Simple Summary
Phthorimaea absoluta (Meyrick) (Lepidoptera: Gelechiidae) is an invasive and destructive pest that significantly threatens global tomato production. Although P. absoluta is known to be resistant to chlorantraniliprole, the long-term effects of this insecticide on multiple generations of the pest, particularly in terms of its development and biochemical parameters, remain poorly understood. This study evaluates the sustained multigenerational effects of chlorantraniliprole on P. absoluta, with a focus on resistance development, life cycle parameters, and changes in nutrient reserves. Our results indicate that the resistance of P. absoluta significantly increased after eight consecutive generations of selection with chlorantraniliprole. The life cycle analysis revealed prolonged developmental times. Additionally, fecundity was reduced. Biochemical parameter analysis in the second-instar larvae showed significant reductions in nutrient reserves. Transcriptome analysis revealed changes in nutritional metabolism related to gene expression and pathways.
Abstract
Phthorimaea absoluta, an important pest of tomato crops, has reportedly developed high levels of resistance to the insecticide chlorantraniliprole, which has a unique mode of action and high efficacy. This study evaluated the sustained multigenerational effects of chlorantraniliprole on P. absoluta, focusing on resistance development, growth, development, reproductive capacity, population parameters, and nutritional indicators. After continuous selection with sublethal chlorantraniliprole for eight generations (CX-Sub8), bioassays showed that CX-Sub8 had 225.37-fold higher resistance than the susceptible strain. The age-stage, two-sex life table analysis revealed that the preadult development time and mean generation time were significantly prolonged, while population reproduction and pupal weight were reduced. Moreover, the relative fitness of CX-Sub8 was 0.62, and changes in the life table parameters correlated with an increase in the serial number of selection cycles. The second-instar larvae of CX-Sub8 presented lower triglyceride, glycerol, trehalose, free fatty acid, and protein contents than the unselected strain (CX-S8). Transcriptome analysis identified 2517 differentially expressed genes, with most being enriched in nutrient metabolism-related pathways, such as amino acid biosynthesis and fatty acid degradation metabolism. These results indicate that multigenerational sublethal chlorantraniliprole treatment disrupts the nutritional metabolism, and inhibits the growth, development, and reproduction of P. absoluta.
1. Introduction
The tomato leafminer, Phthorimaea absoluta (Meyrick) (Lepidoptera: Gelechiidae), is an invasive and destructive pest that threatens the global tomato industry [1]. Phthorimaea absoluta was originally restricted to South America [2]; however, since 2006, it has been introduced to 110 countries and territories throughout Europe, Africa, and Asia [3,4]. In China, P. absoluta was first reported in the Xinjiang Uygur Autonomous Region in August 2017, and then spread to 13 provinces [5]. According to @RISK model estimates, the economic loss of the tomato industry may range from 80 to 400 billion in China if no preventive measures are taken [6]. Since the larvae feed on the mesophyll tissues of tomato leaves, leaving the epidermis intact, the control efficacy of insecticides is significantly reduced [7,8]. Studies have shown that P. absoluta can complete 10–12 generations in a conventional tomato crop season [9]. To minimize yield losses, farmers frequently apply excessive amounts of insecticides, which has led to the development of serious resistance in P. absoluta to various types of insecticides, including diamides [10,11,12,13].
Over the past decade, diamide insecticides have become crucial in global agriculture, but their heavy use against P. absoluta has reduced their field efficacy, highlighting the need to understand resistance mechanisms [14]. Chlorantraniliprole is a diamide insecticide with a unique mode of action on the targeted ryanodine receptor, leading to uncontrolled calcium release, muscle paralysis, and death [15,16]. The high-frequency and large-area use of chlorantraniliprole imposes high and continuous selection pressure on P. absoluta, which may be responsible for the rapid evolution of substantial resistance [11,13,17]. The first report of chlorantraniliprole resistance in P. absoluta was recorded in a field-collected population from Europe [18], and so far, the resistance has evolved in Brazil, Italy, Israel, and Pakistan with a resistance ratio of 2 to 22,573-fold [12,16,19]. In China, resistance of P. absoluta to chlorantraniliprole has been identified in the field populations of 13 regions, with the highest resistance reaching 76.9-fold [14].
Insecticide resistance often involves enhanced metabolic activity associated with detoxification [20]. Previous studies have shown that under short-term environmental stress, insects strategically partition energy between detoxification and reproduction to maximize fitness and sustain population growth [21,22]. However, in most cases, since detoxification metabolism needs a large amount of energy, prioritizing the consumption of energy for detoxification usually leads to growth and development inhibition in insects [23]. Nutrients used by insects, including triglycerides, glycerol, trehalose, free fatty acids, proteins, and amino acids, play an important role in this process [24]. Sublethal deltamethrin and bistrifluron can increase the total protein, lipid, and carbohydrate content in Corcyra cephalonica and Spodoptera exigua, respectively [25,26]. In both cases, the nutrient reserves are significantly higher in later generations compared to earlier generations, with larval growth and development delayed. In contrast, it has also been proposed that different stress factors can reduce the total protein content in the hemolymph of silkworms, which may be due to its decomposition being caused by chemical stress [27,28]. In addition, when Anopheles mosquitoes are exposed to sublethal DDT for multiple generations, they have fewer lipids, sugars, and energetic reserves than sensitive ones [29]. In the field, insecticides are used repeatedly over long periods [12]. Studying pest nutrient reserve changes after multigenerational sublethal exposure helps us understand insect nutritional metabolism, growth, and development under long-term insecticide stress. However, there are still a lack of reports on P. absoluta in terms of the above aspects.
Insecticide resistance usually develops at the cost of a decrease in reproductive ability and relative fitness [30]. Recent reports have shown that sublethal concentrations of insecticides can inhibit insect reproduction and decrease fitness. For example, the resistance of Plutella xylostella and Musca domestica to chlorantraniliprole and Spodoptera exigua to deltamethrin lead to extended development, decreased survival rates, and decreased fecundity [31,32,33]. In contrast, some studies have also shown that target insects enhance their reproductive ability and fitness after exposure to insecticides. For example, when Nilaparvata lugens and Sogatella furcifera are exposed to sublethal concentrations of nitenpyram and triazophos, respectively, their fecundities are significantly increased [34,35]. Phthorimaea absoluta has multiple generations in a year [9]. Despite the known resistance of P. absoluta to chlorantraniliprole, the effects of this insecticide on the life history traits, relative fitness, and nutrient metabolism of the pest across multiple generations remain poorly understood still.
Here, we attempt to verify the hypothesis that multigenerational treatment with sublethal chlorantraniliprole will disrupt nutritional metabolism and simultaneously inhibit the growth, development, and reproduction of P. absoluta. This is achieved through an in-depth examination of four key aspects. First, we assess the evolution of resistance in P. absoluta following sublethal chlorantraniliprole treatment during the second larval stage across multiple generations. Second, we investigate the resulting growth, development, and reproduction of the pest. Third, we analyze the physiological features of the second-instar larvae, with a focus on the nutrient reserves. Fourth, we analyze the expression levels of differentially expressed genes associated with nutritional metabolism in the transcriptome.
2. Materials and Methods
2.1. Insects and Chemicals
The P. absoluta susceptible strain (SS) used for establishing the susceptibility baseline in this study was collected in 2020 from Aksu Prefecture (41.09° N, 80.24° E), Xinjiang, and maintained for >20 generations without exposure to insecticides. More than 1000 fourth-instar larvae of a resistant population of P. absoluta (CX, F0 generation) were collected from Tsabchal Xibe Autonomous County (43.87° N, 81.26° E), lli Kazakh Autonomous Prefecture, Xinjiang Uygur Autonomous Region, in 2022. All test insects (SS and CX) were reared on fresh tomato plants (Tian Fen 2, only in a vegetative state) in the laboratory in insect-rearing cages (0.75 × 0.75 × 0.75 m) at 25 ± 1 °C, with a relative humidity of 30 ± 5%, and with a 16 L:8 D photoperiod.
Chlorantraniliprole (purity ≥ 99%; Shanghai Macklin Biochemical Technology Co., Ltd., Shanghai, China), dimethyl sulfoxide (DMSO; Tianjin Beilian Fine Chemicals Development Co., Ltd., Tianjin, China), and Triton X-100 (Beijing Solarbio Science Technology Co., Ltd., Beijing, China) were used.
2.2. Bioassays
The whole-leaf dip method was used to determine susceptibility to chlorantraniliprole in submerged tomato leaves according to the Insecticide Resistance Action Committee method No. 022 (https://irac-online.org/methods/tuta-absoluta-larvae/, accessed on 7 October 2023) [10,17]. Accurate dilutions at 500, 125, 50, 12.5, 3.125, and 0.78125 mg·L−1 were prepared with a 0.1% Triton X-100 aqueous solution control containing DMSO. Briefly, round tomato leaf discs with a diameter of 4–5 cm or entire leaves were immersed in serial insecticide concentrations for 20 s. Treated leaves were allowed to dry for 0.5–1 h at room temperature (25 ± 1 °C) and then inserted into pre-solidified six-well plates containing 1% agar. Five second-instar larvae were placed in each well, and the edges of the six-well plates were sealed with a sealing film. All bioassays were incubated (25 ± 1 °C, 30 ± 5% relative humidity, 16 L:8 D photoperiod) for 48 h to assess larval survival. The larvae were considered dead if they did not respond or were severely deformed by touching the body with the tip of a brush. Each concentration treatment was repeated six times.
2.3. Selection Experiments
In the selection experiments, the concentration of chlorantraniliprole used to select each subsequent generation was LC25, based on the bioassay results from the previous generation. The larvae of the CX population were divided into two sub-populations during the F1 generation. One sub-population was selected for eight consecutive generations by exposing second-instar larvae to tomato leaves treated with LC25 chlorantraniliprole and was named the CX-Sub strain. Strain CX-Sub-n represented the strain selected for n generations consecutively. The second sub-population of larvae was the CX-S strain, and it was fed the solvent-treated tomato leaves without exposure to insecticides (eight generations were unselected and named CX-S1 to CX-S8, with CX-S8 acting as the control strain). The CX-S strain was reared in the laboratory in parallel with the CX-Sub strain. Resistance ratio (RR) values were estimated as the LC50 of the resistant strain divided by the LC50 of the susceptible strain (SS).
2.4. Life Table Data, Study, and Analysis
An age-stage, two-sex life table was constructed using the CX-Sub2, CX-Sub4, CX-Sub8, and control strains. First, 150 eggs were randomly collected within 12 h of the peak laying period of female adults, and hatched under the same insect-rearing conditions as described above. Next, 100 newly hatched larvae were randomly selected, placed in Petri dishes (9 cm diameter, one individual/Petri dish, No. 1–100), and provided with fresh tomato leaves. The egg incubation and larval development times were recorded every day until pupation. The development times of all life stages were recorded. Thirty randomly selected pupae were weighed and placed in individual Petri dishes until adult emergence, and male and female individuals that emerged on the same day were paired and allowed to lay eggs. Finally, the duration of the pupal stage, longevity, survival, and oviposition of P. absoluta were recorded daily until the adult died. We used the age-stage, two-sex life table theory, and the Twosex-MSChart program to analyze the life table raw data [36,37]. The basic life table parameters included the age-stage survival rate (Sxj) (where x is the age and j is the stage), age-specific survival rate (lx), female age-specific fecundity (fxj), age-specific fecundity (mx), age-stage life expectancy (exj), and age-stage reproductive value (vxj). The sxj, lx, mx, exj, and vxj values were calculated using Equations (1)–(5):
where n01 is the number of eggs used at the beginning of the life table study, nxj is the number of individuals surviving to age x and stage j, and β is the number of stages. The s’iy represents the probability that the individual survives to age i and stage y. The fiy represents the number of eggs laid by an individual of age i and stage y [37].
The net reproductive rate (R0), intrinsic rate of increase (r), finite rate of increase (λ), and mean generation time (T) are important parameters for describing population characteristics and were calculated using Equations (6)–(9):
Life table parameters were mapped using Origin 2021 software (Northampton, MA, USA). The mean value and standard error of the life table parameters were calculated using the bootstrap method with 100,000 replications [38]. The significance of the differences between the parameters was calculated using a paired bootstrap test program.
The relative fitness (Rf) was analyzed using the method outlined by Abbas et al. [39] and calculated as Rf = R0 of the CX-Sub strain/R0 of the control strain.
Pearson’s correlation analysis was employed to analyze the correlations between the life table parameters and P. absoluta resistance selection across generations. All data were obtained using the bootstrap method with 100,000 replications, including the life table parameters of the CX-Sub2, CX-Sub4, and CX-Sub8 strains. Origin 2021 software (Northampton, MA, USA) was utilized to perform the Pearson correlation analysis [40].
2.5. Determination of Nutrient Reserves
Healthy second-instar larvae of the same size were randomly selected from the control and CX-Sub8 strains. Firstly, 100 mg of numerous intact second-instar larvae were homogenized in 1000 μL of the extraction solution according to the instruction manual. Then, the nutrient reserves were determined using specific substance determination kits (Suzhou Grace Biotechnology Co., Ltd., Suzhou, China). The measured nutrients included triglyceride (G0910W), glycerol (G0912W), trehalose (G0553W96), free fatty acids (G0927W96), proteins (G0418W), and amino acids (G0415W). An Epoch-cn microplate reader (Agilent BioTek, Winooski, VT, USA) was used to determine the absorbance of the reaction fluid of the nutrients. Each assay was performed in triplicate and repeated three times. The results were calculated according to the manufacturer’s instructions.
2.6. Transcriptome Sequencing
Transcriptome sequencing was performed by Biomarker Technologies (Beijing, China). Total RNA was extracted from three replicate pools of 50 whole second-instar larvae of each strain (CX-Sub8 and Control) with TRIzol reagent (Life technologies, Carlsbad, CA, USA). The concentration and purity of the RNA were measured using a NanoDrop 2000 (Thermo Fisher Scientific, Wilmington, DE, USA). RNA integrity was assessed using the RNA Nano 6000 Assay Kit with an Agilent 2100 Bioanalyzer System (Agilent Technologies, Santa Clara, CA, USA). The cDNA libraries were sequenced on the Illumina NovaSeq 6000 platform and analyzed using the bioinformatics analysis tool on the BMK Cloud online platform (www.biocloud.net) [41]. Briefly, high-quality clean reads were generated by removing low-quality fragments and junctions from the raw reads, which were mapped to the reference genome using HISAT2 (GenBank no. GCA_027580185.1). The reads were assembled using StringTie to reconstruct the transcriptome for subsequent analyses [42]. Gene transcript levels were calculated using the fragments per kilobase of exon model per million mapped reads (FPKM) method [43]. The genes with a fold change (FC) ≥ 2 and false discovery rate (FDR) ≤ 0.01 were designated as differentially expressed genes (DEGs), which were subjected to enrichment analysis by Gene Ontology (GO) and Kyoto Encyclopedia Genes of Genomes (KEGG) annotations. Rich factor = DEG number/total gene number identified from the transcriptome of a certain process.
2.7. Validation of Transcriptomic Data with Quantitative Real-Time PCR (qRT-PCR)
To verify the transcriptome data, nine genes (from the top three KEGG-enriched entries: biosynthesis of amino acids, fatty acid degradation, and glycine, serine, threonine metabolism) were randomly selected from the DEG list for qRT-PCR. Total RNA was extracted from the samples in Section 2.6, following the standard protocol of the Total RNA Extraction Reagent (Vazyme, Nanjing, China). First-strand cDNA was generated from 1 μg total RNA using HiScript III RT SuperMix (Vazyme, Nanjing, China). The reaction volume of the qRT-PCR was 20 μL: RNase-free water (8.2 μL), qPCR SYBR Green Master Mix (10 μL), forward primer (0.4 μL, 10 μM), reverse primer (0.4 μL, 10 μM), and cDNA template (1 μL). The qPCR procedure was as follows: pre-denaturation at 95 °C for 30 s; then 40 cycles were conducted (denaturation at 95 °C for 10 s, annealing at 60 °C for 30 s). The stably expressed genes TaEF1α (GenBank: MZ054826) and TaRPL28 (GenBank: MZ054829) were used as the reference genes [44]. qRT-PCR was conducted using three biological and technical replicates. The relative expression levels were calculated using the Ct (2−ΔΔCt) method [45]. Primer sequences for all genes are presented in Table 1.

Table 1.
Primers used in the qRT-PCR synthesis.
2.8. Statistical Analysis
Bioassays were analyzed by probit analysis via SPSS version 22.0 software (SPSS Inc., Chicago, IL, USA) [46]. The relative expression levels and nutrient reserve results were compared by Student’s t-test using the SPSS 22.0 software as well. All data were expressed as the mean ± standard error of the mean (SEM).
3. Results
3.1. Selection of P. absoluta Resistance to Chlorantraniliprole
CX-Sub8 exhibited a 225.37-fold higher resistance to chlorantraniliprole than a susceptible strain (SS; Table 2). The LC50 value increased from 6.741 to 6.933 mg·L−1 during the first two generations, with a relatively slow increase in resistance. Subsequently, the resistance level increased rapidly in the third generation, with the LC50 increasing to 36.5 mg·L−1 and reaching a high resistance level (RR = 214.73-fold). The resistance level tended to stabilize between the fourth and eighth generations. Despite experiencing upward and downward fluctuations, the overall trend demonstrated a gradual increase. The LC50 values of the unselected strain (CX-S) for chlorantraniliprole are shown in Table S1.

Table 2.
Resistance levels of P. absoluta to chlorantraniliprole during selection.
3.2. Inhibitory Effect of Chlorantraniliprole on the Growth, Development, and Reproduction of P. absoluta
CX-Sub completed development and produced offspring. However, the biological parameters of CX-Sub differed significantly among generations (Table 3). The preadult duration of CX-Sub4 and CX-Sub8 was significantly prolonged by 0.98 and 2.45 days, respectively, compared to the control. Meanwhile, the average number of eggs per female adult in the CX-Sub8 was 48.56, which was 2.01-fold lower than the control, and the oviposition time was shortened by 1.66 days. The preadult time, female adult longevity, female adult ratio, adult preoviposition period (APOP), and total preoviposition period (TPOP) of CX-Sub increased gradually with the increase in the serial number of the selection cycles, while egg production was reduced. Finally, compared to the control group, the average pupal weight of CX-Sub8 decreased significantly by 1.15 mg (Figure 1A). Compared to the dorsal and ventral surfaces of the pupae in the control group, the pupae of CX-Sub8 were significantly smaller in size (Figure 1B,C).

Table 3.
Development times of various life stages and fecundity for the control, CX-Sub2, CX-Sub4, and CX-Sub8 strains of P. absoluta.
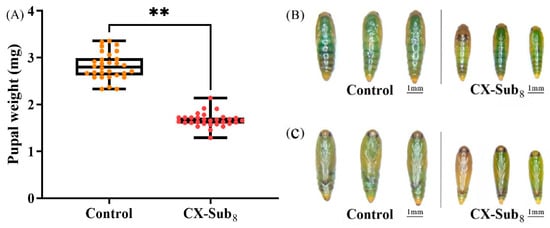
Figure 1.
Effects of chlorantraniliprole on pupae of different strains of P. absoluta. (A) Pupal weight, (B) dorsal surface of control and CX-Sub8 pupae, (C) ventral surface of control and CX-Sub8 pupae. The pupal weight was assessed using a Student’s t-test. ** indicates a significantly different at the level of p < 0.01.
3.3. Effects of Chlorantraniliprole on the Life Table Parameters of the F2, F4, and F8 Generations of P. absoluta
The age-stage survival (Sxj) in the different CX-Sub generations assessed the probability of egg survival to age x and stage j (Figure 2). Under sublethal concentration conditions, no significant effect was observed on the survival rate of P. absoluta larvae with an increase in the serial number of selection cycles. However, the peak survival of adult males decreased by 0.08. In addition, the Sxj curves of adult females ended later in the CX-Sub strain than the control, suggesting that LC25 treatment prolonged the survival of P. absoluta adult females.
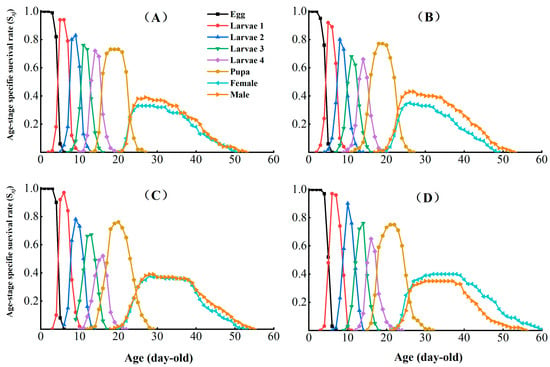
Figure 2.
Effects of chlorantraniliprole on the age-stage survival rate (Sxj) of different strains of P. absoluta. (A) Control, (B) CX-Sub2, (C) CX-Sub4, (D) CX-Sub8.
The control had the highest peak female age-specific fecundity value (fx7) = 17.42, occurring on day 25 (Figure 3). In contrast, CX-Sub8 had the lowest peak fx7 = 10.97 on day 27. The control strain had the highest peak age-specific net reproductive rate of population (lxmx) value = 5.75 on day 25, while CX-Sub8 had the lowest lxmx peak (3.84) on day 27. Additionally, the peak age-specific fecundity of population (mx) values for the control, CX-Sub2, CX-Sub4, and CX-Sub8 were 7.88, 7.05, 4.96, and 5.05, respectively, aligning with the occurrence times of the peak female age-specific fecundity values of fx7.
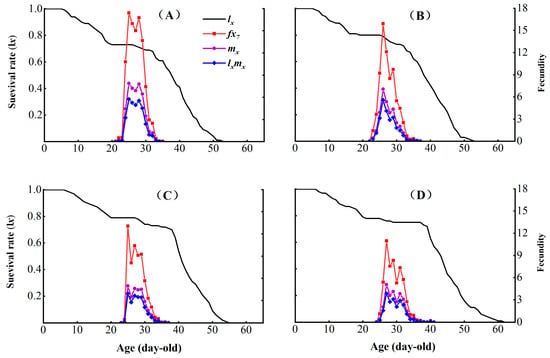
Figure 3.
Effects of chlorantraniliprole on the age-specific survival rate (lx), female age-specific fecundity (fx), age-specific fecundity of population (mx), and age-specific net reproductive rate of the population (lx mx) of different strains of P. absoluta. (A) Control, (B) CX-Sub2, (C) CX-Sub4, (D) CX-Sub8.
The age-stage-specific life expectancy (exj) indicates the expected life span of an individual of age x and stage j in the same stage after age x (Figure 4). The exj of the egg, larval, pupal, and adult stages were increased in CX-Sub2, CX-Sub4, and CX-Sub8 compared to the control.
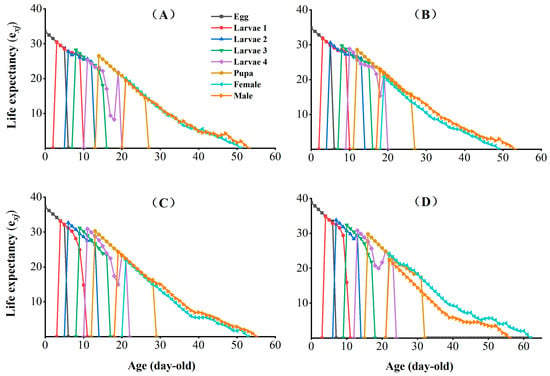
Figure 4.
Effects of chlorantraniliprole on the life expectancy (exj) of different strains of P. absoluta. (A) Control, (B) CX-Sub2, (C) CX-Sub4, (D) CX-Sub8.
The age-stage reproductive value (Vxj) represents the individual contribution of age x and stage j to the future of the population (Figure 5). The peak reproductive values of CX-Sub2, CX-Sub4, and CX-Sub8 females were lower than those for the control strain. The sublethal concentration of chlorantraniliprole significantly reduced the Vxj of P. absoluta with an increase in the serial number of the selection cycles.
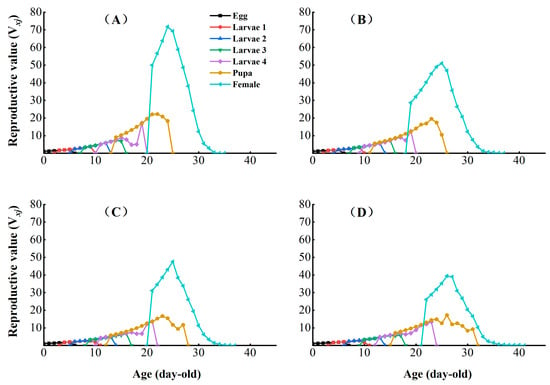
Figure 5.
Effects of chlorantraniliprole on the age-stage reproductive value (Vxj) of different strains of P. absoluta. (A) Control, (B) CX-Sub2, (C) CX-Sub4, (D) CX-Sub8.
In CX-Sub, the preadult duration (R = 0.98), longevity (R = 0.77), TPOP (R = 0.95), and T (R = 0.88) were positively correlated with the serial number of the selection cycles (Figure 6). Meanwhile, the fecundity (R = −0.83), male-to-female ratio (R = −0.61), oviposition days (R = −0.83), r (R = −0.66), and λ (R = −0.66) were negatively correlated with the selection cycle.
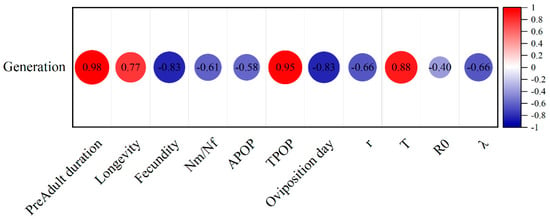
Figure 6.
Pearson’s correlation heatmap of each life table parameter analyzed in three strains of P. absoluta (CX-Sub2, CX-Sub4, and CX-Sub8).
3.4. Effects of Chlorantraniliprole on the Population Parameters of the F2, F4, and F8 Generations of P. absoluta
The GRR, R0, ri, and λ values slightly decreased in CX-Sub2, but significantly decreased in CX-Sub4 and CX-Sub8 compared to the control. The mean generation time (T) in CX-Sub8 was 30.17 d, which was 2.35 d longer than that in the control strain (Table 4). The relative fitness (Rf) values of CX-Sub2, CX-Sub4, and CX-Sub8 were 0.74, 0.65, and 0.62 lower than for the control strain, respectively (Table 4). This suggests that the fitness of CX-Sub suffered a cost due to resistance.

Table 4.
Life table parameters for the control, CX-Sub2, CX-Sub4, and CX-Sub8 strains of P. absoluta.
3.5. Chlorantraniliprole Reduces Nutrient Reserves in the Second-Instar Larvae of P. absoluta
Compared to the control strain, the content of triglycerides, glycerol, trehalose, free fatty acids, and proteins were significantly reduced (p < 0.05) in CX-Sub8 larvae by 44.4%, 43.9%, 53.1%, 47.1%, and 4.5%, respectively. In contrast, the amino acid content increased slightly, but not significantly (Figure 7). These data showed that after continuous selection with chlorantraniliprole for eight generations, significant changes occurred in the larval nutrient reserves of P. absoluta.
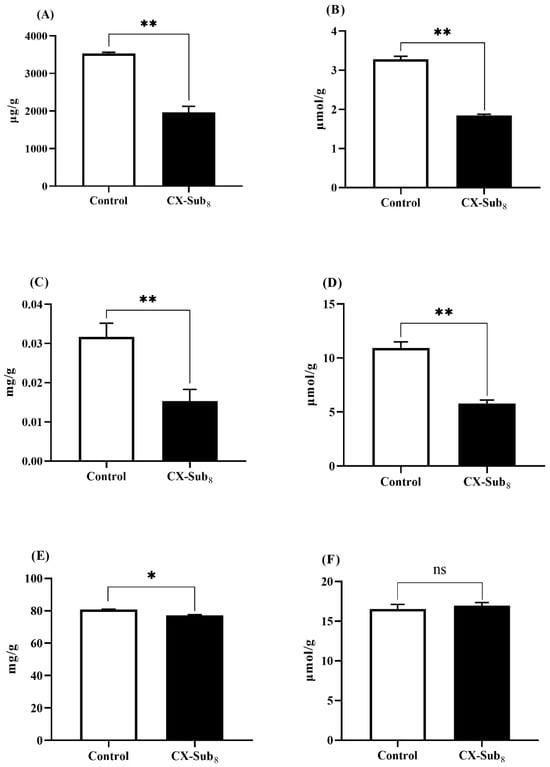
Figure 7.
Effects of chlorantraniliprole on P. absoluta nutrient reserves. (A) Triglycerides, (B) glycerol, (C) trehalose, (D) free fatty acids, (E) proteins, and (F) amino acids. * indicates a significantly different at the level of p < 0.05, ** indicates a significantly different at the level of p < 0.01, and ns is not significantly different (p > 0.05).
3.6. Disruption of Nutritional Metabolism in P. absoluta by Chlorantraniliprole Revealed Through Transcriptome Sequencing
Sequencing generated 38.11 Gb of clean data with an average of 6.18 Gb per data library. The proportion of Q30 bases was >93.61%. The alignment rate to the reference sequence was 79.60–82.64%. A total of 2517 DEGs were detected, with 1831 upregulated and 686 downregulated, in CX-Sub8 versus the control (Figure 8A). GO annotation analysis showed that the primary biological processes associated with the DEGs were cellular, metabolic, and biological. DEGs were enriched in cellular components related to cellular anatomical entities, as well as intracellular and protein-containing complexes. Under molecular functions, binding, catalytic activity, and structural molecule activity were associated with most of the DEGs (Figure 8B). In addition, KEGG pathway enrichment analysis revealed that the DEGs were enriched in the biosynthesis of amino acids; fatty acid degradation; glycine, serine, and threonine metabolism; other glycan degradation; carbon metabolism; and the biosynthesis of unsaturated fatty acids (Figure 8C, Table S2). qRT-PCR was used to verify the transcriptome data by analyzing the expression levels of nine randomly selected upregulated genes (Table 1). The variations in gene expression levels were consistent with the transcriptome data (p < 0.05), demonstrating the reliability of the RNA-seq results (Figure 9).
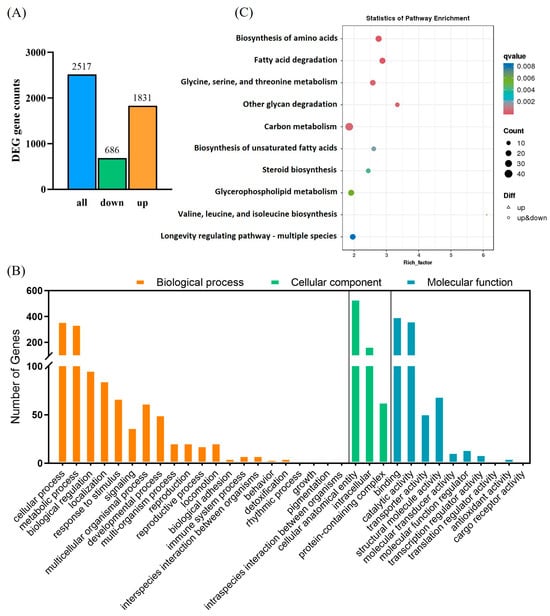
Figure 8.
Analysis of differentially expressed genes (DEGs) between the control and CX-Sub8 strains of P. absoluta. (A) Number of upregulated and downregulated DEGs in the control and CX-Sub8 strains. (B) Gene Ontology (GO) analysis of DEGs in the control and CX-Sub8 strains. (C) Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis of DEGs in the control and CX-Sub8 strains. Processes with a q value < 0.01 are significantly enriched.
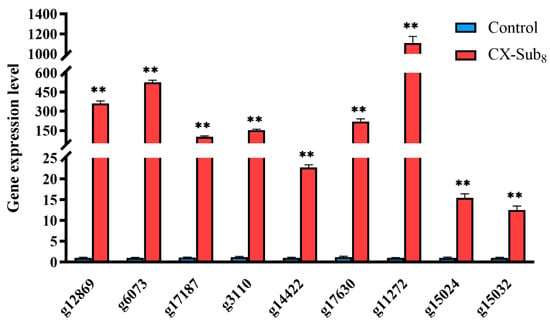
Figure 9.
Comparison of the RNA-seq and qPCR validated expression of differentially expressed genes (DEGs) of the control and CX-Sub8 strains of P. absoluta. ** indicates a significantly different at the level of p < 0.01.
4. Discussion
In this study, the resistance level of CX-Sub to chlorantraniliprole increased from medium to high levels through three generations (RR = 214.73-fold). This is consistent with the results of Silva et al. [47] and Jallow et al. [48], who reported that resistance to chlorantraniliprole in P. absoluta developed rapidly after six to eight generations of selection in Kuwait and the United States, respectively. The field populations of P. absoluta in Italy developed high levels of resistance to chlorantraniliprole (RR = 742-fold) after only four generations [15]. Similar results were reported for P. xylostella in China and the United Kingdom [49,50].
When a pest is resistant to a pesticide, identifying the resistance mechanisms and suppressing the resistance in the field are key to pest management [51]. A fitness cost is typically required for insects to develop resistance [30]. In the current study, CX-Sub showed a gradual decrease in R0 and GRR with an increase in the serial number of the selection cycles, which is consistent with the significant decrease in average fecundity, R0, ri, and λ in an S. exigua chlorantraniliprole-resistant strain [52]. However, unlike P. xylostella Sub strains (selected by LC25 spinosad for multiple generations), despite its higher resistance, the sublethal effect on the Sub strains decreased as the serial number of the selection cycles increased. Compared to the susceptible strain, the Sub-5 strains showed no differences in rm, R0, λ, and GRR. Notably, the Sub-10 strain had higher fecundity and shorter larval development. Thus, multigeneration spinosad selection imposed no corresponding energy or fitness costs on P. xylostella [53]. In this study, the effect of sublethal chlorantraniliprole on the P. absoluta Sub strains did not decrease as selection cycle serial numbers increased. One possible explanation is that chlorantraniliprole disrupts the normal neural tissues of insects [54], leading to motor disorders and feeding disruption [55]. This may cause larvae to spend more time feeding on leaves, compensating for their low energy reserves by consuming more food, resulting in extended preadult periods and decreased fecundity [56]. In addition, after exposure to insecticides, the balance between detoxification and development is disrupted [57]. Some of the insect’s energy is directed toward the metabolism of detoxification, reducing the energy essential for insect development and reproduction [24].
Generally, fecundity reflects the development of insect populations [58]. Previous studies have found that after P. absoluta and H. armigera are exposed to tetraniliprole and chlorantraniliprole, respectively, the female adults’ fecundity will be significantly reduced [59,60]. Conversely, these findings suggest that exposure to low doses of a pesticide might increase the fecundity of insects, but rising fecundity and offspring population could be inhibited by the LC50 of insecticides [61]. For example, Tetranychus urticae exposed to the LC10 of chlorfenapyr had increased fecundity and longer oviposition days, but these traits decreased at higher concentrations [62]. Similarly, an increase in the fecundity of second-generation Daphnia carinata was observed at low concentrations of chlorpyrifos [63]. Our results showed that multigeneration LC25 chlorantraniliprole application significantly reduced the fecundity of CX-Sub compared to the control. The decrease in female offspring production might be attributable to the following factors: First, the relatively high sublethal concentration of chlorantraniliprole [64]. Second, insecticide exposure damages most viable oocytes [65]. Third, chlorantraniliprole significantly disrupts insect mating behavior [66]. Moreover, the pupal weight of P. absoluta, which is closely related to egg production, enables females with higher pupal weights to lay more eggs for the next generation [67]. This positive correlation has also been reported in P. xylostella [68], H. armigera [69,70], and other species [71]. However, the reasons for the reduced fecundity of P. absoluta treated through more generations with sublethal chlorantraniliprole require further investigation.
When target insects are exposed to pesticides during the preadult stage, the survival rates of the sexes differ [13]. Although sexually reproducing species typically have a sex ratio of 1:1, several mechanisms may cause this ratio to deviate, such as when sex-asymmetric inbreeding occurs or through exposure to the insecticide [72,73]. The female ratio of CX-Sub increased gradually with the serial number of the selection cycles, exhibiting a significant positive correlation. In contrast, treating P. absoluta with indoxacarb, carbendazim, and abamectin increased the male ratio [74]. Differences in the sex ratio might result from insecticide exposure affecting the mating frequencies and conception rates of male and female insects [75] or causing significant differences in insecticide susceptibility due to locomotory and physiological responses [76].
Previous findings regarding insect nutritional reserves were that females exposed to insecticides yield heavier offspring with more lipid storage than non-exposed ones [77]. For instance, sublethal phosphine significantly increased protein, lipid, and carbohydrate content across generations of C. cephalonica [25]. Despite many insecticides reducing insect feeding efficiency, insects might boost their nutritional reserves and reduce body metabolic components to counter insecticide-induced energy stress [78]. This potentially explains the post-exposure increase in their offspring’s nutritional reserves. In contrast to the findings described above, our results showed that the multigeneration sublethal chlorantraniliprole treatment disrupted nutritional metabolism in P. absoluta, leading to changes in nutrient reserves. Compared to the control strain, nutrient reserves (triglyceride, glycerol, trehalose, free fatty acid, and protein content) in the CX-Sub8 strain were significantly decreased. Similarly, Piri et al. documented that sublethal concentrations of spinosad decreased the carbohydrate, protein, and lipid content in Glyphodes pyloalis [79]. They suggested that this phenomenon may be due to the food rejection effect of spinosad. Food rejection may also occur in the response of P. absoluta to chlorantraniliprole. However, the reduced energy reserves of carbohydrates, lipids, proteins, and glycogen in P. absoluta also reflect the high energetic cost of the detoxification mechanism [80]. Moreover, previous research has shown that metabolic resistance to insecticides uses resources essential for development [24]. These changes in biochemical parameters also supported the KEGG analysis in finding that the amino acid biosynthesis, fatty acid degradation, and carbon metabolism significantly enriched DEGs. Significant differences in amino acid content were not observed between CX-Sub8 and the control. One probable reason is that under insecticidal stress, when insects face insufficient energy reserves, they consume proteins to maintain the free amino acid content in the hemolymph [27,81]. Whether this phenomenon also exists in P. absoluta warrants further investigation.
Numerous studies have shown that increased insecticide resistance leads to reduced relative fitness, which may be associated with nutrient reserves and metabolism [30]. Changes in the fitness of resistant insects can disrupt normal physiological functions and create an imbalance in nutrient and energy metabolism allocation [60,68]. However, a more thorough analysis from a molecular perspective may be necessary to explore the relationship between the characteristics, such as the prolonged larval development period, reduced fecundity, and decreased pupal weight in P. absoluta after multigenerational sublethal treatment with chlorantraniliprole and the nutritional metabolism mechanism.
5. Conclusions
Our results show that resistance to chlorantraniliprole developed rapidly in P. absoluta, with medium to high resistance levels developed in only three generations. Moreover, multigenerational treatment with chlorantraniliprole led to longer larval development, reduced fecundity and relative fitness, and significant decreases in nutrient-related biochemical parameters compared to the unselected strain. These results provide a new perspective for further analysis of the relationship between resistance and nutrient metabolism, offering a theoretical basis for the effective application of insecticides in pest management.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects16050524/s1, Table S1: Resistance levels of the unselected strain (CX-S) of P. absoluta to chlorantraniliprole during unselection; Table S2: KEGG analysis of DEG number in control and CX-Sub8 strains of P. absoluta.
Author Contributions
Conceptualization: L.L., Z.J. and W.G. designed the research framework and formulated the experimental plan; investigation and data curation: L.L., Z.J., K.F., X.D., X.W., T.A., Y.W. and X.Y. carried out the experiments, collected data, and curated the datasets to ensure data integrity and accuracy; writing—original draft: L.L., Z.J., K.F., W.J. and J.W. drafted the initial manuscript; funding acquisition: W.G. and Z.J. jointly secured the research funding; supervision—review and editing: both W.G. and H.H. reviewed, revised, and finalized the manuscript, and also supervised the overall research progress while ensuring the accuracy of the experimental results. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Xinjiang Academy of Agricultural Sciences Public Welfare Fundamental Scientific Research Project (KY2022043), “Tianshan Talents-Science and Technology Leading Talents” Project of Xinjiang and “Tianchi Talent Program for Young Doctoral Scholars” Project of Xinjiang.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors express many thanks to the Xinjiang Academy of Agricultural Sciences for their support. We are grateful to the anonymous reviewers for their useful comments.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SE | Standard error |
| ri | Intrinsic rate of increase |
| λ | Finite rate of increase |
| R0 | Net reproductive rate |
| T | Mean generation time |
| GRR | Gross reproductive rate |
| Rf | Relative fitness |
| CX-Sub8 | Chlorantraniliprole-resistant strain continuously selected for eight generations |
| CX-Sub-n | Where n = 1 to n = 8 and represents the serial numbers of generations |
| CX-S | Unselected strains |
| CX-S8 | The eighth generation of the unselected strain, the control strain |
| DEG | Differentially expressed gene |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| APOP | Adult preoviposition period |
| TPOP | Total preoviposition period |
| Nm/Nf | Number of males/Number of females |
References
- Chang, P.E.C.; Metz, M.A. Classification of Tuta absoluta (Meyrick, 1917) (Lepidoptera: Gelechiidae: Gelechiinae: Gnorimoschemini) based on cladistic analysis of morphology. Proc. Entomol. Soc. Wash. 2021, 123, 41–54. [Google Scholar]
- Desneux, N.; Luna, M.G.; Guillemaud, T.; Urbaneja, A. The invasive South American tomato pinworm, Tuta absoluta, continues to spread in Afro-Eurasia and beyond: The new threat to tomato world production. J. Pest Sci. 2011, 84, 403–408. [Google Scholar] [CrossRef]
- Guillemaud, T.; Blin, A.; Le Goff, I.; Desneux, N.; Reyes, M.; Tabone, E.; Tsagkarakou, A.; Niño, L.; Lombaert, E. The tomato borer, Tuta absoluta, invading the Mediterranean Basin, originates from a single introduction from Central Chile. Sci. Rep. 2015, 5, 8371. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.R.; Biondi, A.; Adiga, A.; Guedes, R.N.; Desneux, N. From the western Palaearctic region to beyond: Tuta absoluta 10 years after invading Europe. J. Pest Sci. 2017, 90, 787–796. [Google Scholar] [CrossRef]
- Zhang, G.F.; Xian, X.Q.; Zhang, Y.B.; Liu, W.X.; Liu, H.; Feng, X.D.; Ma, D.Y.; Wang, Y.S.; Gao, Y.H.; Zhang, R.; et al. Outbreak of the South American tomato leafminer Tuta absoluta on the Chinese mainland: Geographic and potential host range expansion. Pest Manag. Sci. 2021, 77, 5475–5488. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Y.; Zhang, Y.; Sun, Z.; Li, Y.; Ding, J.; Zhang, G.; Du, E.; Zi, X.; Tian, C.; et al. Role of Enterococcus mundtii in gut of the tomato leaf miner (Tuta absoluta) to detoxification of Chlorantraniliprole. Pestic. Biochem. Physiol. 2024, 204, 106060. [Google Scholar] [CrossRef]
- Garzia, T.G.; Siscaro, G.; Biondi, A.; Zappala, L. Tuta absoluta, a South American pest of tomato now in the EPPO region: Biology, distribution and damage. Bulletin OEPP. EPPO Bull. 2012, 42, 205–210. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Siqueira, H.A.A. The tomato borer Tuta absoluta: Insecticide resistance and control failure. CAB Rev. 2012, 7, 1–7. [Google Scholar] [CrossRef]
- Barrientos, R.Z.; Apablaza, H.J.; Norero, S.A.; Estay, P.P. Temperatura base y constante térmica de desarrollo de la polilla del tomate, Tuta absoluta (Lepidoptera: Gelechiidae). Cienc. Investig. Agrar. 1998, 25, 133–137. [Google Scholar] [CrossRef]
- Silva, G.A.; Picanço, M.C.; Bacci, L.; Crespo, A.L.B.; Rosado, J.F.; Guedes, R.N.C. Control failure likelihood and spatial dependence of insecticide resistance in the tomato pinworm, Tuta absoluta. Pest Manag. Sci. 2011, 67, 913–920. [Google Scholar] [CrossRef]
- Gontijo, P.C.; Picanço, M.C.; Pereira, E.J.G.; Martins, J.C.; Chediak, M.; Guedes, R.N.C. Spatial and temporal variation in the control failure likelihood of the tomato leaf miner, Tuta absoluta. Ann. Appl. Biol. 2013, 162, 50–59. [Google Scholar] [CrossRef]
- Grant, C.; Jacobson, R.; Ilias, A.; Berger, M.; Vasakis, E.; Bielza, P.; Zimmer, C.T.; Williamson, M.S.; Ffrench-Constant, R.H.; Vontas, J.; et al. The evolution of multiple-insecticide resistance in UK populations of tomato leafminer, Tuta absoluta. Pest Manag. Sci. 2019, 75, 2079–2085. [Google Scholar] [CrossRef] [PubMed]
- Guedes, R.N.C.; Roditakis, E.; Campos, M.R.; Haddi, K.; Bielza, P.; Siqueira, H.A.A.; Tsagkarakou, A.; Vontas, J.; Nauen, R. Insecticide resistance in the tomato pinworm Tuta absoluta: Patterns, spread, mechanisms, management and outlook. J. Pest Sci. 2019, 92, 1329–1342. [Google Scholar] [CrossRef]
- Ma, X.; Qu, C.; Yao, J.; Xia, J.; Luo, C.; Guedes, R.N.C.; Wang, R. Resistance monitoring of diamide insecticides and characterization of field-evolved chlorantraniliprole resistance among Chinese populations of the tomato pinworm Phthorimaea (=Tuta) absoluta (Lepidoptera: Gelechiidae). Pestic. Biochem. Physiol. 2024, 205, 106140. [Google Scholar] [CrossRef] [PubMed]
- Sattelle, D.B.; Cordova, D.; Cheek, T.R. Insect ryanodine receptors: Molecular targets for novel pest control chemicals. Invertebr. Neurosci. 2008, 8, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Roditakis, E.; Steinbach, D.; Moritz, G.; Vasakis, E.; Stavrakaki, M.; Ilias, A.; García-Vidal, L.; Martínez-Aguirre, M.D.R.; Bielza, P.; Morou, E.; et al. Ryanodine receptor point mutations confer diamide insecticide resistance in tomato leafminer, Tuta absoluta (Lepidoptera: Gelechiidae). Insect Biochem. Mol. Biol. 2017, 80, 11–20. [Google Scholar] [CrossRef]
- Siqueira, H.A.A.; Guedes, R.N.C.; Picanço, M.C. Insecticide resistance in populations of Tuta absoluta (Lepidoptera: Gelechiidae). Agric. For. Entomol. 2000, 2, 147–153. [Google Scholar] [CrossRef]
- Roditakis, E.; Skarmoutsou, C.; Staurakaki, M.; Martínez-Aguirre, R.; García-Vidal, L.; Bielza, P.; Haddi, K.; Rapisarda, C.; Rison, J.L.; Bassi, A.; et al. Determination of baseline susceptibility of European populations of Tuta absoluta (Meyrick) to indoxacarb and chlorantraniliprole using a novel dip bioassay method. Pest Manag. Sci. 2013, 69, 217–227. [Google Scholar] [CrossRef]
- Silva, W.M.; Berger, M.; Bass, C.; Balbino, V.Q.; Amaral, M.H.P.; Campos, M.R.; Siqueira, H.A.A. Status of pyrethroid resistance and mechanisms in Brazilian populations of Tuta absoluta. Pestic. Biochem. Physiol. 2015, 122, 8–14. [Google Scholar] [CrossRef]
- Nauen, R.; Bass, C.; Feyereisen, R.; Vontas, J. The role of cytochrome P450s in insect toxicology and resistance. Annu. Rev. Entomol. 2022, 67, 105–124. [Google Scholar] [CrossRef]
- Kliot, A.; Ghanim, M. Fitness costs associated with insecticide resistance. Pest Manag. Sci. 2012, 68, 1431–1437. [Google Scholar] [CrossRef]
- Li, X.Y.; Wan, Y.; Yuan, G.; Hussain, S.; Xu, B.; Xie, W.; Wang, S.; Zhang, Y.; Wu, Q. Fitness trade-off associated with Spinosad resistance in Frankliniella occidentalis (Thysanoptera: Thripidae). J. Econ. Entomol. 2017, 110, 1755–1763. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Sanada-Morimura, S.; Matsukura, K.; Van Chien, H.; Cuong, L.Q.; Loc, P.M.; Estoy, G.F.; Matsumura, M. Energy reserve compensating for trade-off between metabolic resistance and life history traits in the brown planthopper (Hemiptera: Delphacidae). J. Econ. Entomol. 2020, 113, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Hardstone, M.C.; Huang, X.; Harrington, L.C.; Harrington, L.C.; Scott, J.G. Differences in development, glycogen, and lipid content associated with cytochrome P450-mediated permethrin resistance in Culex pipiens quinquefasciatus (Diptera: Culicidae). J. Med. Entomol. 2010, 2, 188. [Google Scholar] [CrossRef]
- Nath, A.; Gadratagi, B.G.; Maurya, R.P.; Ullah, F.; Patil, N.B.; Adak, T.; Govindharaj, G.P.P.; Ray, A.; Mahendiran, A.; Desneux, N.; et al. Sublethal phosphine fumigation induces transgenerational hormesis in a factitious host, Corcyra cephalonica. Pest Manag. Sci. 2023, 79, 3548–3558. [Google Scholar] [CrossRef]
- Hafeez, M.; Li, X.; Yousaf, H.K.; Khan, M.M.; Imran, M.; Zhang, Z.; Huang, Y.; Zhang, J.; Shah, S.; Wang, L.; et al. Sublethal effects of bistrifluron on key biological traits, macronutrients contents and vitellogenin (SeVg) expression in Spodoptera exigua (Hübner). Pestic Biochem. Physiol. 2021, 174, 104802. [Google Scholar] [CrossRef]
- Nath, B.S.; Suresh, A.; Varma, B.M.; Kumar, R.P. Changes in protein metabolism in hemolymph and fat body of the silkworm, Bombyx mori (Lepidoptera: Bombycidae) in response to organophosphorus insecticides toxicity. Ecotoxicol. Environ. Saf. 1997, 36, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A. Biochemical response in phosphine resistant and susceptible adult beetles of Trogoderma granarium to the sublethal dose. J. Entomol. Zool. Stud. 2016, 4, 582–587. [Google Scholar]
- Kulma, K.; Saddler, A.; Koella, J.C. Effects of Age and Larval Nutrition on Phenotypic Expression of Insecticide-Resistance in Anopheles Mosquitoes. PLoS ONE 2013, 8, e0058322. [Google Scholar] [CrossRef]
- Freeman, J.C.; Smith, L.B.; Silva, J.J.; Fan, Y.; Sun, H.; Scott, J.G. Fitness studies of insecticide resistant strains: Lessons learned and future directions. Pest Manag. Sci. 2021, 77, 3847–3856. [Google Scholar] [CrossRef]
- Ribeiro, L.M.; Wanderley-Teixeira, V.; Ferreira, H.N.; Teixeira, A.A.; Siqueira, H.A. Fitness costs associated with field-evolved resistance to chlorantraniliprole in Plutella xylostella (Lepidoptera: Plutellidae). Bull. Entomol. Res. 2014, 104, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, M.; Liu, S.; Jan, S.; Ali, B.; Shahid, M.; Fernández-Grandon, G.M.; Nawaz, M.; Ahmad, A.; Wang, M. Gossypol-induced fitness gain and increased resistance to deltamethrin in beet armyworm, Spodoptera exigua (Hübner). Pest Manag. Sci. 2019, 75, 683–693. [Google Scholar] [CrossRef]
- Shah, R.M.; Shad, S.A. House fly resistance to chlorantraniliprole: Cross resistance patterns, stability and associated fitness costs. Pest Manag. Sci. 2020, 76, 1866–1873. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Yang, X.B.; Yang, H.; Long, G.Y.; Jin, D.C. Effects of sublethal concentrations of insecticides on the fecundity of Sogatella furcifera (Hemiptera: Delphacidae) via the regulation of vitellogenin and its receptor. J. Insect Sci. 2020, 20, 14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gong, Y.; Cheng, S.; Desneux, N.; Gao, X.; Xiu, X.; Wang, F.; Hou, M. Transgenerational hormesis effects of nitenpyram on fitness and insecticide tolerance/resistance of Nilaparvata lugens. J. Pest Sci. 2023, 96, 161–180. [Google Scholar] [CrossRef]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H.; Kavousi, A.; Gharekhani, G.; Atlihan, R.; Salih Özgökçe, M.S.; Güncan, A.; Gökçe, A.; Smith, C.L.; Benelli, G.; Guedes, R.N.C.; et al. Advances in theory, data analysis, and application of the age-stage, two-sex life table for demographic research, biological control, and pest management. Entomol. Gen. 2023, 43, 705–732. [Google Scholar] [CrossRef]
- Reddy, G.V.P.; Chi, H. Demographic comparison of sweetpotato weevil reared on a major host, Ipomoea batatas, and an alternative host, I. triloba. Sci. Rep. 2015, 5, 11871. [Google Scholar] [CrossRef]
- Abbas, N.; Shad, S.A.; Razaq, M. Fitness cost, cross resistance and realized heritability of resistance to Imidacloprid in Spodoptera litura (Lepidoptera: Noctuidae). Pestic. Biochem. Physiol. 2012, 103, 181–188. [Google Scholar] [CrossRef]
- Nie, Z.; Zuo, L.; Tang, S.; Fang, C.; Ma, Y.; Li, X.; Zhang, J.; Su, J. Effects of Prey Switching at Different Stages on Life Parameters of Neoseiulus bicaudus. Agriculture 2024, 14, 728. [Google Scholar] [CrossRef]
- Biocloud. net. “Transcriptome Analysis Platform for Eukaryotes with Reference Genomes.” n.d. Available online: https://www.biocloud.net/ (accessed on 20 January 2025).
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhang, Y.; Xu, K.; Wang, Y.; Yang, W. Selection and validation of reference genes for gene expression analysis in Tuta absoluta Meyrick (Lepidoptera: Gelechiidae). Insects 2021, 12, 589. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- IBM Corp. IBM SPSS Statistics for Windows, Version 22.0; IBM Corp.: Chicago, IL, USA, 2013.
- Silva, J.E.; Ribeiro, L.; Vinasco, N.; Guedes, R.N.C.; Siqueria, H.A.A. Field-evolved resistance to chlorantraniliprole in the tomato pinworm Tuta absoluta: Inheritance, cross-resistance profile, and metabolism. J. Pest Sci. 2019, 92, 1421–1431. [Google Scholar] [CrossRef]
- Jallow, M.F.A.; Dahab, A.A.; Albaho, M.S.; Devi, V.Y.; Awadh, D.G.; Thomas, B.M. Baseline susceptibility and assessment of resistance risk to flubendiamide and chlorantraniliprole in Tuta absoluta (Lepidoptera: Gelechiidae) populations from Kuwait. Appl. Entomol. Zool. 2019, 54, 91–99. [Google Scholar] [CrossRef]
- Gong, W.; Yan, H.H.; Gao, L.; Guo, Y.Y.; Xue, C.B. Chlorantraniliprole resistance in the diamondback moth (Lepidoptera: Plutellidae). J. Econ. Entomol. 2014, 107, 806–814. [Google Scholar] [CrossRef]
- Troczka, B.J.; Williams, A.J.; Williamson, M.S.; Field, L.M.; Lüemmen, P.; Davies, T.G.E. Stable expression and functional characterisation of the diamondback moth ryanodine receptor G4946E variant conferring resistance to diamide insecticides. Sci. Rep. 2015, 5, 14680. [Google Scholar] [CrossRef]
- Ffrench-Constant, R.H.; Bass, C. Does resistance really carry a fitness cost? Curr. Opin. Insect Sci. 2017, 21, 39–46. [Google Scholar] [CrossRef]
- Liu, S.; Yao, X.; Xiang, X.; Yang, Q.; Wang, X.; Xin, T.; Yu, S. Fitness costs associated with chlorantraniliprole resistance in Spodoptera exigua (Lepidoptera: Noctuidae). Pest Manag. Sci. 2021, 77, 1739–1747. [Google Scholar] [CrossRef]
- Yin, X.; Wu, Q.; Li, X.; Zhang, Y.; Xu, B. Demographic Changes in Multigeneration Plutella xylostella (Lepidoptera: Plutellidae) After Exposure to Sublethal Concentrations of Spinosad. J. Econ. Entomol. 2009, 1, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Teixeira, L.A.; Andaloro, J.T. Diamide insecticides: Global efforts to address insect resistance stewardship challenges. Pestic. Biochem. Physiol. 2013, 106, 76–78. [Google Scholar] [CrossRef]
- Erb, S.L.; Bourchier, R.S.; Van Frankenhuyzen, K.; Smith, S.M. Sublethal effects of Bacillus thuringiensis Berliner subsp. kurstaki on Lymantria dispar (Lepidoptera: Lymantriidae) and the Tachinid Parasitoid Compsilura concinnata (Diptera: Tachinidae). Environ. Entomol. 2001, 30, 1174–1181. [Google Scholar] [CrossRef]
- Hannig, G.T.; Ziegler, M.; Marçon, P.G. Feeding cessation effects of chlorantraniliprole, a new anthranilic diamide insecticide, in comparison with several insecticides in distinct chemical classes and mode-of-action groups. Pest Manag. Sci. 2009, 65, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Awmack, C.S.; Leather, S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef]
- Qu, C.; Yao, J.; Zhan, Q.; Zhang, D.; Li, Y.; Huang, J.; Wang, R. Risk assessment, cross-resistance, biochemical mechanism and fitness cost of tetraniliprole resistance in the tomato pinworm Tuta absoluta. Crop Prot. 2024, 183, 106756. [Google Scholar] [CrossRef]
- Zhang, R.; Dong, J.; Chen, J.; Ji, Q.; Cui, J. The sublethal effects of chlorantraniliprole on Helicoverpa armigera (Lepidoptera: Noctuidae). J. Integr. Agric. 2013, 12, 457–466. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Z.; Wang, W.; Pan, Y. Effects of sublethal fumigation with phosphine on the reproductive capacity of Liposcelis entomophila (End.) (Psocoptera: Liposcelididae). Int. J. Pest Manag. 2020, 66, 75–81. [Google Scholar] [CrossRef]
- Bozhgani, N.S.; Ghobadi, H.; Riahi, E. Sublethal effects of chlorfenapyr on the life table parameters of two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae). Syst. Appl. Acarol. 2018, 23, 1342–1351. [Google Scholar] [CrossRef]
- Zalizniak, L.; Nugegoda, D. Effect of sublethal concentrations of chlorpyrifos on three successive generations of Daphnia carinata. Ecotoxicol. Environ. Saf. 2006, 64, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Nayak, M.K.; Collins, P.J. Influence of concentration, temperature and humidity on the toxicity of phosphine to the strongly phosphine-resistant psocid Liposcelis bostrychophila Badonnel (Psocoptera: Liposcelididae). Pest Manag. Sci. 2010, 64, 9. [Google Scholar] [CrossRef]
- Ridley, A.W.; Schlipalius, D.I.; Daglish, G.J. Reproduction of phosphine resistant Rhyzopertha dominica (F.) following sublethal exposure to phosphine. J. Stored Prod. Res. 2012, 48, 106–110. [Google Scholar] [CrossRef]
- Knight, A.L.; Flexner, L. Disruption of mating in codling moth (Lepidoptera: Tortricidae) by chlorantranilipole, an anthranilic diamide insecticide. Pest Manag. Sci. 2007, 63, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Pereyra, P.C.; Sánchez, N.E. Effect of two solanaceous plants on developmental and population parameters of the tomato leaf miner, Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Neotrop. Entomol. 2006, 35, 671–676. [Google Scholar] [CrossRef]
- Han, W.; Zhang, S.; Shen, F.; Liu, M.; Ren, C.; Gao, X. Residual toxicity and sublethal effects of chlorantraniliprole on Plutella xylostella (Lepidoptera: Plutellidae). Pest Manag. Sci. 2012, 68, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gong, P.; Li, M.; Qiu, X.; Wang, K. Sublethal effects of Spinosad on survival, growth and reproduction of Helicoverpa armigera (Lepidoptera: Noctuidae). Pest Manag. Sci. 2009, 65, 223–227. [Google Scholar] [CrossRef]
- Dong, J.; Wang, K.; Li, Y.; Wang, S. Lethal and sublethal effects of cyantraniliprole on Helicoverpa assulta (Lepidoptera: Noctuidae). Pestic. Biochem. Physiol. 2017, 136, 58–63. [Google Scholar] [CrossRef]
- Bessin, R.T.; Reagan, T.E. Fecundity of Sugarcane Borer (Lepidoptera: Pyralidae), as affected by larval development on gramineous host plants. Environ. Entomol. 1990, 19, 635–639. [Google Scholar] [CrossRef]
- Kobayashi, K.; Hasegawa, E.; Yamamoto, Y.; Kawatsu, K.; Vargo, E.L.; Yoshimura, J.; Matsuura, K. Sex ratio biases in termites provide evidence for kin selection. Nat. Commun. 2013, 4, 2048. [Google Scholar] [CrossRef]
- Haddi, K.; Mendes, M.V.; Barcellos, M.S.; Lino-Neto, J.; Freitas, H.L.; Guedes, R.N.; Oliveira, E.E. Sexual Success After Stress? Imidacloprid-Induced Hormesis in Males of the Neotropical Stink Bug Euschistus heros. PLoS ONE 2016, 11, e0156616. [Google Scholar] [CrossRef]
- Nozad-Bonab, Z.; Hejazi, M.J.; Iranipour, S.; Arzanlou, M. Lethal and sublethal effects of some chemical and biological insecticides on Tuta absoluta (Lepidoptera: Gelechiidae) eggs and neonates. J. Econ. Entomol. 2017, 110, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lü, L.; He, Y. Effects of insecticides on sex pheromone communication and mating behavior in Trichogramma chilonis. J. Pest Sci. 2018, 91, 65–78. [Google Scholar] [CrossRef]
- Andreazza, F.; Haddi, K.; Nörnberg, S.D.; Guedes, R.N.; Nava, D.E.; Oliveira, E.E. Sex-dependent locomotion and physiological responses shape the insecticidal susceptibility of parasitoid wasps. Environ. Pollut. 2020, 264, 114605. [Google Scholar] [CrossRef]
- Margus, A.; Piiroinen, S.; Lehmann, P.; Tikka, S.; Karvanen, J.; Lindström, L. Sublethal pyrethroid insecticide exposure carries positive fitness effects over generations in a pest insect. Sci. Rep. 2019, 9, 11320. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, C.; Wang, Q.; Wei, Y.; Liu, F.; Xu, S.; Zhang, Z.; Mu, W. Effects of the microbial secondary metabolite benzothiazole on the nutritional physiology and enzyme activities of Bradysia odoriphaga (Diptera: Sciaridae). Pestic. Biochem. Physiol. 2016, 129, 49–55. [Google Scholar] [CrossRef]
- Piri, F.; Sahragard, A.; Ghadamyari, M. Sublethal effects of Spinosad on some biochemical and biological parameters of Glyphodes pyloalis Walker (Lepidoptera: Pyralidae). Plant Prot. Sci. 2014, 50, 135–144. [Google Scholar] [CrossRef]
- Arambourou, H.; Gismondi, E.; Branchu, P.; Beisel, J.N. Biochemical and morphological responses in Chironomus riparius (Diptera, Chironomidae) larvae exposed to lead-spiked sediment. Environ. Toxicol. Chem. 2013, 32, 2558–2564. [Google Scholar] [CrossRef]
- Etebari, K.; Mirhoseini, S.Z.; Matindoost, L. A study on interaspecific biodiversity of eight groups of silkworm (Bombyx mori) by biochemical markers. Insect Sci. 2005, 12, 87–94. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).