin-9 promotes baculovirus infection in cotton bollworm Helicoverpa armigera. PLOS Pathog. 2017, 13, e1006645. [Google Scholar] [CrossRef]
- Ji, J.; Shen, D.; Zhang, S.; Wang, L.; An, C. Serpin-4 facilitates baculovirus infection by inhibiting melanization in asian corn borer, Ostrinia furnacalis (Guenée). Front. Immunol. 2022, 13, 905357. [Google Scholar] [CrossRef]
- Krapić, M.; Kavazović, I.; Wensveen, F.M. Immunological mechanisms of sickness behavior in viral infection. Viruses 2021, 13, 2245. [Google Scholar] [CrossRef] [PubMed]
- Aderinto, N.; Abdulbasit, M.O.; Tangmi, A.D.E.; Okesanya, J.O.; Mubarak, J.M. Unveiling the growing significance of metabolism in modulating immune cell function exploring mechanisms and implications a review. Ann. Med. Surg. 2023, 85, 5511–5522. [Google Scholar] [CrossRef] [PubMed]
- Dolezal, T.; Krejcova, G.; Bajgar, A.; Nedbalova, P.; Strasser, P. Molecular regulations of metabolism during immune response in insects. Insect Biochem. Mol. Biol. 2019, 109, 31–42. [Google Scholar] [CrossRef]
- Bonora, M.; Patergnani, S.; Rimessi, A.; De Marchi, E.; Suski, J.M.; Bononi, A.; Giorgi, C.; Marchi, S.; Missiroli, S.; Poletti, F.; et al. ATP synthesis and storage. Purinergic Signal. 2012, 8, 343–357. [Google Scholar] [CrossRef]
- Senior, A.E. ATP synthesis by oxidative phosphorylation. Physiol. Rev. 1988, 68, 177–231. [Google Scholar] [CrossRef]
- Nakashima, R.A.; Paggi, M.G.; Pedersen, P.L. Contributions of glycolysis and oxidative phosphorylation to adenosine 5′-triphosphate production in AS-30D hepatoma cells. Cancer Res. 1984, 44, 5702–5706. [Google Scholar] [PubMed]
- Pfeiffer, T.; Schuster, S.; Bonhoeffer, S. Cooperation and competition in the evolution of ATP-producing pathways. Science 2001, 292, 504–507. [Google Scholar] [CrossRef]
- Nascimento, J.M.; Shi, L.Z.; Tam, J.; Chandsawangbhuwana, C.; Durrant, B.; Botvinick, E.L.; Berns, M.W. Comparison of glycolysis and oxidative phosphorylation as energy sources for mammalian sperm motility, using the combination of fluorescence imaging, laser tweezers, and real-time automated tracking and trapping. J. Cell Physiol. 2008, 217, 745–751. [Google Scholar] [CrossRef]
- Pinto, M.M.; Paumard, P.; Bouchez, C.; Ransac, S.; Duvezin-Caubet, S.; Mazat, J.P.; Rigoulet, M.; Devin, A. The Warburg effect and mitochondrial oxidative phosphorylation: Friends or foes? Biochim. Biophys. Acta. Bioenerg. 2023, 1864, 148931. [Google Scholar]
- Krejcova, G.; Danielova, A.; Nedbalova, P.; Kazek, M.; Strych, L.; Chawla, G.; Tennessen, J.M.; Lieskovska, J.; Jindra, M.; Dolezal, T.; et al. Drosophila macrophages switch to aerobic glycolysis to mount effective antibacterial defense. eLife 2019, 8, e50414. [Google Scholar] [CrossRef]
- Bland, M.L. Regulating metabolism to shape immune function: Lessons from Drosophila, Semin. Cell Dev. Biol. 2023, 138, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Korb, J.; Belles, X. Juvenile hormone and hemimetabolan eusociality: A comparison of cockroaches with termites. Curr. Opin. Insect Sci. 2017, 22, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Feng, Y.; Zou, X.; Zhou, Y.; Cao, W. Transcriptome and proteome analyses reveal genes and signaling pathways involved in the response to two insect hormones in the insect-fungal pathogen Hirsutella satumaensis. mSystems 2024, 9, e00166-24. [Google Scholar] [CrossRef]
- Richard, G.; Jaquiéry, J.; Le Trionnaire, G. Contribution of epigenetic mechanisms in the regulation of environmentally-induced polyphenism in insects. Insects 2021, 12, 649. [Google Scholar] [CrossRef]
- Nijhout, H.F. Development and evolution of adaptive polyphenisms. Evol. Dev. 2003, 5, 9–18. [Google Scholar] [CrossRef]
- Gomez, J.M.; Gonzalez-Megias, A.; Armas, C.; Narbona, E.; Navarro, L.; Perfectti, F. Selection maintains a nonadaptive floral polyphenism. Evol. Lett. 2024, 8, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, B.; Johansson, A. Seasonal polyphenism and developmental trade-offs between flight ability and egg laying in a pierid butterfly. Proc. Biol. Sci. 2008, 275, 2131–2136. [Google Scholar] [CrossRef]
- Teder, T.; Esperk, T.; Remmel, T.; Sang, A.; Tammaru, T. Counterintuitive size patterns in bivoltine moths: Late-season larvae grow larger despite lower food quality. Oecologia 2010, 162, 117–125. [Google Scholar] [CrossRef]
- Stockton, D.G.; Wallingford, A.K.; Brind’Amore, G.; Diepenbrock, L.; Burrack, H.; Leach, H.; Isaacs, R.; Iglesias, L.E.; Liburd, O.; Drummond, F.; et al. Seasonal polyphenism of spotted-wing Drosophila is affected by variation in local abiotic conditions within its invaded range, likely influencing survival and regional population dynamics. Ecol. Evol. 2020, 10, 7669–7685. [Google Scholar] [CrossRef]
- Baudach, A.; Lee, K.Z.; Vogel, H.; Vilcinskas, A. Immunological larval polyphenism in the map butterfly Araschnia levana reveals the photoperiodic modulation of immunity. Ecol. Evol. 2018, 8, 4891–4898. [Google Scholar] [CrossRef]
- Freitak, D.; Tammaru, T.; Sandre, S.; Meister, H.; Esperk, T. Longer life span is associated with elevated immune activity in a seasonally polyphenic butterfly. J. Evol. Biol. 2019, 32, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Schwenke, R.A.; Lazzaro, B.P.; Wolfner, M.F. Reproduction-Immunity Trade-Offs in Insects. Annu. Rev. Entomol. 2016, 61, 239–256. [Google Scholar] [CrossRef] [PubMed]



| Type | Number |
|---|---|
| Total CCS | 116,535 |
| Average CCS length (bp) | 203,132,661 |
| Mean read length of CCS | 1743 |
| Undesired primer reads | 18,534 |
| Full-length non-chimeric (FLNC) reads | 93,175 |
| Full-length non-chimeric percentage (FLNC%) | 79.95% |
| High-quality isoforms | 35,503 |
| Low-quality isoforms | 5 |
| Average consensus isoforms read length | 1724 |
| Full-length non-redundant transcripts (FLNRTs) | 30,097 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, J.; Gao, X.; Hu, Z.; Ma, R.; Zhao, L. Morphotype-Specific Antifungal Defense in Cacopsylla chinensis Arises from Metabolic and Immune Network Restructuring. Insects 2025, 16, 541. https://doi.org/10.3390/insects16050541
Ji J, Gao X, Hu Z, Ma R, Zhao L. Morphotype-Specific Antifungal Defense in Cacopsylla chinensis Arises from Metabolic and Immune Network Restructuring. Insects. 2025; 16(5):541. https://doi.org/10.3390/insects16050541
Chicago/Turabian StyleJi, Jiayue, Xin Gao, Zengli Hu, Ruiyan Ma, and Longlong Zhao. 2025. "Morphotype-Specific Antifungal Defense in Cacopsylla chinensis Arises from Metabolic and Immune Network Restructuring" Insects 16, no. 5: 541. https://doi.org/10.3390/insects16050541
APA StyleJi, J., Gao, X., Hu, Z., Ma, R., & Zhao, L. (2025). Morphotype-Specific Antifungal Defense in Cacopsylla chinensis Arises from Metabolic and Immune Network Restructuring. Insects, 16(5), 541. https://doi.org/10.3390/insects16050541