Nature-Identical Safranal and Dihydrocoumarin from Ageratina adenophora ((Spreng., 1970) King and H. Rob.) Target Energy Metabolism to Control Solenopsis invicta Buren, 1972 (Hymenoptera: Formicidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. S. invicta Collection and Rearing
2.2. Plant Materials and Preparation
2.3. Toxicity Determination of Ethanol Extracts of A. adenophora to S. invicta
2.4. Effects of Ethanol Extracts from A. adenophora Leaves on the Behavior of S. invicta
- Grasping ability: Evaluated as per Xing et al. (2022) [28], briefly, 50 workers were placed in a talcum-coated plastic cup. After 10 s of inactivity, the cup was inverted over paper for 5–8 s. Workers remaining inside were counted as lacking grasping ability.where P1 = the number of workers with grasping ability and P2 = the total number of workers in the test.Grasping (%) = (P1/P2) × 100%
- Climbing ability: Adapted from Shan et al. (2022) [23], ants were coaxed onto a marked bamboo stick (20 cm long, 2 cm wide). Individuals climbing ≥3 cm were recorded as competent.where P1 = the number of workers with climbing ability and P2 = the total number of workers in the test.Climbing (%) = (P1/P2) × 100%
- Aggression determination: We took a thin bamboo stick and touched the antennae of the workers so that the worker’s upper jaw tightly bit the bamboo stick. We lifted the bamboo stick 5 cm above the table. If it did not fall, it was considered to have aggressive ability.where P1 = the number of workers with attacking ability and P2 = the total number of workers in the experiment.Attacking (%) = (P1/P2) × 100%
2.5. Detection of Bioactive Compounds from A. adenophora
2.5.1. Sample Extraction
2.5.2. UPLC-MS Conditions
2.6. Toxicity Determination of Safranal and Dihydrocoumarin to S. invicta
2.7. Effects of Safranal and Dihydrocoumarin on the Metabolism of S. invicta
2.8. Effects of Safranal and Dihydrocoumarin on the Behavior of S. invicta
2.9. Effects of Safranal and Dihydrocoumarin on Enzyme Activities of S. invicta
3. Data Analysis
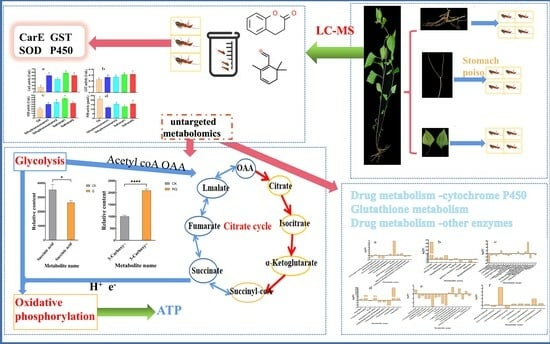
4. Results
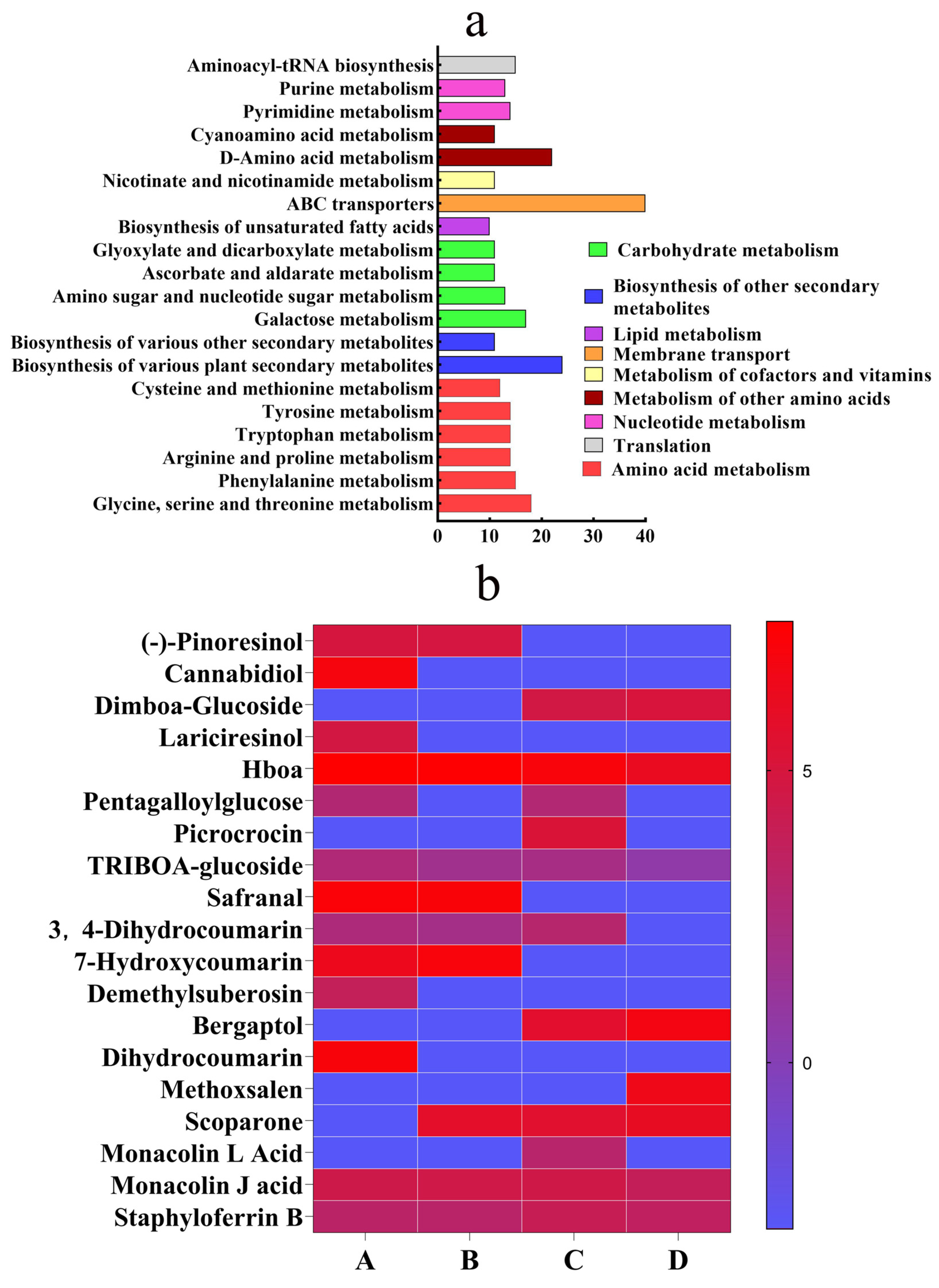
4.1. LC-MS Analysis to Explore Bioactive Compounds from A. adenophora
4.2. Toxicity and Behavioral Effects of Ethanol Extracts of A. adenophora on S. invicta
4.3. Toxicity and Behavioral Effects of Safranal and Dihydrocoumarin on S. invicta
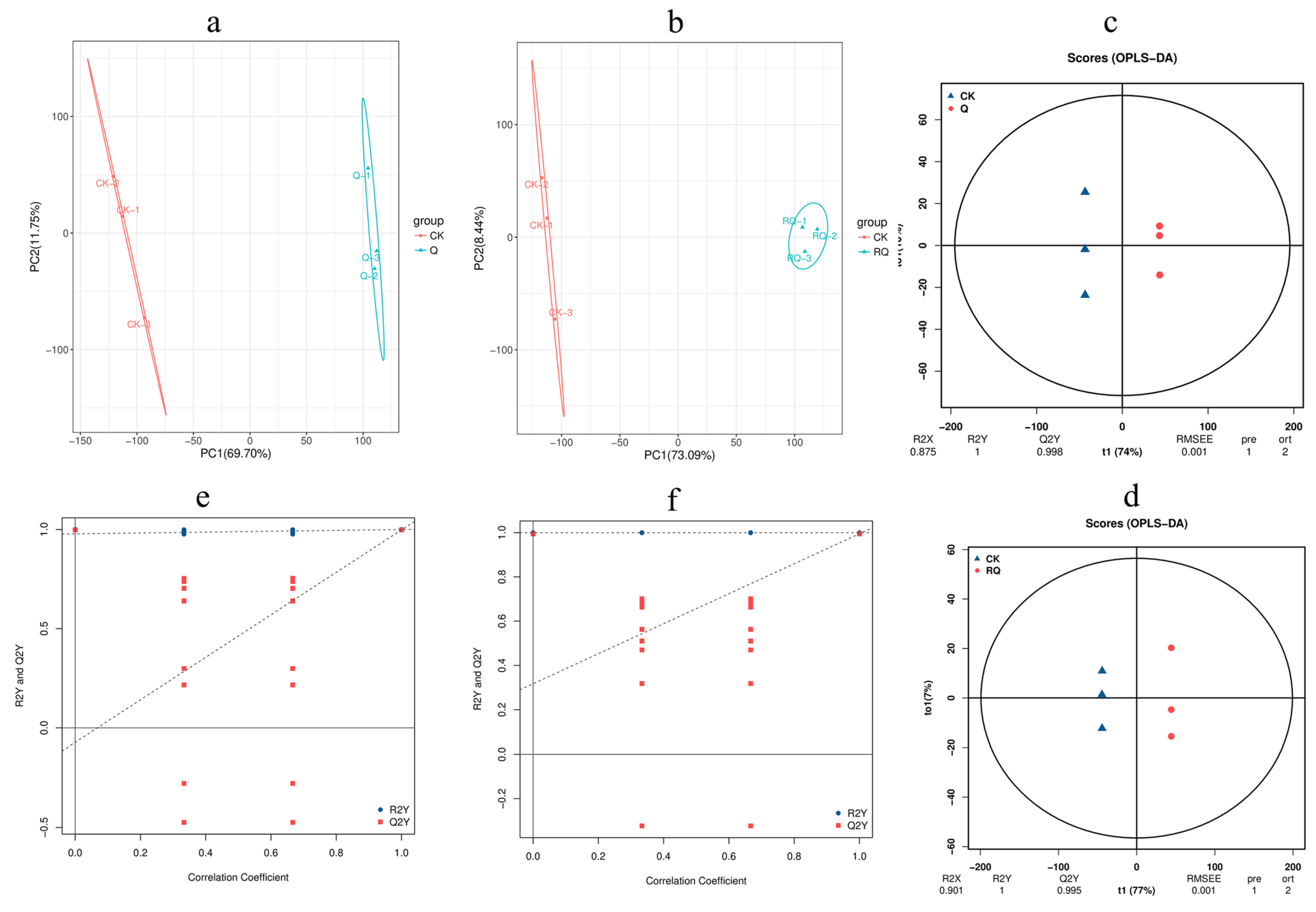
4.4. Metabolic Profiling of S. invicta Against Safranal and Dihydrocoumarin
4.4.1. Principal Component Analysis
4.4.2. Orthogonal Partial Least Squares Discriminant Analysis
4.4.3. Metabolic Mechanism Analysis
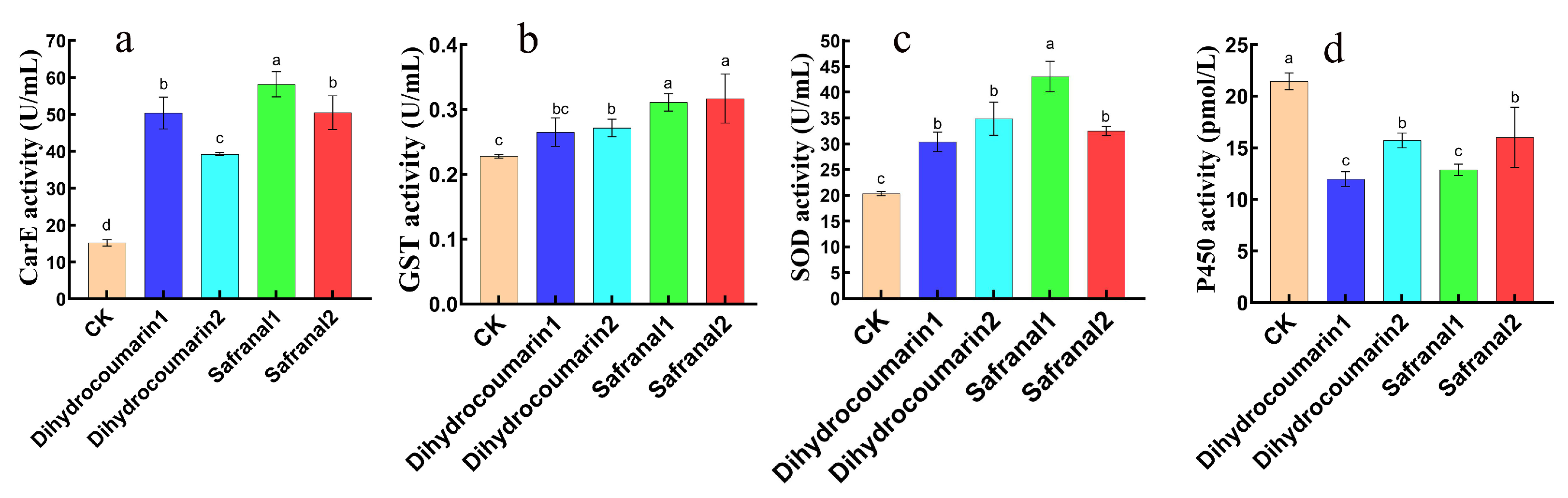
4.5. Effects of Safranal and Dihydrocoumarin on Activities of Four Enzymes of S. invicta
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LC-MS | Liquid chromatography–mass spectrometry |
| LC50 | 50% lethal concentration |
| UPLC | Ultra-high-performance liquid chromatography |
| S.E. | Standard error |
| PCA | Principal component analysis |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| SOD | Superoxide dismutase |
| GST | Glutathione S-transferase |
| CarE | Carboxylesterase |
| QC | Quality control |
| ESI | Electrospray ionization |
| GSII | Gas II |
| CUR | Curtain gas |
| DP | Declustering potential |
| CE | Collision energy |
| Q | Safranal |
| RQ | Dihydrocoumarin |
| ANOVA | One-way analysis of variance |
| PMDB | Plant Metabolome Database |
| TCA | Tricarboxylic acid cycle |
References
- Ascunce, M.; Yang, C.; Oakey, J.; Calcaterra, L.; Wu, W.; Shih, C.; Goudet, J.; Ross, K.; Shoemaker, D. Global invasion history of the fire ant Solenopsis invicta. Science 2011, 331, 1066–1068. [Google Scholar] [CrossRef] [PubMed]
- Pitts, J.P.; Camacho, G.P.; Gotzek, D.; McHugh, J.V.; Ross, K.G. Revision of the Fire Ants of the Solenopsis saevissima Species Group (Hymenoptera: Formicidae). Proc. Entomol. Soc. Wash. 2018, 120, 308–411. [Google Scholar] [CrossRef]
- Swartwout, M.; Willson, J. Southeastern US Snake Species are Vulnerable to Egg Predation by Red Imported Fire Ants (Solenopsis invicta). Herpetologica 2022, 78, 139–144. [Google Scholar] [CrossRef]
- Chen, S.; Chen, H.; Xu, Y. Safe chemical repellents to prevent the spread of invasive ants. Pest Manag. Sci. 2018, 75, 821–827. [Google Scholar] [CrossRef]
- Les, G.; Kabashima, J.; Allison, C.; Rust, M.; Klotz, J.; Jean-Pierre, H.; Paine, T. Lethality of Red Imported Fire Ant Venom to Argentine Ants and Other Ant Species. Ann. Entomol. Soc. Am. 2008, 101, 1162–1168. [Google Scholar]
- Gunawardana, D. Solenopsis invicta (red imported fire ant). CABI Compend. 2022. [Google Scholar] [CrossRef]
- Liang, Y.; Liang, M.; Li, P.; Song, Y.; Lu, Y. The effect of chlorfenapyr exposure on Solenopsis invicta Buren (Hymenoptera: Formicidae). J. Asia-Pac. Entomol. 2023, 26, 102063. [Google Scholar] [CrossRef]
- Adams, C.; Lofgren, C. Red imported fire ants (Hymenoptera: Formicidae): Frequency of sting attacks on residents of Sumter County, Georgia. J. Med. Entomol. 1981, 18, 378–382. [Google Scholar] [CrossRef]
- Kaushal, V.; Dawra, R.; Sharma, O.; Kurade, N. Hepatotoxicity in rat induced by partially purified toxins from Eupatorium adenophorum (Ageratina adenophora). Toxicon 2001, 39, 615–619. [Google Scholar] [CrossRef]
- Solley, G.; Vanderwoude, C.; Knight, G. Anaphylaxis due to Red Imported Fire Ant sting. Med. J. Aust. 2012, 49, 237–238. [Google Scholar] [CrossRef]
- Sakamoto, H.; Goka, K. Acute toxicity of typical ant control agents to the red imported fire ant, Solenopsis invicta (Hymenoptera: Formicidae). Appl. Entomol. Zool. 2021, 56, 217–224. [Google Scholar] [CrossRef]
- Cheng, D.; Huang, C.; Li, W.; Tian, Y.; Zhang, Z. Toxicities comparison of rotenone and acetone extract of Tephrosiavogelii and Derristrifoliate against Solenopsis invicta. Sociobiology 2015, 62, 474–480. [Google Scholar] [CrossRef]
- Li, Y.; Zou, H.; Zhu, N.; Li, W.; Na, X.; Tang, S.; Yang, Y. Insecticidal activity of different fractions of distilled oil extracted from Eupatorium adenophorum against 4 species of stored grain insects. J. Southwest. Univ. 2000, 22, 331–332. [Google Scholar]
- Stankovic, M.S.; Niciforovic, N.; Mihailovic, V.; Topuzovic, M.; Solujic, S. Antioxidant activity, total phenolic content and flavonoid concentrations of different plant parts of Teucrium polium L. subsp polium. Acta Soc. Bot. Pol. 2012, 81, 117–122. [Google Scholar] [CrossRef]
- Giri, S.; Sahu, R.; Paul, P.; Nandi, G.; Dua, T. An updated review on Eupatorium adenophorum Spreng. [Ageratina adenophora (Spreng.)]: Traditional uses, phytochemistry, pharmacological activities and toxicity. Pharmacol. Res.-Mod. Chin. Med. 2022, 2, 100068. [Google Scholar] [CrossRef]
- Shrestha, K.; Wilson, E.; Gay, H. Ecological and environmental study of Eupatorium adenophorum Sprengel (banmara) with reference to its gall formation in Gorkha-langtang route, Nepal. J. Nat. Hist. Mus. 2009, 23, 108–124. [Google Scholar] [CrossRef]
- Muniappan, R.; Raman, A.; Reddy, G.V.P. Biological Control of Tropical Weeds Using Arthropods, 1st ed.; Cambridge University Press: Cambridge, UK, 2009; pp. 63–73. [Google Scholar]
- Evans, H.; Crocoll, C.; Bajpai, D.; Kaur, R.; Feng, Y.L.; Silva, C.; Trevino, C.J.; Valiente, B.A.; Gershenzon, J. Volatile chemicals from leaf litter are associated with invasiveness of a Neotropical weed in Asia. Ecology 2011, 92, 316–324. [Google Scholar]
- Wang, W.B.; Wang, R.F.; Lei, Y.B.; Liu, C.; Han, L.H.; Shi, X.D.; Feng, Y.L. High resource capture and use efficiency and prolonged growth season contribute to invasiveness of Eupatorium adenophorum. Plant Ecol. 2013, 214, 857–868. [Google Scholar] [CrossRef]
- Xu, Y.; Vargo, E.; Tsuji, K.; Wylie, R. Exotic Ants of the Asia-Pacific: Invasion, National Response, and Ongoing Needs. Annu. Rev. Entomol. 2022, 67, 27–42. [Google Scholar] [CrossRef]
- Vargas-Soto, F.A.; Céspedes-Acuña, C.L.; Aqueveque-Muñoz, P.M.; Alarcón-Enos, J.E. Toxicity of coumarins synthesized by Pechmann-Duisberg condensation against Drosophila melanogaster larvae and antibacterial effects. Food Chem. Toxicol. 2017, 109, 1118–1124. [Google Scholar] [CrossRef]
- Fuertes, M.; Witkowski, J.; Streeter, D.; Robins, R. Synthesis and enzymic activity of 1,2,4-triazole-3-carboxamide 6’-deoxyhomoribonucleoside-6’-phosphonic acid and related compounds. Journal of Medicinal Chemistry. J. Mol. Struct. 1974, 17, 642–645. [Google Scholar]
- Shan, X.; Lv, M.; Wang, J.; Qin, Y.; Xu, H. Acaricidal and insecticidal efficacy of new esters derivatives of a natural coumarin osthole. Ind. Crop Prod. 2022, 182, 114855. [Google Scholar] [CrossRef]
- Qasim, M.; Islam, W.; Rizwan, M.; Hussain, D.; Noman, A.; Khan, K.A.; Ghramh, H.A.; Han, X. Impact of plant monoterpenes on insect pest management and insect-associated microbes. Heliyon 2024, 10, e39120. [Google Scholar] [CrossRef] [PubMed]
- Gallo, L.G.; Allee, L.L.; Gibson, D.M. Insecticidal effectiveness of Mammea Americana (Guttiferae) extracts on larvae of Dlabrotica Virgifera Virgifera (Coleoptera:Chrysomelidae) and Trichoplusia Ni (Lepidoptera:Noctuidae). Econ. Bot 1996, 50, 236–242. [Google Scholar] [CrossRef]
- Banks, W.A.; Lofgren, C.S.; Jouvenaz, D.P. Techniques for Collecting, Rearing, and Handling Imported Fire Ants; USDA-ARS (Science and Education Administration. Agricultural Research. Southern Region), US Department of Agriculture: Washington, DC, USA, 1981; Volume 21, pp. 1–9.
- Shao, L.; Wang, W.; Gong, X.; Yu, Y.; Xue, J.; Zeng, X.; Liu, J. The Toxicity Differences of Fluralaner against the Red Imported Fire Ant (Solenopsis invicta) at Different Developmental Stages. Int. J. Mol. Sci. 2023, 24, 15627. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Hu, Y.; Yang, L.; Lin, J.; Bai, H.; Li, Y.; Tanvir, R.; Li, L.; Bai, M.; Zhang, Z. Fumigation activity of essential oils of Cinnamomum loureirii toward red imported fire ant workers. J. Pest Sci. 2022, 96, 647–662. [Google Scholar] [CrossRef]
- Lin, S.; Qin, D.; Zhang, Y.; Zheng, Q.; Yang, L.; Cheng, D.; Huang, S.; Chen, J.; Zhang, Z. Toxicity and Sublethal Effects of Autumn. Crocus (Colchicum autumnale) Bulb Powder on Red Imported Fire Ants (Solenopsis invicta). Toxins 2020, 12, 731. [Google Scholar] [CrossRef]
- Ryabova, A.; Cornette, R.; Cherkasov, A.; Watanabe, M.; Gusev, O. Combined metabolome and transcriptome analysis reveals key components of complete desiccation tolerance in an anhydrobiotic insect. Proc. Natl. Acad. Sci. USA 2020, 117, 19209–19220. [Google Scholar] [CrossRef]
- Lian, W.; Wang, Y.; Zhang, J.; Yan, Y.; Xia, C.; Gan, H.; Wang, X.; Yang, T.; Xu, J.; He, J.; et al. The genus Datura L. (Solanaceae): A systematic review of botany, traditional use, phytochemistry, pharmacology, and toxicology. Phytochemistry 2022, 204, 113446. [Google Scholar] [CrossRef]
- Esmaealzadeh, D.; Moodi Ghalibaf, A.; Shariati Rad, M.; Rezaee, R.; Razavi, B.M.; Hosseinzadeh, H. Pharmacological effects of Safranal: An updated review. Iran. J. Basic. Med. Sci. 2023, 26, 1131–1143. [Google Scholar] [CrossRef]
- Du, Y.; Grodowitz, M.; Chen, J. Insecticidal and Enzyme Inhibitory Activities of Isothiocyanates against Red Imported Fire Ants. Biomolecules 2020, 10, 716. [Google Scholar] [CrossRef] [PubMed]
- Junaid, A.; Siddiqui, Y.; Yuanyuan, L.; Bamisope, S.B.; Naveed, U.R.; Waqar, I.; Muhammad, Q. Comprehensive Detoxification Mechanism Assessment of Red Imported Fire Ant (Solenopsis invicta) against Indoxacarb. Mol. Cancer 2022, 27, 870. [Google Scholar]





| Different Parts Extracts | Regression Equation | 50% Lethal Concentration (mg/mL) | 95% Fiducial Limits | p-Value |
|---|---|---|---|---|
| Leaf | Y = 0.008X − 1.366 | 166.253 | 144.867~194.392 | p < 0.05 |
| Stems | Y = 0.008X − 1.429 | 188.256 | 141.716~284.943 | p < 0.05 |
| Root | Y = 0.006X − 1.851 | 331.847 | 270.168~452.323 | p < 0.05 |
| Metabolite Name | Regression Equation | 50% Lethal Concentration (mg/L) | 95% Fiducial Limits | p-Value |
|---|---|---|---|---|
| Safranal | Y = 3.44X − 8.759 | 349.042 | 282.542~461.604 | p < 0.05 |
| Dihydrocoumarin | Y = 3.232X − 6.701 | 118.336 | 16.541~673.671 | p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Luo, R.; Hussain, M.; Wu, W.; Li, S.; Guo, Z.; Jia, B.; Bi, G.; Gao, X.; Wu, G.; et al. Nature-Identical Safranal and Dihydrocoumarin from Ageratina adenophora ((Spreng., 1970) King and H. Rob.) Target Energy Metabolism to Control Solenopsis invicta Buren, 1972 (Hymenoptera: Formicidae). Insects 2025, 16, 540. https://doi.org/10.3390/insects16050540
Wu M, Luo R, Hussain M, Wu W, Li S, Guo Z, Jia B, Bi G, Gao X, Wu G, et al. Nature-Identical Safranal and Dihydrocoumarin from Ageratina adenophora ((Spreng., 1970) King and H. Rob.) Target Energy Metabolism to Control Solenopsis invicta Buren, 1972 (Hymenoptera: Formicidae). Insects. 2025; 16(5):540. https://doi.org/10.3390/insects16050540
Chicago/Turabian StyleWu, Mingqi, Rongchao Luo, Mehboob Hussain, Wenmei Wu, Shini Li, Zijun Guo, Boyu Jia, Gaofeng Bi, Xi Gao, Guoxing Wu, and et al. 2025. "Nature-Identical Safranal and Dihydrocoumarin from Ageratina adenophora ((Spreng., 1970) King and H. Rob.) Target Energy Metabolism to Control Solenopsis invicta Buren, 1972 (Hymenoptera: Formicidae)" Insects 16, no. 5: 540. https://doi.org/10.3390/insects16050540
APA StyleWu, M., Luo, R., Hussain, M., Wu, W., Li, S., Guo, Z., Jia, B., Bi, G., Gao, X., Wu, G., & Qin, D. (2025). Nature-Identical Safranal and Dihydrocoumarin from Ageratina adenophora ((Spreng., 1970) King and H. Rob.) Target Energy Metabolism to Control Solenopsis invicta Buren, 1972 (Hymenoptera: Formicidae). Insects, 16(5), 540. https://doi.org/10.3390/insects16050540