Insect Pest Control from Chemical to Biotechnological Approach: Constrains and Challenges
Simple Summary
Abstract
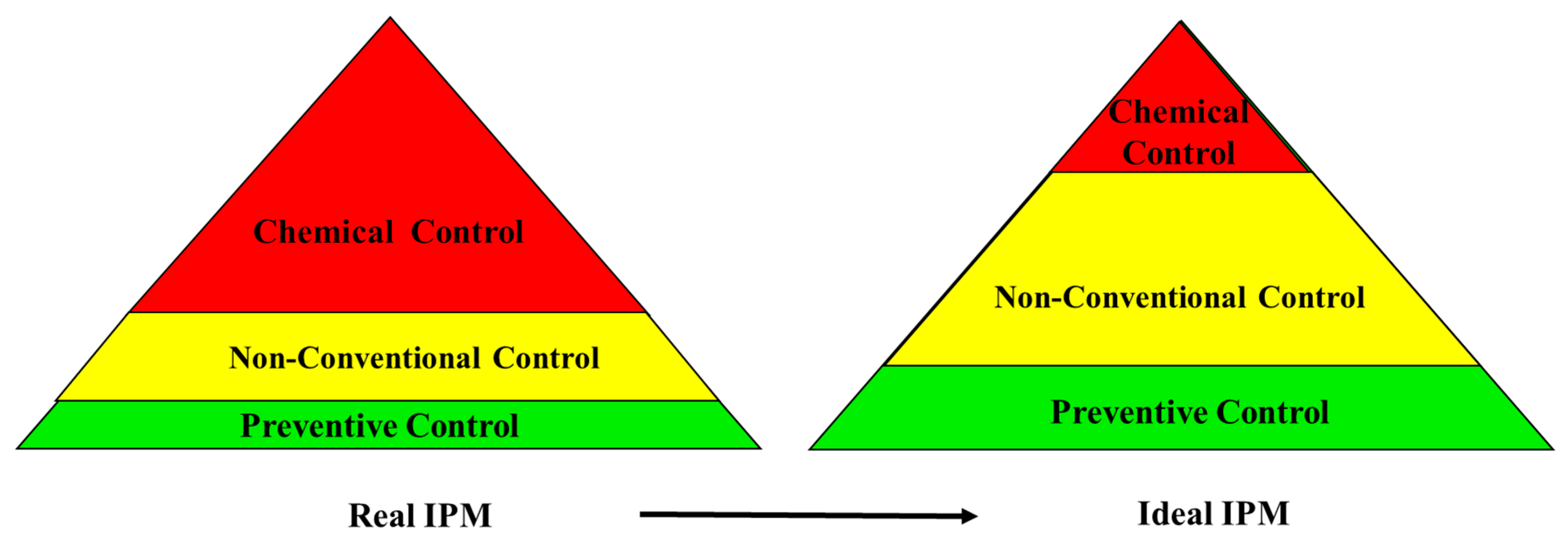
1. Introduction
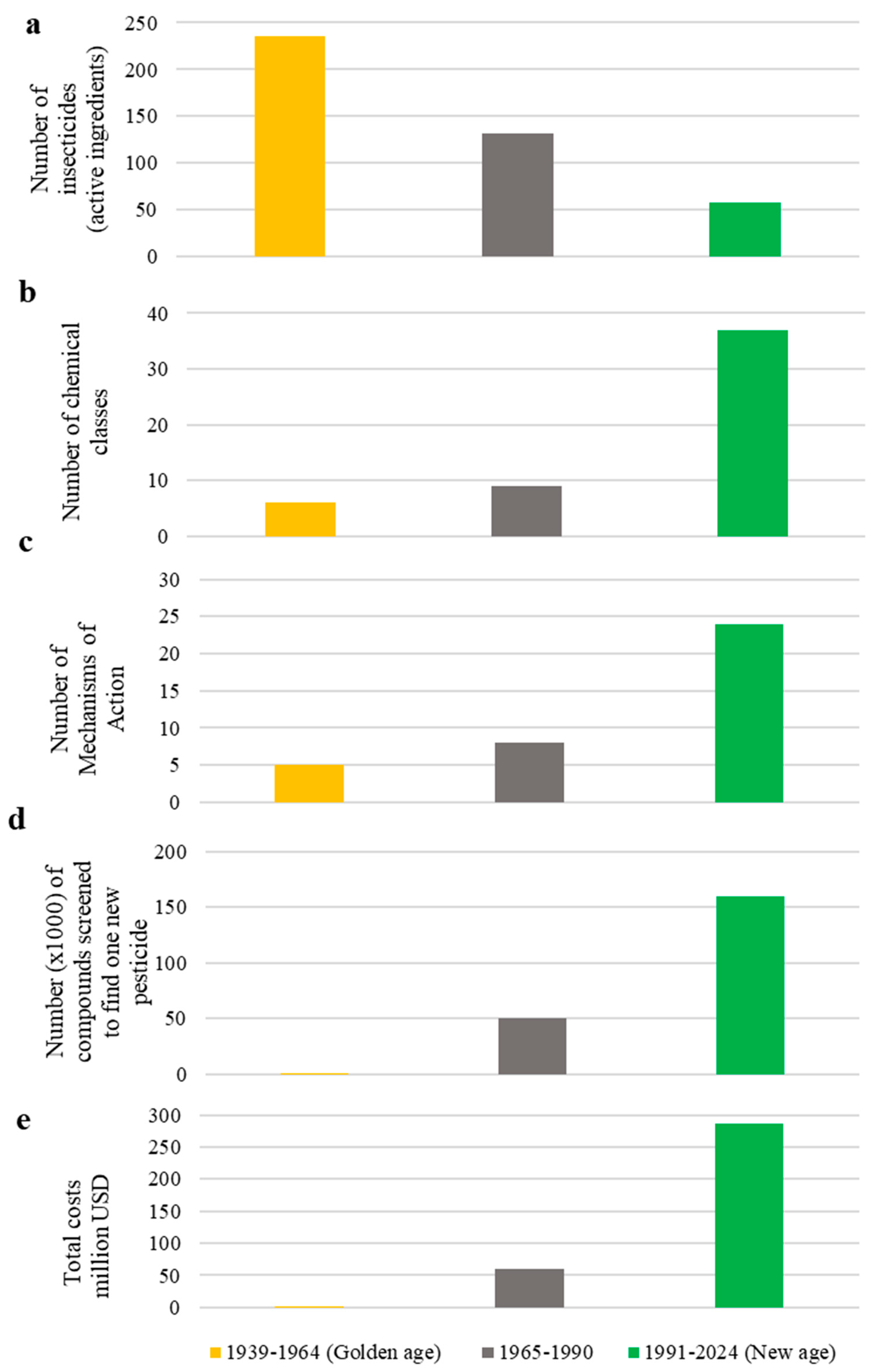
2. Chemical Control
3. Non-Conventional Control
3.1. Natural Substances
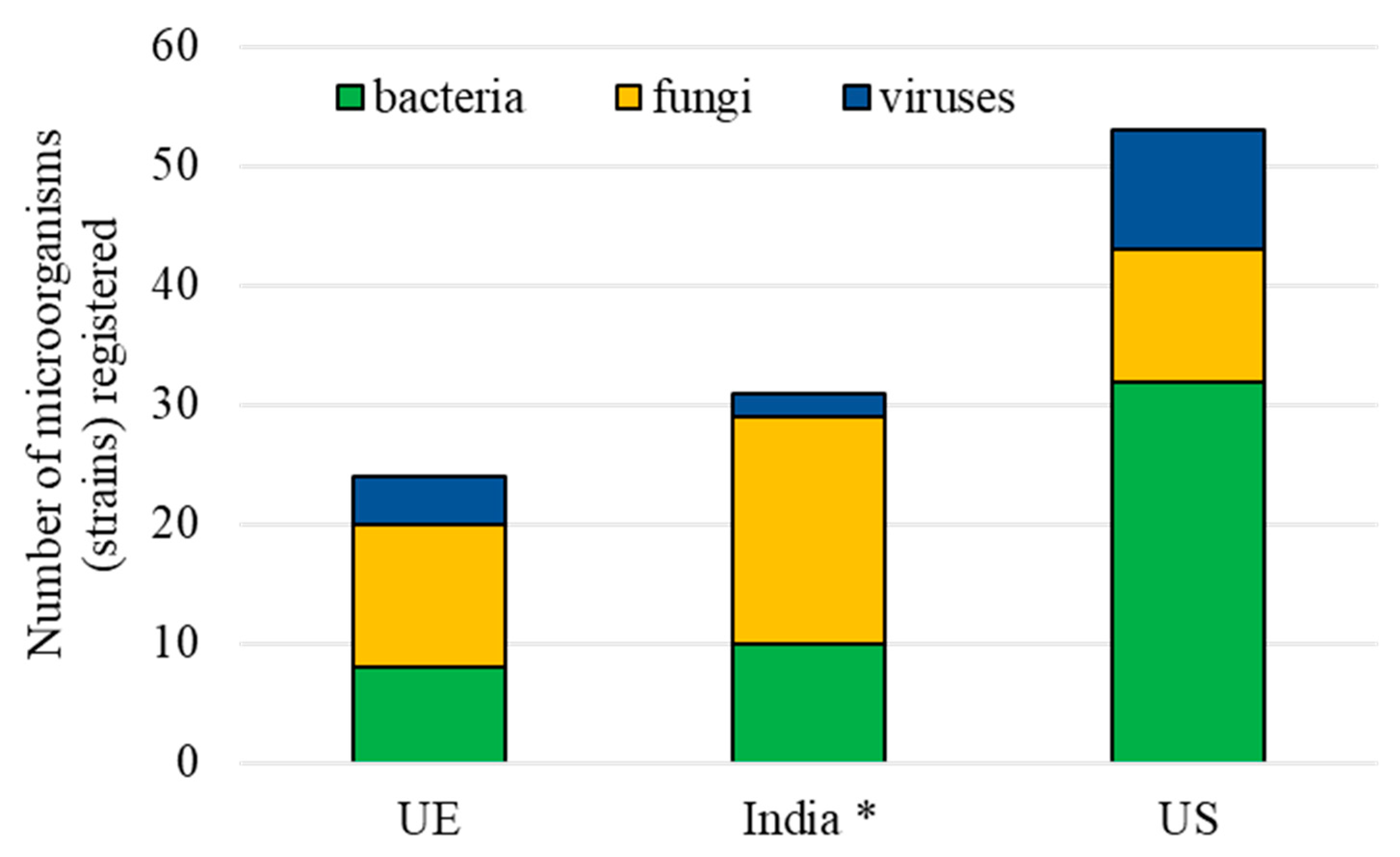
3.2. Entomopathogenic Microorganisms
3.3. Semiochemical
3.4. Biological Control
3.5. Biotechnological Control
3.5.1. Synthetic Peptides
3.5.2. RNA Interference
3.6. Genetic Control
3.7. Symbiotic Control and Paratransgenic Insect
3.8. Insect Pest-Resistant Transgenic Plants
3.9. Transgenic Entomopathogenic Microorganisms
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Sharma, S.; Kooner, R.; Arora, R. Insect Pests and Crop Losses. In Breeding Insect Resistant Crops for Sustainable Agriculture; Arora, R., Sandhu, S., Eds.; Springer: Singapore, 2017. [Google Scholar]
- Deguine, J.P.; Aubertot, J.N.; Flor, R.J.; Lescourret, F.; Wyckhuys, K.A.G.; Ratnadass, A. Integrated pest management: Good intentions, hard realities. A review. Agron. Sustain. Dev. 2021, 41, 38. [Google Scholar] [CrossRef]
- Zhou, W.; Arcot, Y.; Medina, R.F.; Bernal, J.; Cisneros-Zevallos, L.; Akbulut, M.E.S. Integrated Pest Management: An Update on the Sustainability Approach to Crop Protection. ACS Omega 2024, 9, 41130–41147. [Google Scholar] [CrossRef] [PubMed]
- Maienfisch, P.; Koerber, K. Recent innovations in crop protection research. Pest Manag. Sci. 2024, 81, 2406–2418. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Food & Agriculture Organization. FAO Publications Series 2021—Pesticides Use and Trade 1990–2022. 2023. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/a8a8c2c8-ee36-42e8-a619-7e73c8daf8a6/content (accessed on 30 April 2025).
- Sparks, T.C. Insecticide mixtures—Uses, benefits and considerations. Pest Manag. Sci. 2025, 81, 1137–1144. [Google Scholar] [CrossRef]
- Sparks, T.C. Insecticide discovery: An evaluation and analysis. Pest. Biochem. Physiol. 2013, 107, 8–17. [Google Scholar] [CrossRef]
- Sparks, T.C.; Crossthwaite, A.J.; Nauen, R.; Banba, S.; Cordova, D.; Earley, F.; Ebbinghaus-Kintscher, U.; Fujioka, S.; Hirao, A.; Karmon, D.; et al. Insecticides, biologics and nematicides: Updates to IRAC’s mode of action classification a tool for resistance management. Pest. Biochem. Physiol. 2020, 167, 104588. [Google Scholar] [CrossRef]
- Sparks, T.C.; Storer, N.; Porter, A.; Slater, R.; Nauen, R. Insecticide resistance management and industry: The origins and evolution of the Insecticide Resistance Action Committee (IRAC) and the mode of action classification scheme. Pest Manag. Sci. 2021, 77, 2609–2619. [Google Scholar] [CrossRef]
- Kathage, J.; Castañera, P.; Alonso-Prados, J.L.; Gómez-Barbero, M.; Rodríguez-Cerezo, E. The impact of restrictions on neonicotinoid and fipronil insecticides on pest management in maize, oilseed rape and sunflower in eight European Union regions. Pest Manag. Sci. 2018, 74, 88–99. [Google Scholar] [CrossRef]
- Casida, J.E.; Quistad, G.B. Golden age of insecticide research: Past, present, or future? Ann. Rev. Entomol. 1998, 43, 1–16. [Google Scholar] [CrossRef]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Kogan, M. Integrated pest management: Historical perspective and contemporary developments. Annu. Rev. Entomol. 1998, 43, 243–270. [Google Scholar] [CrossRef] [PubMed]
- Drew, K.L.; Baiman, H.; Khwaounjoo, P.; Yu, B.; Reynisson, J. Size estimation of chemical space: How big is it? J. Pharm. Pharmacol. 2012, 64, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.C.; Lorsbach, B.A. Perspectives on the agrochemical industry and agrochemical discovery. Pest Manag. Sci. 2017, 73, 672–677. [Google Scholar] [CrossRef]
- Marrone, P.G. Pesticidal natural products-status and future potential. Pest Manag. Sci. 2019, 75, 2325–2340. [Google Scholar] [CrossRef]
- Sparks, T.C.; Bryant, R.J. Crop protection compounds—Trends and perspective. Pest Manag. Sci. 2021, 77, 3608–3616. [Google Scholar] [CrossRef]
- Sparks, T.C.; Bryant, R.J. East meets west: Regional impact on agrochemical discovery and innovation. Pest Manag. Sci. 2021, 77, 4211–4223. [Google Scholar] [CrossRef]
- Sparks, T.C.; Lorsbach, B.A. Insecticide discovery—“Chance favors the prepared mind”. Pest. Biochem. Physiol. 2023, 192, 105412. [Google Scholar] [CrossRef]
- Jeschke, P. Recent developments in fluorine-containing pesticides. Pest Manag. Sci. 2024, 80, 3065–3087. [Google Scholar] [CrossRef]
- Jeschke, P. The continuing significance of chiral agrochemicals. Pest Manag. Sci. 2025, 81, 1697–1716. [Google Scholar] [CrossRef]
- Umetsu, N.; Shirai, Y. Development of novel pesticides in the 21st century. J. Pest. Sci. 2020, 45, 54–74. [Google Scholar] [CrossRef]
- Swale, D.R. Perspectives on new strategies for the identification and development of insecticide targets. Pest. Biochem. Physiol. 2019, 161, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.L.; Groom, C.R. The druggable genome. Nat. Rev. Drug Discov. 2002, 1, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, G.; Austin, C.; Anderson, J.; Pawlyk, A.; Colvis, C.; Margolis, R.; Baker, J. Glimmers in illuminating the druggable genome. Nat. Rev. Drug Discov. 2018, 17, 301–302. [Google Scholar] [CrossRef]
- Santos, R.; Ursu, O.; Gaulton, A.; Bento, A.P.; Donadi, R.S.; Bologa, C.G.; Karlsson, A.; Al-Lazikani, B.; Hersey, A.; Oprea, T.I.; et al. A comprehensive map of molecular drug targets. Nat. Rev. Drug Discov. 2017, 16, 19–34. [Google Scholar] [CrossRef]
- Casida, J.E. Pesticide mode of action: Evidence for and implications of a finite number of biochemical targets. In Pesticides and Alternatives: Innovative Chemical and Biological Approaches to Pest Control; Casida, J.E., Ed.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Sparks, T.C.; Bryant, R.J. Impact of natural products on discovery of, and innovation in, crop protection compounds. Pest Manag. Sci. 2022, 78, 399–408. [Google Scholar] [CrossRef]
- Wing, K.D. Pharmaceutical technologies with potential application to insecticide discovery. Pest Manag. Sci. 2021, 77, 3617–3625. [Google Scholar] [CrossRef]
- Yang, D.; Zhou, Q.; Labroska, V.; Qin, S.; Darbalaei, S.; Wu, Y.; Yuliantie, E.; Xie, L.; Tao, H.; Cheng, J.; et al. G protein-coupled receptors: Structure- and function-based drug discovery. Signal Transduct. Target Ther. 2021, 6, 7. [Google Scholar]
- Gressel, J. Perspective: It is time to consider new ways to attack unpesticidable (undruggable) target sites by designing peptide pesticides. Pest Manag. Sci. 2022, 78, 2108–2112. [Google Scholar] [CrossRef]
- Altstein, M.; Ben-Aziz, O.; Schefler, I.; Zeltser, I.; Gilon, C. Advances in the application of neuropeptides in insect control. Crop Prot. 2000, 19, 547–555. [Google Scholar] [CrossRef]
- Scherkenbeck, J.; Zdobinsky, T. Insect neuropeptides: Structures, chemical modifications and potential for insect control. Bioorganic Med. Chem. 2009, 17, 4071–4084. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Kumar, S.; Singh, A.; Raghava, G.P.S.; Singh, I.K. NeuroPIpred: A tool to predict, design and scan insect neuropeptides. Sci. Rep. 2019, 9, 5129. [Google Scholar] [CrossRef]
- Sparks, T.C.; Hahn, D.R.; Garizi, N.V. Natural products, their derivatives, mimics and synthetic equivalents. Pest Manag. Sci. 2017, 73, 700–715. [Google Scholar] [CrossRef]
- Chandler, D.; Bailey, A.S.; Tatchell, G.M.; Davidson, G.; Greaves, J.; Grant, W.P. The development, regulation and use of biopesticides for integrated pest management. Philosophical transactions of the Royal Society of London. Biol. Sci. 2011, 366, 1987–1998. [Google Scholar] [CrossRef]
- Czaja, K.; Góralczyk, K.; Struciński, P.; Hernik, A.; Korcz, W.; Minorczyk, M.; Łyczewska, M.; Ludwicki, J.K. Biopesticides-towards increased consumer safety in the European Union. Pest Manag. Sci. 2015, 71, 3–6. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical Insecticides in the Twenty-First Century-Fulfilling Their Promise? Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar] [CrossRef]
- Mordue, A.J.; Blackwell, A. Azadirachtin: An update. J. Insect Physiol. 1993, 39, 903–924. [Google Scholar] [CrossRef]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential oils in insect control: Low-risk products in a high-stakes world. Annu. Rev. Entomol. 2012, 57, 405–425. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B.; Tak, J.-H. Commercialization of insecticides based on plant essential oils: Past, present, and future. In Green Pesticides Handbook: Essential Oils for Pest Control; Nollet, L.M.L., Rathore, H.S., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 27–39. [Google Scholar]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential Oils as Potential Alternative Biocontrol Products against Plant Pathogens and Weeds: A Review. Foods 2020, 9, 365. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Ye, D.X.; Liu, Y.; Zhang, X.Y.; Zhou, Y.L.; Zhang, L.; Yang, X.L. Peptides, new tools for plant protection in eco-agriculture. Adv. Agrochem 2023, 2, 58–78. [Google Scholar] [CrossRef]
- Gressent, F.; Da Silva, P.; Eyraud, V.; Karaki, L.; Royer, C. Pea albumin 1 subunit b (PA1b), a promising bioinsecticide of plant origin. Toxins 2011, 3, 1502–1517. [Google Scholar] [CrossRef] [PubMed]
- Diya, F.; Rahioui, I.; Vallier, A.; Benhamou, S.; Sivignon, C.; Kfoury, L.; Rizk, F.; Da Silva, P. Vicia sativa subsp. sativa native to the Middle East comprises Pea Albumin1 b-like homologs: A promising natural biopesticide. Heliyon 2024, 10, e26903. [Google Scholar] [CrossRef]
- Eyraud, V.; Balmand, S.; Karaki, L.; Rahioui, I.; Sivignon, C.; Delmas, A.F.; Royer, C.; Rahbé, Y.; Da Silva, P.; Gressent, F. The interaction of the bioinsecticide PA1b (Pea albumin 1 subunit b) with the insect V-ATPase triggers apoptosis. Sci. Rep. 2017, 7, 4902. [Google Scholar] [CrossRef]
- Grover, T.; Mishra, R.; Gulati, P.; Mohanty, A. An insight into biological activities of native cyclotides for potential applications in agriculture and pharmaceutic. Peptides 2021, 135, 170430. [Google Scholar] [CrossRef]
- Oguis, G.K.; Gilding, E.K.; Jackson, M.A.; Craik, D.J. Butterfly Pea (Clitoria ternatea), a Cyclotide-Bearing Plant with applications in agriculture and medicine. Front. Plant Sci. 2019, 10, 645. [Google Scholar] [CrossRef]
- Oguis, G.K.; Gilding, E.K.; Huang, Y.-H.; Poth, A.G.; Jackson, M.A.; Craik, D.J. Insecticidal diversity of butterfly pea (Clitoria ternatea) accessions. Ind. Crops Prod. 2020, 147, 112214. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Adeleke, B.S.; Akinola, S.A.; Fayose, C.A.; Adeyemi, U.T.; Gbadegesin, L.A.; Omole, R.K.; Johnson, R.M.; Uthman, Q.O.; Babalola, O.O. Biopesticides as a promising alternative to synthetic pesticides: A case for microbial pesticides, phytopesticides, and nanobiopesticides. Front. Microbiol. 2023, 14, 1040901. [Google Scholar] [CrossRef]
- Damalas, C.A.; Koutroubas, S.D. Botanical pesticides for eco-friendly pest management: Drawbacks and limitations. Pestic. Crop Prod. Physiol. Biochem. Action 2020, 10, 181–193. [Google Scholar]
- Vekemans, M.C.; Marchand, P.A. The fate of biocontrol agents under the European phytopharmaceutical regulation: How this regulation hinders the approval of botanicals as new active substances. Environ. Sci. Pollut. Res. 2020, 27, 39879–39887. [Google Scholar] [CrossRef]
- King, G.F. Tying pest insects in knots: The deployment of spider-venom-derived knottins as bioinsecticides. Pest Manag. Sci. 2019, 75, 2437–2445. [Google Scholar] [CrossRef]
- King, G.F.; Hardy, M.C. Spider-Venom Peptides: Structure, Pharmacology, and Potential for Control of Insect Pests. Annu. Rev. Entomol. 2013, 58, 475–496. [Google Scholar] [CrossRef] [PubMed]
- Lasota, J.A.; Dybas, R.A. Abamectin as a pesticide for agricultural use. Acta Leiden. 1990, 59, 217–225. [Google Scholar] [PubMed]
- Sparks, T.C.; Crouse, G.D.; Benko, Z.; Demeter, D.; Giampietro, N.C.; Lambert, W.; Brown, A.V. The spinosyns, spinosad, spinetoram, and synthetic spinosyn mimics—Discovery, exploration, and evolution of a natural product chemistry and the impact of computational tools. Pest Manag. Sci. 2021, 77, 3637–3649. [Google Scholar] [CrossRef]
- Isman, M.B.; Grieneisen, M.L. Botanical insecticide research: Many publications, limited useful data. Trends Plant Sci. 2014, 19, 140–145. [Google Scholar] [CrossRef]
- Chaudhary, R.; Nawaz, A.; Khattak, Z.; Butt, M.A.; Fouillaud, M.; Dufossé, L.; Munir, M.; ul Haq, I.; Mukhtar, H. Microbial bio-control agents: A comprehensive analysis on sustainable pest management in agriculture. J. Agric. Food Res. 2024, 18, 101421. [Google Scholar] [CrossRef]
- Hernández-Rosas, F.; Figueroa-Rodríguez, K.A.; García-Pacheco, L.A.; Velasco-Velasco, J.; Sangerman-Jarquín, D.M. Microorganisms and Biological Pest Control: An Analysis Based on a Bibliometric Review. Agronomy 2020, 10, 1808. [Google Scholar] [CrossRef]
- Ragasruthi, M.; Balakrishnan, N.; Murugan, M.; Swarnakumari, N.; Harish, S.; Jeya Sundara Sharmila, D. Bacillus thuringiensis (Bt)-based biopesticide: Navigating success, challenges, and future horizons in sustainable pest control. Sci. Total Environ. 2024, 954, 176594. [Google Scholar] [CrossRef]
- Adang, M.J.; Crickmore, N.; Jurat-fuentes, J.L. Diversity of Bacillus thuringiensis crystal toxins and mechanism of action. Adv. Insect Physiol. 2014, 47, 39–87. [Google Scholar]
- Bravo, A.; Likitvivatanavong, S.; Gill, S.S.; Soberón, M. Bacillus thuringiensis: A story of a successful bioinsecticide. Insect Biochem. Mol. Biol. 2011, 41, 423–431. [Google Scholar] [CrossRef]
- Gill, S.S.; Cowles, E.A.; Pietrantonio, P.V. The mode of action of Bacillus thuringiensis endotoxins. Annu. Rev. Entomol. 1992, 37, 615–636. [Google Scholar] [CrossRef] [PubMed]
- Gassmann, A.J.; Reisig, D.D. Management of Insect Pests with Bt Crops in the United States. Annu. Rev. Entomol. 2023, 68, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Romeis, J.; Naranjo, S.E.; Meissle, M.; Shelton, A.M. Genetically engineered crops help support conservation biological control. Biol. Control. 2019, 130, 136–154. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Carrière, Y.; Wu, Y.; Fabrick, J.A. Global perspectives on field-evolved resistance to transgenic Bt crops: A special collection. J. Econ. Entomol. 2023, 116, 269–274. [Google Scholar] [CrossRef]
- Jurat-Fuentes, J.L.; Heckel, D.G.; Ferré, J. Mechanisms of Resistance to Insecticidal Proteins from Bacillus thuringiensis. Annu. Rev. Entomol. 2021, 66, 121–140. [Google Scholar] [CrossRef]
- Matyjaszczyk, E. Products containing microorganisms as a tool in integrated pest management and the rules of their market placement in the European Union. Pest Manag. Sci. 2015, 71, 1201–1206. [Google Scholar] [CrossRef]
- Kumar, K.K.; Sridhar, J.; Murali-Baskaran, R.K.; Senthil-Nathan, S.; Kaushal, P.; Dara, S.K.; Arthurs, S. Microbial biopesticides for insect pest management in India: Current status and future prospects. J. Invert. Path. 2019, 165, 74–81. [Google Scholar] [CrossRef]
- Marchand, P.A. Evolution of plant protection active substances in Europe: The disappearance of chemicals in favour of biocontrol agents. Environ. Sci. Pollut. Res. 2023, 30, 1–17. [Google Scholar] [CrossRef]
- Robin, D.C.; Marchand, P.A. Evolution of the biocontrol active substances in the framework of the European Pesticide Regulation (EC) No. 1107/2009. Pest Manag. Sci. 2019, 75, 950–958. [Google Scholar] [CrossRef]
- Mazzoni, V.; Anfora, G.; Cocroft, R.B.; Fatouros, N.E.; Groot, A.T.; Gross, J.; Hill, P.S.M.; Hoch, H.; Ioriatti, C.; Nieri, R.; et al. Bridging biotremology and chemical ecology: A new terminology. Trends Plant Sci. 2024, 29, 848–855. [Google Scholar] [CrossRef]
- Yanagisawa, R.; Tatsuta, H.; Sekine, T.; Oe, T.; Mukai, H.; Uechi, N.; Koike, T.; Onodera, R.; Suwa, R.; Takanashi, T. Vibrations as a new tool for pest management—A review. Entomol. Exp. Applicata 2024, 172, 1116–1127. [Google Scholar] [CrossRef]
- Nieri, R.; Anfora, G.; Mazzoni, V.; Rossi Stacconi, M.V. Semiochemicals, semiophysicals and their integration for the development of innovative multi-modal systems for agricultural pests’ monitoring and control. Entomol. Gen. 2022, 42, 167–183. [Google Scholar] [CrossRef]
- Cardè, R.T.; Minks, A.K. Control of Moth Pests by Mating Disruption: Successes and Constraints. Annu. Rev. Entomol. 1995, 40, 559–585. [Google Scholar] [CrossRef]
- Howse, P.E.; Stevens, I.D.R.; Jones, O.T. Mass trapping. In Insect Pheromones and Their Use in Pest Management; Springer: Dordrecht, The Netherlands, 1998; pp. 280–299. [Google Scholar]
- Gregg, P.C.; Del Socorro, A.P.; Landolt, P.J. Advances in Attract-and-Kill for Agricultural Pests: Beyond Pheromones. Annu. Rev. Entomol. 2018, 63, 453–470. [Google Scholar] [CrossRef]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The Use of Push-Pull Strategies in Integrated Pest Management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef]
- Gaston, L.K.; Shorey, H.H.; Saario, C.A. Insect population control by the use of sex pheromones to inhibit orientation between the sexes. Nature 1967, 213, 155. [Google Scholar] [CrossRef]
- Masetti, A.; Morelli, A.; Fagioli, L.; Pradolesi, G.; Nicoli, R.; Scagliarini, O.; Tommasini, M.G.; Preti, M. Evaluation of an Attract-and-Kill Strategy Using Long-Lasting Insecticide Nets for the Management of the Brown Marmorated Stink Bug in Northern Italy. Insects 2024, 15, 577. [Google Scholar] [CrossRef]
- Paoli, F.; Iovinella, I.; Barbieri, F.; Sciandra, C.; Sabbatini Peverieri, G.; Mazza, G.; Torrini, G.; Barzanti, G.P.; Benvenuti, C.; Strangi, A.; et al. Effectiveness of field-exposed attract-and-kill devices against the adults of Popillia japonica (Coleoptera: Scarabaeidae): A study on duration, form and storage. Pest Manag. Sci. 2023, 79, 3262–3270. [Google Scholar] [CrossRef]
- Bale, J.S.; van Lenteren, J.C.; Bigler, F. Biological control and sustainable food production. Phil. Trans. R. Soc. B 2008, 363, 761–776. [Google Scholar] [CrossRef]
- Cock, M.J.W.; van Lenteren, J.C.; Brodeur, J.; Barratt, B.I.P.; Bigler, F.; Bolckmans, K.; Cônsoli, F.L.; Haas, F.; Mason, P.G.; Parra, J.R.P. Do new access and benefit sharing procedures under the convention on biological diversity threaten the future of biological control? BioControl 2010, 55, 199–218. [Google Scholar] [CrossRef]
- van Lenteren, J.C. Quality Control and Production of Biological Control Agents: Theory and Testing Procedures; CABI Publishing: Wallingford, UK, 2003. [Google Scholar]
- van Lenteren, J.C. The state of commercial augmentative biological control: Plenty of natural enemies, but a frustrating lack of uptake. BioControl 2012, 57, 1–20. [Google Scholar] [CrossRef]
- Bolckmans, K.J.F. Commercial aspects of biological pest control in greenhouses. In Integrated Pest, Disease Management in Greenhouse Crops; Albajes, R., Gullino, M.L., van Lenteren, J.C., Elad, Y., Eds.; Kluwer Publishers: Dordrecht, The Netherlands, 1999; pp. 310–318. [Google Scholar]
- Tortorici, F.; Bombi, P.; Loru, L.; Mele, A.; Moraglio, S.T.; Scaccini, D.; Pozzebon, A.; Pantaleoni, R.A.; Tavella, L. Halyomorpha halys and its egg parasitoids Trissolcus japonicus and T. mitsukurii: The geographic dimension of the interaction. NeoBiota 2023, 85, 197–221. [Google Scholar] [CrossRef]
- Fellin, L.; Grassi, A.; Puppato, S.; Saddi, A.; Anfora, G.; Ioriatti, C.; Rossi Stacconi, M.V. First report on classical biological control releases of the larval parasitoid Ganaspis brasiliensis against Drosophila suzukii in northern Italy. BioControl 2023, 68, 1–12. [Google Scholar] [CrossRef]
- Schoofs, L.; De Loof, A.; Van Hiel, M.B. Neuropeptides as Regulators of Behavior in Insects. Annu. Rev. Entomol. 2017, 62, 35–52. [Google Scholar] [CrossRef]
- Audsley, N.; Down, R.E. G protein coupled receptors as targets for next generation pesticides. Insect Biochem. Mol. Biol. 2015, 67, 27–37. [Google Scholar] [CrossRef]
- Birgül Iyison, N.; Shahraki, A.; Kahveci, K.; Düzgün, M.B.; Gün, G. Are insect GPCRs ideal next-generation pesticides: Opportunities and challenges. FEBS J. 2021, 288, 2727–2745. [Google Scholar] [CrossRef]
- Masler, E.P.; Kelly, T.J.; Menn, J.J. Insect neuropeptides: Discovery and application in insect management. Arch. Insect Biochem. Physiol. 1993, 22, 87–111. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Zhang, Y.; Zhao, Y.; Xu, W.; Ye, D.; He, Q.; Iqbal, C.; Feng, H.; Li, X.; et al. Insect kinin mimics act as potential control agents for aphids: Structural modifications of Trp4. J. Pept. Sci. 2023, 29, e3444. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, C.; Lin, T.; Ji, Q.; Han, Q.; Liu, A.; Chen, J.; Liu, T.; Ran, W. A Novel Insect Short Neuropeptide sNPF Peptidomimetic Insecticide: Rational Design, Synthesis, and Aphicidal Activity Study. J. Pept. Sci. 2025, 31, e3669. [Google Scholar] [CrossRef]
- Jeffers, L.A.; Shen, H.; Khalil, S.; Bissinger, B.W.; Brandt, A.; Gunnoe, T.B.; Roe, R.M. Enhanced activity of an insecticidal protein, trypsin modulating oostatic factor (TMOF), through conjugation with aliphatic polyethylene glycol. Pest Manag. Sci. 2012, 68, 49–59. [Google Scholar] [CrossRef]
- Wu, W.; Ali, A.; Shen, J.; Ren, M.; Cai, Y.; He, L. Cell Penetrating Peptide Enhances the aphidicidal activity of Spider Venom-Derived Neurotoxin. Toxins 2024, 16, 358. [Google Scholar] [CrossRef] [PubMed]
- Al Musaimi, O.; AlShaer, D.; de la Torre, B.G.; Albericio, F. 2024 FDA TIDES (Peptides and Oligonucleotides) Harvest. Pharmaceuticals 2025, 18, 291. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.J.; de Campos, L.J.; Xing, H.; Conda-Sheridan, M. Peptide-based therapeutics: Challenges and solutions. Med. Chem. Res. 2024, 33, 1275–1280. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Palli, S.R. Mechanisms, Applications, and Challenges of Insect RNA Interference. Annu. Rev. Entomol. 2020, 65, 293–311. [Google Scholar] [CrossRef]
- Meister, G.; Tuschl, T. Mechanisms of gene silencing by double-stranded RNA. Nature 2024, 431, 343–349. [Google Scholar] [CrossRef]
- Christiaens, O.; Sweet, J.; Dzhambazova, T.; Urru, I.; Smagghe, G.; Kostov, K.V.; Arpaia, S. Implementation of RNAi-based arthropod pest control: Environmental risks, potential for resistance and regulatory considerations. J. Pest Sci. 2021, 95, 1–15. [Google Scholar] [CrossRef]
- Cooper, A.M.W.; Silver, K.; Zhang, J.; Parka, Y.; Zhua, K.Y. Molecular mechanisms influencing efficiency of RNA interference in insects. Pest Manag. Sci. 2019, 75, 18–28. [Google Scholar] [CrossRef]
- He, L.; Huang, Y.; Tang, X. RNAi-based pest control: Production, application and the fate of dsRNA. Front. Bioeng. Biotechnol. 2022, 10, 1080576. [Google Scholar] [CrossRef]
- Head, G.P.; Carroll, M.W.; Evans, S.P.; Rule, D.M.; Willse, A.R.; Clark, T.L.; Storer, N.P.; Flannagan, R.D.; Samuel, L.W.; Meinke, L.J. Evaluation of SmartStax and SmartStax PRO maize against western corn rootworm and northern corn rootworm: Efficacy and resistance management. Pest Manag. Sci. 2017, 73, 1883–1899. [Google Scholar] [CrossRef]
- Niu, D.; Hamby, R.; Sanchez, J.N.; Cai, Q.; Yan, Q.; Jin, H. RNAs—A new frontier in crop protection. Curr. Opin. Biotechnol. 2021, 70, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Taning, C.N.; Arpaia, S.; Christiaens, O.; Dietz-Pfeilstetter, A.; Jones, H.; Mezzetti, B.; Sabbadini, S.; Sorteberg, H.G.; Sweet, J.; Ventura, V.; et al. RNA-based biocontrol compounds: Current status and perspectives to reach the market. Pest Manag. Sci. 2020, 76, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Petek, M.; Coll, A.; Ferenc, R.; Razinger, J.; Gruden, K. Validating the potential of double-stranded RNA targeting Colorado potato beetle mesh gene in laboratory and field trials. Front. Plant Sci. 2020, 11, 1250. [Google Scholar] [CrossRef]
- List, F.; Tarone, A.M.; Zhu-Salzman, K.; Vargo, E.L. RNA meets toxicology: Efficacy indicators from the experimental design of RNAi studies for insect pest management. Pest Manag. Sci. 2022, 78, 3215–3225. [Google Scholar] [CrossRef]
- Adeyinka, O.S.; Riaz, S.; Toufiq, N.; Yousaf, I.; Bhatti, M.U.; Batcho, A.; Olajide, A.A.; Nasir, I.A.; Tabassum, B. Advances in exogenous RNA delivery techniques for RNAi-mediated pest control. Mol. Biol. Rep. 2020, 47, 6309–6319. [Google Scholar] [CrossRef]
- Rodrigues, T.B.; Mishra, S.K.; Sridharan, K.; Barnes, E.R.; Alyokhin, A.; Tuttle, R.; Kokulapalan, W.; Garby, D.; Skizim, N.J.; Tang, Y.W.; et al. First Sprayable Double-Stranded RNA-Based Biopesticide Product Targets Proteasome Subunit Beta Type-5 in Colorado Potato Beetle (Leptinotarsa decemlineata). Front. Plant Sci. 2021, 12, 728652. [Google Scholar] [CrossRef]
- Chen, Y.; De Schutter, K. Biosafety aspects of RNAi-based pests control. Pest Manag. Sci. 2024, 80, 3697–3706. [Google Scholar] [CrossRef]
- Dalakouras, A.; Koidou, V.; Papadopoulou, K. DsRNA-based pesticides: Considerations for efficiency and risk assessment. Chemosphere 2024, 352, 141530. [Google Scholar] [CrossRef]
- De Schutter, K.; Taning, C.N.T.; Van Daele, L.; Van Damme, E.J.M.; Dubruel, P.; Smagghe, G. RNAi-Based Biocontrol Products: Market Status, Regulatory Aspects, and Risk Assessment. Front. Insect Sci. 2022, 1, 818037. [Google Scholar] [CrossRef]
- Klassen, W.; Curtis, C.F.; Hendrichs, J. History of the sterile insect technique. In Sterile Insect Technique, 2nd ed.; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 1–44. [Google Scholar]
- Knipling, E.F. Possibilities of insect control or eradication through the use of sexually sterile males. J. Econ. Entomol. 1955, 48, 902–904. [Google Scholar] [CrossRef]
- Franz, G.; Gencheva, E.; Kerremans, P. Improved stability of genetic sex-separation strains for the Mediterranean fruit fly, Ceratitis capitata. Genome 1994, 37, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Ant, T.; Koukidou, M.; Rempoulakis, P.; Gong, H.-F.; Economopoulos, A.; Vontas, J.; Alpheyet, L. Control of the olive fruit fly using genetics-enhanced sterile insect technique. BMC Biol. 2012, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Condon, K.C.; Epton, M.J.; Gong, P.; Jin, L.; Condon, G.C.; Morrison, N.I.; Dafa’alla, T.H.; Alphey, L. Female-specific insect lethality engineered using alternative splicing. Nat. Biotechnol. 2007, 25, 353–357. [Google Scholar] [CrossRef]
- Yan, Y.; Aumann, R.A.; Häcker, I.; Schetelig, M.F. CRISPR-based genetic control strategies for insect pests. J. Integr. Agric. 2023, 22, 651–668. [Google Scholar] [CrossRef]
- Yan, Y.; Ahmed, H.M.M.; Wimmer, E.A.; Schetelig, M.F. Biotechnology-enhanced genetic controls of the global pest Drosophila suzukii. Trends Biotechnol. 2024, 43, 826–837. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, L.; Wei, L.; Wang, Y.; Han, Z. Advancements and Future Prospects of CRISPR-Cas-Based Population Replacement Strategies in Insect Pest Management. Insects 2024, 15, 653. [Google Scholar] [CrossRef]
- Morrison, N.I. Self-Limiting Insects for Pest Management. In Transgenic Insects: Techniques and Applications, 2nd ed.; Benedict, M.Q., Scott, M.J., Eds.; CABI International: Wallingford, UK, 2022; pp. 459–473. [Google Scholar]
- Thizy, D.; Carter, L.; Coche, I.; Delborne, J.A.; Emerson, C.; Kormos, A.; Mankad, A.; Pare Tore, L.; Roberts, A.; Rohwer, Y. Public acceptability and stakeholder engagement for genetic control technologies. In Transgenic Insects: Techniques and Applications, 2nd ed.; Benedict, M.Q., Scott, M.J., Eds.; CABI International: Wallingford, UK, 2022; pp. 474–492. [Google Scholar]
- Beech, C.; Rose, N.; Dass, B. Regulation of Transgenic Insects. In Transgenic Insects: Techniques and Applications, 2nd ed.; Benedict, M.Q., Scott, M.J., Eds.; CABI International: Wallingford, UK, 2022; pp. 493–517. [Google Scholar]
- Hayes, K.R.; Quinlan, M.M. Risk Analysis of Transgenic Insects. In Transgenic Insects: Techniques and Applications, 2nd ed.; Benedict, M.Q., Scott, M.J., Eds.; CABI International: Wallingford, UK, 2022; pp. 552–578. [Google Scholar]
- Klassen, W.; Vreysen, M.J.B. Area-Wide Integrated Pest Management and the Sterile Insect Technique. In Sterile Insect Technique, 2nd ed.; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 75–112. [Google Scholar]
- Pérez-Staples, D.; Díaz-Fleischer, F.; Montoya, P. The Sterile Insect Technique: Success and Perspectives in the Neotropics. Neotrop. Entomol. 2021, 50, 172–185. [Google Scholar] [CrossRef]
- Marec, F.; Vreysen, M.J.B. Advances and challenges of using the sterile insect technique for the management of pest lepidoptera. Insects 2019, 10, 371. [Google Scholar] [CrossRef]
- Homem, R.A.; Mateos-Fierro, Z.; Jones, R.; Gilbert, D.; Mckemey, A.R.; Slade, G.; Fountain, M.T. Field Suppression of Spotted Wing Drosophila (SWD) (Drosophila suzukii Matsumura) Using the Sterile Insect Technique (SIT). Insects 2022, 13, 328. [Google Scholar] [CrossRef]
- Roselli, G.; Anfora, G.; Suckling, D.M.; Mazzoni, V.; Vanoni, V.; Menegotti, L.; Fellin, L.; Rossi Stacconi, M.V.; Ioriatti, C.; Cristofaro, M. Effects of Irradiation on Biology and Mating Behaviour of Wild Males of Brown Marmorated Stink Bug Using a 6 MV Medical Linear Accelerator. Insects 2023, 14, 460. [Google Scholar] [CrossRef]
- Gong, J.T.; Li, T.P.; Wang, M.K.; Hong, X.Y. Wolbachia-based strategies for control of agricultural pests. Curr. Opin. Insect Sci. 2023, 57, 101039. [Google Scholar] [CrossRef] [PubMed]
- Zabalou, S.; Riegler, M.; Theodorakopoulou, M.; Stauffer, C.; Savakis, C.; Bourtzis, K. Wolbachia-induced cytoplasmic incompatibility as a means for insect pest population control. Proc. Natl. Acad. Sci. USA 2004, 101, 15042–15045. [Google Scholar] [CrossRef] [PubMed]
- Neuenschwander, P.; Russ, K.; Hoebaus, E.; Michelakis, S. Ecological studies on Rhagoletis cerasi in Crete for the use of the incompatible insect technique. In Fruit Flies of Economic Importance, Proceedings of the CEC/IOBC International Symposium, Athens, Greece, 16–19 November 1982; Calvalloro, R., Ed.; Balkema: Rotterdam, The Netherlands, 1983; pp. 366–370. [Google Scholar]
- Nikolouli, K.; Colinet, H.; Renault, D.; Enriquez, T.; Mouton, L.; Gibert, P.; Sassu, F.; Cáceres, C.; Stauffer, C.; Pereira, R.; et al. Sterile insect technique and Wolbachia symbiosis as potential tools for the control of the invasive species Drosophila suzukii. J. Pest Sci. 2018, 91, 489–503. [Google Scholar] [CrossRef]
- Kapranas, A.; Collatz, J.; Michaelakis, A.; Milonas, P. Review of the role of sterile insect technique within biologically-based pest control—An appraisal of existing regulatory frameworks. Entomol. Exp. Appl. 2022, 170, 385–393. [Google Scholar] [CrossRef]
- Rupawate, P.S.; Roylawar, P.; Khandagale, K.; Gawande, S.; Ade, A.B.; Jaiswal, D.K.; Borgave, S. Role of gut symbionts of insect pests: A novel target for insect-pest control. Front. Microbiol. 2023, 14, 1146390. [Google Scholar] [CrossRef]
- Gonella, E.; Alma, A. The Role of Symbiont-Targeted Strategies in the Management of Pentatomidae and Tephritidae Pests under an Integrated Vision. Agronomy 2023, 13, 868. [Google Scholar] [CrossRef]
- Dho, M.; Gonella, E.; Alma, A. Field evaluation of symbiont-targeted control of Halyomorpha halys in hazelnut crop. Crop Prot. 2025, 187, 106952. [Google Scholar] [CrossRef]
- Gonella, E.; Orrù, B.; Alma, A. Egg masses treatment with micronutrient fertilizers has a suppressive effect on newly-emerged nymphs of the brown marmorated stink bug Halyomorpha halys. Entomol. Gen. 2019, 39, 231–238. [Google Scholar] [CrossRef]
- Tungadi, T.D.; Powell, G.; Shaw, B.; Fountain, M.T. Factors influencing oviposition behaviour of the invasive pest, Drosophila suzukii, derived from interactions with other Drosophila species: Potential applications for control. Pest Manag. Sci. 2023, 79, 4132–4139. [Google Scholar] [CrossRef]
- Gonella, E.; Crotti, E.; Mandrioli, M.; Daffonchio, D.; Alma, A. Asaia symbionts interfere with infection by Flavescence dorée phytoplasma in leafhoppers. J. Pest Sci. 2018, 91, 1033–1046. [Google Scholar] [CrossRef]
- Arora, A.K.; Pesko, K.N.; Quintero-Hernández, V.; Possani, L.D.; Miller, T.A.; Durvasula, R.V. A paratransgenic strategy to block transmission of Xylella fastidiosa from the glassy-winged sharpshooter Homalodisca vitripennis. BMC Biotechnol. 2018, 18, 50. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. Strategies for Enhanced Crop Resistance to Insect Pests. Ann. Rev. Plant Biol. 2018, 69, 637–660. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.M.; Clement, S.L. Molecular Bases of Plant Resistance to Arthropods. Annu. Rev. Entomol. 2012, 57, 309–328. [Google Scholar] [CrossRef]
- Legan, A.W.; Allan, C.W.; Jensen, Z.N.; Tabashnik, B.E. Mismatch between lab-generated and field-evolved resistance to transgenic Bt crops in Helicoverpa zea. Proc. Natl. Acad. Sci. USA 2024, 121, e2416091121. [Google Scholar] [CrossRef]
- Baranek, J.; Pogodziński, B.; Szipluk, N.; Zielezinski, A. TOXiTAXi: A web resource for toxicity of Bacillus thuringiensis protein compositions towards species of various taxonomic groups. Sci. Rep. 2020, 10, 19767. [Google Scholar] [CrossRef]
- Chalivendra, S. Microbial Toxins in Insect and Nematode Pest Biocontrol. Int. J. Mol. Sci. 2021, 22, 7657. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; Bhatnagar, N.B.; Bhatnagar, R. Bacterial Insecticidal Toxins. Crit. Rev. Microb. 2004, 30, 33–54. [Google Scholar] [CrossRef]
- Reinders, J.D.; Moar, W.J.; Head, G.P.; Hassan, S.; Meinke, L.J. Effects of SmartStax® and SmartStax® PRO maize on western corn rootworm (Diabrotica virgifera virgifera LeConte) larval feeding injury and adult life history parameters. PLoS ONE 2023, 18, e0288372. [Google Scholar] [CrossRef]
- Chougule, N.P.; Bonning, B.C. Toxins for transgenic resistance to hemipteran pests. Toxins 2012, 4, 405–429. [Google Scholar] [CrossRef]
- Napoleão, T.H.; Albuquerque, L.P.; Santos, N.D.; Nova, I.C.V.; Lima, T.A.; Paiva, P.M.G.; Pontual, E.V. Insect midgut structures and molecules as targets of plant-derived protease inhibitors and lectins. Pest Manag. Sci. 2019, 75, 1212–1222. [Google Scholar] [CrossRef]
- Xue, Q.; Swevers, L.; Taning, C.N.T. Plant and insect virus-like particles: Emerging nanoparticles for agricultural pest management. Pest Manag. Sci. 2023, 79, 2975–2991. [Google Scholar] [CrossRef] [PubMed]
- Fitches, E.; Audsley, N.; Gatehouse, J.A.; Edwards, J.P. Fusion proteins containing neuropeptides as novel insect contol agents: Snowdrop lectin delivers fused allatostatin to insect haemolymph following oral ingestion. Insect Biochem. Mol. Biol. 2002, 32, 1653–1661. [Google Scholar] [CrossRef]
- Lu, H.P.; Luo, T.; Fu, H.W.; Wang, L.; Tan, Y.Y.; Huang, J.Z.; Wang, Q.; Ye, G.-Y.; Gatehouse, A.M.R.; Lou, Y.-G.; et al. Resistance of rice to insect pests mediated by suppression of serotonin biosynthesis. Nat. Plants 2018, 4, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Malone, L.A.; Barraclough, E.I.; Lin-Wang, K.; Stevenson, D.E.; Allan, A.C. Effects of red-leaved transgenic tobacco expressing a MYB transcription factor on two herbivorous insects, Spodoptera litura and Helicoverpa armigera. Entomol. Exp. Appl. 2009, 133, 117–127. [Google Scholar] [CrossRef]
- Tyagi, S.; Kesiraju, K.; Saakre, M.; Rathinam, M.; Raman, V.; Pattanayak, D.; Sreevathsa, R. Genome Editing for Resistance to Insect Pests: An Emerging Tool for Crop Improvement. ACS Omega 2020, 5, 20674–20683. [Google Scholar] [CrossRef]
- Turnbull, C.; Lillemo, M.; Hvoslef-Eide, T.A.K. Global Regulation of Genetically Modified Crops Amid the Gene Edited Crop Boom—A Review. Front. Plant Sci. 2021, 12, 630396. [Google Scholar] [CrossRef]
- European Court of Justice. Judgment of 25 July 2018, Confédération Paysanne a.o., C- 528/16. ECLI:EU:C:2018:583; Court of Justice of the European Union: Luxembourg, 2018. [Google Scholar]
- Sánchez, M.A. The Global Advance of Genome-Edited Plants to the Market: The Key Role of Chile in Its Development. Plants 2024, 13, 3597. [Google Scholar] [CrossRef]
- Sankar, S.S.H.; Rani, O.P.R. Genetic improvement of entomopathogenic microbes: A retrospection. J. Entomol. Zool. St. 2018, 6, 646–651. [Google Scholar]
- Azizoglu, U.; Jouzani, G.S.; Yılmaz, N.; Baz, E.; Ozkok, D. Genetically modified entomopathogenic bacteria, recent developments, benefits and impacts: A review. Sci. Total Environ. 2020, 734, 139–169. [Google Scholar] [CrossRef]
- Gelaye, Y.; Negash, B. The role of baculoviruses in controlling insect pests: A review. Cogent Food Agric. 2023, 9, 2254139. [Google Scholar] [CrossRef]
- Zhao, H.; Lovett, B.; Fang, W. Genetically Engineering Entomopathogenic Fungi. Adv. Genet. 2016, 94, 137–163. [Google Scholar]

| Active Ingredient | Chemical Class (Nr of Insecticides per Class) | MoA (IRAC) | Commercial Name | Company | Date and Country of 1st Registration | EU Registration |
|---|---|---|---|---|---|---|
| spirotetramat | Tetronic and Tetramic acid derivatives (5) | 23 | Movento® | 1 | 2011 Japan | Yes |
| cyantraniliprole | Diamides (5) | 28 | Benevia® Exirel® | 2 | 2012 Argentina, 2013 Canada | Yes |
| sulfoxaflor | Sulfoximines (1) | 4C | Closer® | 2 | 2013 USA | Yes |
| flupyradifurone | Butenolides (1) | 4D | Sivanto® | 1 | 2014 Guatemala and Honduras, (2015 USA and Japan) | Yes |
| pyflubumide | Carboxanilides (1) | 25B | Danikong® | 3 | 2015 Japan | No |
| cyclaniliprole | Diamides (5) | 28 | Teppan® | 4 | 2017 Japan | No |
| pyrifluquinazon | Pyridine azomethine derivatives | 9B | Colt® | 3 | 2017 Japan | No |
| triflumezopyrim | Mesoionic compounds (3) | 4E | Pexalon® | 2 | 2017 India | No |
| afidopyropen | Pyropenes (1) | 9D | Inscalis® | 4, 5 | 2018 Australia, India | No |
| flometoquin | Phenoxy-quinoline (1) | 34 | Finesave® | 6 | 2018 Japan | No |
| flupyrimin | Pyridylidenes (1) | 4F | Lydia®, Emylia® Kevuka® | 7 | 2019 Japan | No |
| fluxametamid | Isoxazoline | 30 | Gracia® | 8 | 2019 Japan | No |
| acynonapyr | Acynonapyr | 33 | Danyote® | 9 | 2020 Japan | No |
| benzpyrimoxan | Benzyloxypyrimidines | Unkown MoA | Orchestra® | 3 | 2020 Japan, (2021 India) | No |
| broflanilide | Meta-diamides (3) | 30 | Exponus® | 7, 4 | 2020 Australia, (2021 USA) | No |
| spiropidion | Tetronic and Tetramic acid derivatives (5) | 23 | Elestal® | 10 | 2020 Guatemala | No |
| tetraniliprole | Diamides (5) | 28 | Vayego® | 1 | 2021 USA | No |
| dimpropyridaz | Pyrazole carboxamide (1) | 36 | Efficon® Cimegra® | 4 | 2022 Australia | probable |
| oxazosulfyl | Ethyl sulfones (1) | 37 | Alles® | 11 | 2022 Japan | No |
| isocycloseram | Meta-diamides (3) | 30 | Plinazolin® | 10 | 2023 Brasil | No |
| fenmezoditiaz | Mesoionic compounds (3) | 4E | Prexio® | 4 | 2025 India | No |
| spidoxamat | Tetronic and Tetramic acid derivatives (5) | 23 | Plenexos® | 1 | probably 2025 | Not known |
| Extract Origin | Main Active Ingredient | Product Name | Company | Date and Country of 1st Registration | UE Registration |
|---|---|---|---|---|---|
| Nicotiana tabacum | nicotine | 1940 | no | ||
| Lonchocarpus, Derris, Tefrosia spp. | rotenone | 1947 | no | ||
| Tanacetum cinerariifolium | pyrethrin I | 1950 | yes | ||
| Azadirachta indica | azadiractin | Margosan O ® | 1 | 1985 USA | yes |
| Veratrum sabadilla | veratrine and related cevadine alkaloids | Veratran D® | 2 | (1961) 2004 USA | |
| Annona squamosa | acetogenine | Anosom® | 3 | 2008 India | no |
| Chenopodium ambrosoides | terpinene, limonene, cymene | Requiem® | 4, 5 | 2008 USA | yes |
| Celastrus angulatus | celanguline and related dihydroagarofuran sesquiterpenes | Celangulin ® | 6 | 2010 China | no |
| Capsicum and Garlic | capsaicin and allicin | Captiva® | 7 | 2014 USA | no |
| Citrus x sinensis | limonene | Prev-AM®, | 8 | 2015 South Africa, USA | yes |
| Rosmary and peppermint | geraniol | Ecotec® | 9 | 2016 USA | no |
| Clitoria ternatea | Cter M (Cliotide T3) | Sero-X® | 10 | 2017 Australia | no |
| Sophora flavescens | matrine and related quinolizidine alkaloids | CE Matrine SL® | 11 | 2018 China | no |
| Veratrum nigrum | veratrine and related cevadine alkaloids | CE Veratrum Rhizome Extract ® | 11 | 2021 China | no |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Civolani, S.; Bariselli, M.; Osti, R.; Bernacchia, G. Insect Pest Control from Chemical to Biotechnological Approach: Constrains and Challenges. Insects 2025, 16, 528. https://doi.org/10.3390/insects16050528
Civolani S, Bariselli M, Osti R, Bernacchia G. Insect Pest Control from Chemical to Biotechnological Approach: Constrains and Challenges. Insects. 2025; 16(5):528. https://doi.org/10.3390/insects16050528
Chicago/Turabian StyleCivolani, Stefano, Massimo Bariselli, Riccardo Osti, and Giovanni Bernacchia. 2025. "Insect Pest Control from Chemical to Biotechnological Approach: Constrains and Challenges" Insects 16, no. 5: 528. https://doi.org/10.3390/insects16050528
APA StyleCivolani, S., Bariselli, M., Osti, R., & Bernacchia, G. (2025). Insect Pest Control from Chemical to Biotechnological Approach: Constrains and Challenges. Insects, 16(5), 528. https://doi.org/10.3390/insects16050528








