A Little Peek May Be Enough: How Small Hive Beetle Estimates Can Help Address Immediate Colony Management Needs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Sites
2.2. Sampling Protocol
- Inspection began by lifting the whole hive with the cover on and without smoking the entrance. Because the hive and the bottom board were not physically attached to any of the colonies we sampled, the hive’s floor was then exposed. Adult beetles found on the hive floor were captured, and the bottom board was then considered “SHB-free”. Beetles caught at this stage were assigned to BB, which stands for bottom board before inspection.
- The hive was then placed back over the bottom board.
- The within-colony inspection began by sampling the cover, which, depending on the site, could include the lid and an inner cover or just a cover. Covers were visually inspected and/or banged on a hard substrate to dislodge any SHB adults. When the cover is removed, elements that affect the beetle’s behavior (sunlight, frame movement, and smoke) begin to impact the results. Consequently, the cover inspection was performed quickly, and the observers began the inspection of the first set of side frames almost simultaneously to ensure that these two areas were as accurate a representation of the SHB distribution as possible.
- The side frames (SFs), the two most lateral frames in a box, could belong to a honey box or a brood box, depending on the configuration of the colony. Beetles found on the side frames were removed by tapping the frames on a solid surface and collecting beetles directly from the frames. The SF of the box immediately under the cover was labeled SfT, indicating that this was the “top” box of this colony. For colonies consisting of only a brood box, the brood box itself was considered the top box.
- After the SFs were examined, a single frame from the center of each box was inspected for beetles. The center frame (Cf) could be from either a honey box or a brood box, depending on the hive configuration. The percent SHB infestation that is strictly based on a center frame from the brood box is labeled Cfb.
- After all boxes were inspected, the hive was reassembled, and the cover was placed back on. The observers looked for beetles that may have moved down to the cover during the manipulation of the hive, but none were found.
- The final step of the inspection involved lifting the whole hive off the bottom board again and collecting all beetles found on the floor. Beetles caught on the floor of the hive at this stage were assigned to BA, which stands for bottom board after inspection.
2.3. Statistics
3. Results
3.1. SHB Abundance in Honeybee Colonies in Hawai’i, Mexico, and Costa Rica
3.2. Presence of SHB Larvae in Colonies
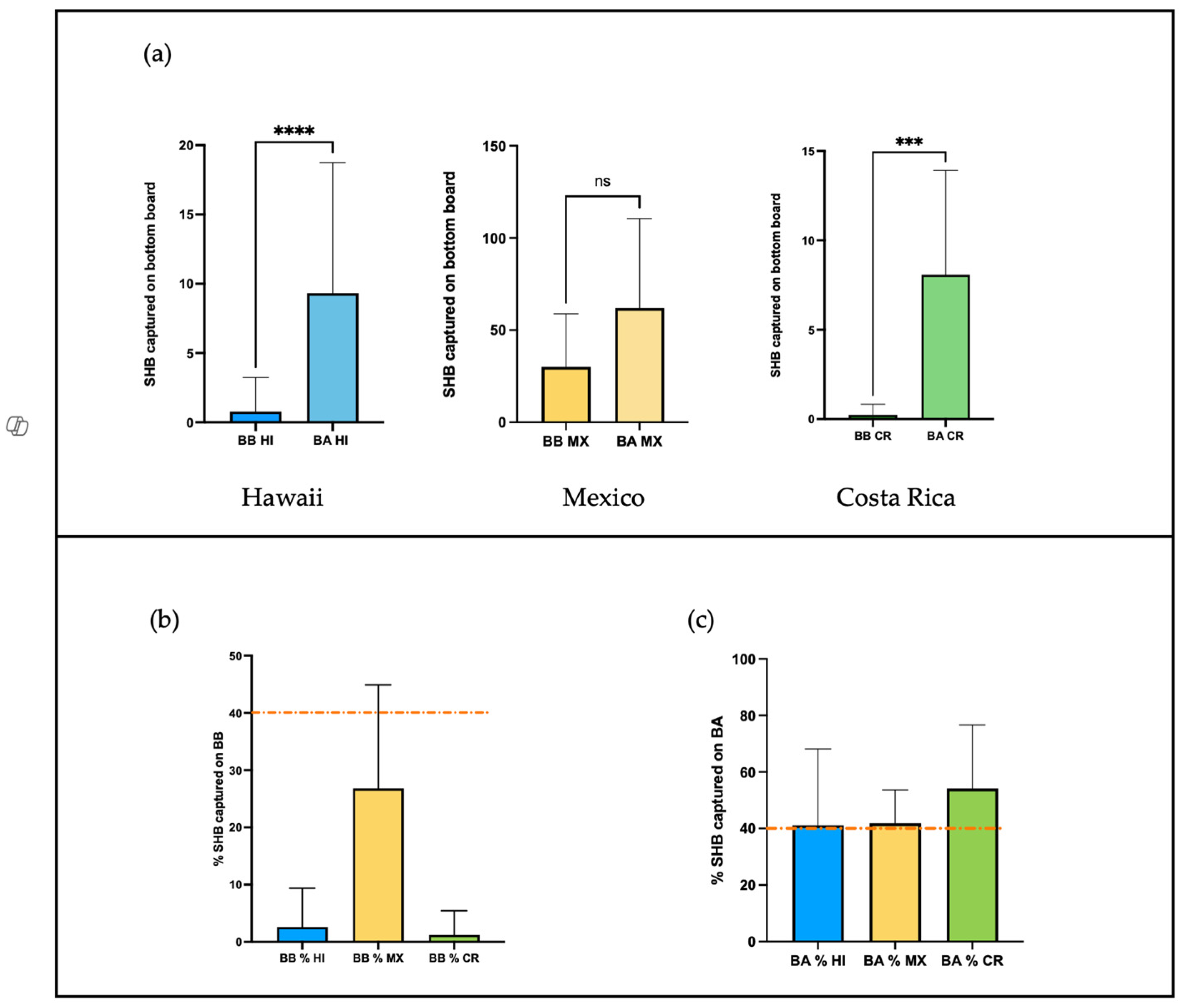
3.3. Bottom Board Before and After Colony Inspection
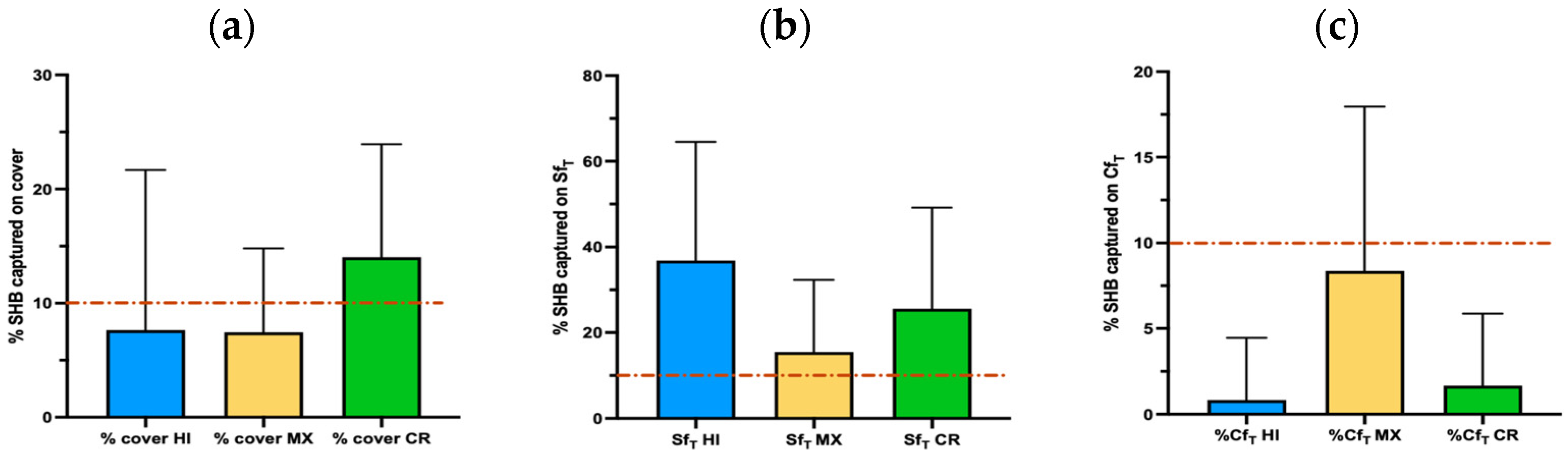
3.4. Inspection of the Cover and First Box
3.4.1. Cover Inspection
3.4.2. Side Frame Inspection
3.4.3. Center Frame Inspection
3.5. Defense of the Brood Nest
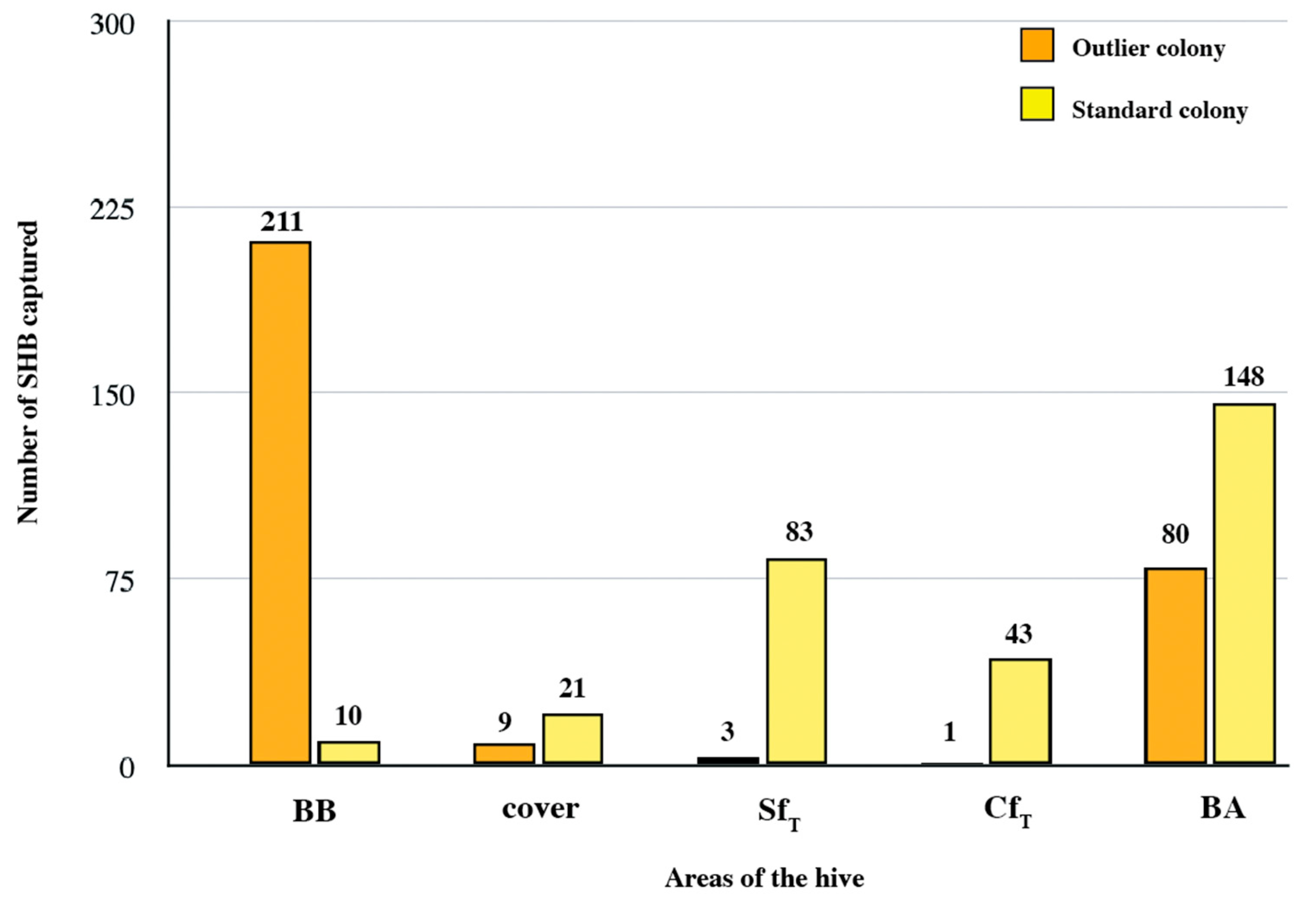
3.6. Summary: Overall SHB Distribution in the Colonies
3.7. Targeted Sampling and Relative SHB Infestation Correlates
3.8. Notes on Effective Field Sampling of SHBs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hood, W.M. Overview of the small hive beetle, Aethina tumida, in North America. Bee World 2000, 81, 129–137. [Google Scholar] [CrossRef]
- Cordeiro, E.M.G.; Soares, P.L.; Alves, D.A.; Corrêa, A.S. Updating the saga of the small hive beetle (Aethina tumida): Molecular inference of the origin of the South American invasion. Apidologie 2019, 50, 273–276. [Google Scholar] [CrossRef]
- Idrissou, F.O.; Huang, Q.; Yañez, O.; Neumann, P. International beeswax trade facilitates small hive beetle invasions. Sci. Rep. 2019, 9, 10665. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.O.; Cardaio, I.; Cilia, G.; Cornelissen, B.; Crailsheim, K.; Formato, G.; Lawrence, A.K.; Le Conte, Y.; Mutinelli, F.; Nanetti, A.; et al. How to slow the global spread of small hive beetles, Aethina tumida. Biol. Invasions 2019, 21, 1451–1459. [Google Scholar] [CrossRef]
- Robson, J.D. Small Hive Beetle Aethina tumida Murray (Coleoptera: Nitidulidae), Pest Alert 12-01; Plant Pest Control Branch, Division of Plant Industry, State of Hawai´i Department of Agriculture: Honolulu, HI, USA, 2012. Available online: https://hdoa.hawaii.gov/pi/files/2013/01/NPA-SHB-1-12.pdf (accessed on 28 April 2025).
- Cervancia, C.R.; de Guzman, L.I.; Polintan, E.A.; Dupo, A.L.B.; Locsin, A.A. Current status of small hive beetle infestation in the Philippines. J. Apic. Res. 2016, 55, 74–77. [Google Scholar] [CrossRef]
- Namin, S.M.; Koh, Y.; Osabutey, A.F.; Jung, C. Invasion pathway of the honeybee pest, small hive beetle, Aethina tumida (Coleoptera: Nitidulidae) in the Republic of Korea inferred by mitochondrial DNA sequence analysis. J. Asia Pac. Entomol. 2019, 22, 963–968. [Google Scholar] [CrossRef]
- Gillespie, P.; Staples, J.; King, C.; Fletcher, M.J.; Dominiak, B.C. Small hive bettle, ‘Aethina tumida’ (Murray) (Coleoptera: Nitidulidae) in New South Wales. Gen. Appl. Ent. J. Ent. Soc. N. S. W. 2003, 32, 5–7. [Google Scholar]
- Liu, Y.; Han, W.; Gao, J.; Su, S.; Beaurepaire, A.; Yañez, O.; Neumann, P. Out of Africa: Novel source of small hive beetles infesting Eastern and Western honey bee colonies in China. J. Apic. Res. 2021, 60, 108–110. [Google Scholar] [CrossRef]
- Granato, A.; Zecchin, B.; Baratto, C.; Duquesne, V.; Negrisolo, E.; Chauzat, M.P.; Ribière-Chabert, M.; Cattoli, G.; Mutinelli, F. Introduction of Aethina tumida (Coleoptera: Nitidulidae) in the regions of Calabria and Sicily (southern Italy). Apidologie 2017, 48, 194–203. [Google Scholar] [CrossRef]
- Mutinelli, F.; Montarsi, F.; Federico, G.; Granato, A.; Ponti, A.M.; Grandinetti, G.; Ferrè, N.; Franco, S.; Duquesne, V.; Rivière, M.P.; et al. Detection of Aethina tumida Murray (Coleoptera: Nitidulidae.) in Italy: Outbreaks and early reaction measures. J. Apic. Res. 2014, 53, 569–575. [Google Scholar] [CrossRef]
- Palmeri, V.; Scirtò, G.; Malacrinò, A.; Laudani, F.; Campolo, O. A scientific note on a new pest for European honeybees: First report of small hive beetle Aethina tumida, (Coleoptera: Nitidulidae) in Italy. Apidologie 2015, 46, 527–529. [Google Scholar] [CrossRef]
- Kumaranag, K.M.; Garain, P.K.; Chandran, N.; Shashank, P.R.; Kukkamudi, M.R.; Deka, M.K.; Das, P.P.G.; Jasrotia, P.; Suroshe, S.S. First report of invasive small hive beetle, Aethina tumida Murray (Coleoptera: Nitidulidae) from India. J. Apic. Res. 2025, 64, 1–11. [Google Scholar] [CrossRef]
- Pettis, J.; Schäfer, M.; Neumann, P. Small hive beetles in the Americas. In The Small Hive Beetle—A Growing Problem in the 21st Century; Carreck, N.L., Ed.; International Bee Research Association: Bristol, UK, 2017; pp. 41–50. [Google Scholar]
- Bulacio Cagnolo, N.; Aldea-Sánchez, P.; Branchiccela, B.; Calderón-Fallas, R.A.; Medina-Medina, L.A.; Palacio, M.A.; Velarde, R.; Teixeira, E.W.; Antúnez, K. Current status of the small hive beetle Aethina tumida in Latin America. Apidologie 2023, 54, 23. [Google Scholar] [CrossRef]
- Orozco, J. First record of the beekeeping pest Aethina tumida Murray (Coleoptera: Nitidulidae) for Honduras. Insecta Mundi 2024, 1049, 1–3. [Google Scholar]
- Neumann, P.; Pettis, J.S.; Schäfer, M.O. Quo vadis Aethina tumida? Biology and control of small hive beetles. Apidologie 2016, 47, 427–466. [Google Scholar] [CrossRef]
- Martin, S. Double trouble in paradise: Small hive beetle joins Varroa in Hawaii. Am. Bee J. 2013, 153, 529–532. [Google Scholar]
- Del Valle Molina, J.A. Small Hive Beetle Infestation (Aethina tumida) in Mexico: Immediate Notification Report; Ref OIE: 6397; SENASICA: Mexico City, Mexico, 2007. [Google Scholar]
- Bai, W.F.; Liu, J.; Liu, Y.; Han, W.; Evans, J.D.; Huang, Q. Phylogenetic Analysis of Small Hive Beetles From Native to Introduced Populations. Front. Genet. 2022, 13, 900795. [Google Scholar] [CrossRef] [PubMed]
- Lounsberry, Z.; Spiewok, S.; Pernal, S.F.; Sonstegard, T.S.; Hood, W.M.; Pettis, J.; Neumann, P.; Evans, J.D. Worldwide Diaspora of Aethina tumida (Coleoptera: Nitidulidae), a Nest Parasite of Honey Bees. Ann. Entomol. Soc. Am. 2010, 103, 671–677. [Google Scholar] [CrossRef]
- Calderón-Fallas, R.A.; Sánchez-Chaves, L.A.; Hernández-Ching, P. Strategies for detection and monitoring of the small hive beetle (Aethina tumida) in Africanized honeybee colonies in Costa Rica. Cienc. Vet. 2024, 42, 1–8. [Google Scholar] [CrossRef]
- Chauzat, M.P.; Laurent, M.; Brown, M.; Kryger, P.; Mutinelli, F.; Roelandt, S.; Roels, S.; Van Der Stede, Y.; Schaefer, M.; Franco, S.; et al. Guidelines for the Surveillance of the Small Hive Beetle (Aethina tumida) Infestation (Updated Version); European Union Reference Laboratory for Honey Bee Health: Antibes, France, 2016. [Google Scholar]
- Di Ruggiero, C.; Mezher, Z.; Mutinelli, F.; De Carolis, A.; Pocci, N.; Formato, G. Updates on the Mobile Divider and Its Use in Calabria Region to Monitor and Control Aethina tumida Infestation. Appl. Sci. 2021, 11, 10637. [Google Scholar] [CrossRef]
- Li, D.; Waite, D.W.; Fan, Q.H.; George, S.; Semeraro, L.; Blacket, M.J. Molecular detection of small hive beetle Aethina tumida Murray (Coleoptera: Nitidulidae): DNA barcoding and development of a real-time PCR assay. Sci. Rep. 2018, 8, 9623. [Google Scholar] [CrossRef]
- Ponting, S.; Tomkies, V.; Stainton, K. Rapid identification of the invasive small hive beetle (Aethina tumida) using LAMP. Pest Manag. Sci. 2021, 77, 1476–1481. [Google Scholar] [CrossRef]
- Calderón, R.; Sánchez-Chaves, L.A. Small Hive Beetle, Aethina tumida, in Africanized Honey Bees in Costa Rica: Sentinel apiaries, epidemiological surveillance and training programs as strategies for early detection or to prevent its spread. Bee World. 2023, 100, 27–30. [Google Scholar] [CrossRef]
- Bernier, M.; Fournier, V.; Eccles, L.; Giovenazzo, P. Control of Aethina tumida (Coleoptera: Nitidulidae) using in-hive traps. Can. Entomol. 2015, 147, 97–108. [Google Scholar] [CrossRef]
- Neumann, P.; Evans, J.D.; Pettis, J.S.; Pirk, C.W.; Schäfer, M.O.; Tanner, G.; Ellis, J.D. Standard methods for small hive beetle research. J. Apic. Res. 2013, 52, 1–32. [Google Scholar] [CrossRef]
- Schäfer, M.O.; Pettis, J.S.; Ritter, W.; Neumann, P. A scientific note on quantitative diagnosis of small hive beetles, Aethina tumida, in the field. Apidologie 2008, 39, 564–565. [Google Scholar] [CrossRef]
- Saldaña, L.M.; Lara, L.G.; Antonio, D.J. Manual de Nuevos Manejos en la Apicultura para el Control del Pequeño Escarabajo de la Colmena Aethina tumida Murray, 2nd ed.; SAGARPA: Mexico City, Mexico, 2014. [Google Scholar]
- Keeling, M.J.; Datta, S.; Franklin, D.N.; Flatman, I.; Wattam, A.; Brown, M.; Budge, G.E. Efficient use of sentinel sites: Detection of invasive honeybee pests and diseases in the UK. J. R. Soc. Interface 2017, 14, 20160908. [Google Scholar] [CrossRef]
- Salvioni, C.; Formato, G. The sustainable management of invasive alien species: The Case of Small Hive Beetle. In Proceedings of the European Association of Agricultural Economists, 166th Seminar, 30–31 August 2018; AgEcon Search: Galway, Ireland, 2018. [Google Scholar]
- Formato, G.; Federico, G.; Di Ruggiero, C.; Pietropaoli, M.; Milito, M.; Mutinelli, F. Definition of a Protocol to Manage and Officially Confirm SHB Presence in Sentinel Honeybee Colonies. Appl. Sci. 2021, 11, 8260. [Google Scholar] [CrossRef]
- Cornelissen, B.; Neumann, P. How to catch a small beetle: Top tips for visually screening honey bee colonies for small hive beetles. Bee World 2018, 95, 99–102. [Google Scholar] [CrossRef]
- Muturi, M.N.; Papach, A.; Lattorff, H.M.G.; Neumann, P. A scientific note on in-hive positioning determines small hive beetle trap efficacy. J. Apic. Res. 2022, 61, 315–316. [Google Scholar] [CrossRef]
- Torto, B.; Arbogast, R.T.; Van Engelsdorp, D.; Willms, S.; Purcell, D.; Boucias, D.; Tumlinson, J.H.; Teal, P.E. Trapping of Aethina tumida Murray (Coleoptera: Nitidulidae) from Apis mellifera L. (Hymenoptera: Apidae) colonies with an in-hive baited trap. Environ. Entomol. 2014, 36, 1018–1024. [Google Scholar] [CrossRef]
- Ouessou Idrissou, F.; Huang, Q.; Yañez, O.; Akinwande, K.L.; Neumann, P. PCR diagnosis of small hive beetles. Insects 2018, 9, 24. [Google Scholar] [CrossRef]
- Ribani, A.; Taurisano, V.; Utzeri, V.J.; Fontanesi, L. Honey environmental DNA can be used to detect and monitor honey bee pests: Development of methods useful to identify Aethina tumida and Galleria mellonella infestations. Vet. Sci. 2022, 9, 213. [Google Scholar] [CrossRef]
- Spiewok, S.; Duncan, M.; Spooner-Hart, R.; Pettis, J.S.; Neumann, P. Small hive beetle, Aethina tumida, populations II: Dispersal of small hive beetles. Apidologie 2008, 39, 683–693. [Google Scholar] [CrossRef]
- Mustafa, S.G.; Spiewok, S.; Duncan, M.; Spooner-Hart, R.; Rosenkranz, P. Susceptibility of small honey bee colonies to invasion by the small hive beetle, Aethina tumida (Coleoptera, Nitidulidae). J. Appl. Entomol. 2014, 138, 547–550. [Google Scholar] [CrossRef]
- Annand, N. Investigations on small hive beetle biology to develop better control options. Master’s Thesis, University of Western Sydney, Sydney, Australia, 2011. [Google Scholar]
- Neumann, P.; Hoffmann, D. Small hive beetle diagnosis and control in naturally infested honeybee colonies using bottom board traps and CheckMite+ strips. J. Pest. Sci. 2008, 81, 43–48. [Google Scholar] [CrossRef]
- Schäfer, M.; Pettis, J.; Ritter, W.; Neumann, P. Simple Small Hive Beetle Diagnosis. Am. Bee. J. 2010, 150, 371–372. [Google Scholar]
- Duehl, A.J.; Arbogast, R.T.; Sheridan, A.B.; Teal, P.E. The influence of light on small hive beetle (Aethina tumida) behavior and trap capture. Apidologie 2012, 43, 417–424. [Google Scholar] [CrossRef]
- Villalobos, E.M.; Nikaido, S.; Ito, T.; Wong, J. Monitoring strategies during the establishment phase of Aethina tumida on Oahu, Hawaii. J. Appl. Entomol. 2024, 148, 708–711. [Google Scholar] [CrossRef]
- Spiewok, S.; Pettis, J.S.; Duncan, M.; Spooner-Hart, R.; Westervelt, D.; Neumann, P. Small hive beetle, Aethina tumida, populations I: Infestation levels of honeybee colonies, apiaries and regions. Apidologie 2007, 38, 595–605. [Google Scholar] [CrossRef]
- Lundie, A.E. The Small Hive Beetle, Aethina tumida; Science Bulletin; Department of Agriculture and Forestry, Union of South Africa: Pretoria, South Africa, 1940; Volume 220, 30p.
- Ellis, J.D., Jr.; Hepburn, R.; Delaplane, K.S.; Neumann, P.; Elzen, P.J. The effects of adult small hive beetles, Aethina tumida (Coleoptera: Nitidulidae), on nests and flight activity of Cape and European honey bees (Apis mellifera). Apidologie 2003, 34, 399–408. [Google Scholar] [CrossRef][Green Version]
- Levot, G.; Somerville, D.; Annand, N.; Collins, D.; Barchia, I. A six-month-long assessment of the health of bee colonies treated with APITHOR™ hive beetle insecticide. J. Apic. Res. 2022, 54, 386–393. [Google Scholar] [CrossRef]
- Sabella, G.; Mulè, R.; Robba, L.; Agrò, A.; Manachini, B. Could Europe apply a suitable control method for the small hive beetle (Coleoptera: Nitidulidae)? J. Econ. Entomol. 2022, 115, 401–411. [Google Scholar] [CrossRef]
- Torto, B.; Arbogast, R.T.; Alborn, H.; Suazo, A.; Van Engelsdorp, D.; Boucias, D.; Tumlinson, J.H.; Teal, P.E. Composition of volatiles from fermenting pollen dough and attractiveness to the small hive beetle Aethina tumida, a parasite of the honeybee Apis mellifera. Apidologie 2007, 38, 380–389. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Small hive beetle diagnosis and risk management options. EFSA J. 2015, 13, 4048. [Google Scholar] [CrossRef]
- Cornelissen, B.; Neumann, P.; Schweiger, O. Global warming promotes biological invasion of a honey bee pest. Glob. Change Biol. 2019, 25, 3642–3655. [Google Scholar] [CrossRef]
- Cornelissen, B.; Ellis, J.D.; Gort, G.; Hendriks, M.; van Loon, J.J.; Stuhl, C.J.; Neumann, P. The small hive beetle’s capacity to disperse over long distances by flight. Sci. Rep. 2024, 14, 14859. [Google Scholar] [CrossRef]
- Conrad, K.M.; Valerie, E.P.; Sandra, M.R. Tropical bee species abundance differs within a narrow elevational gradient. Sci. Rep. 2021, 11, 23368. [Google Scholar] [CrossRef]
- Vercelli, M.; Novelli, S.; Ferrazzi, P.; Lentini, G.; Ferracini, C. A qualitative analysis of beekeepers’ perceptions and farm management adaptations to the impact of climate change on honey bees. Insects 2021, 12, 228. [Google Scholar] [CrossRef]


| Sites | Total Number of Colonies Sampled | Colonies with a Single Brood Box | Colonies with Brood Box and Supers |
|---|---|---|---|
| HAWAII | 31 | 10 | 21 |
| MEXICO | 14 | 14 | 0 |
| COSTA RICA | 20 | 7 | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villalobos, E.M.; Medina Medina, L.; Zhang, Z.; Nikaido, S.; Miranda, E.; Wong, J.; Santamaria, J.; Buteler, M. A Little Peek May Be Enough: How Small Hive Beetle Estimates Can Help Address Immediate Colony Management Needs. Insects 2025, 16, 517. https://doi.org/10.3390/insects16050517
Villalobos EM, Medina Medina L, Zhang Z, Nikaido S, Miranda E, Wong J, Santamaria J, Buteler M. A Little Peek May Be Enough: How Small Hive Beetle Estimates Can Help Address Immediate Colony Management Needs. Insects. 2025; 16(5):517. https://doi.org/10.3390/insects16050517
Chicago/Turabian StyleVillalobos, Ethel M., Luis Medina Medina, Zhening Zhang, Scott Nikaido, Emanuel Miranda, Jason Wong, Jessika Santamaria, and Micaela Buteler. 2025. "A Little Peek May Be Enough: How Small Hive Beetle Estimates Can Help Address Immediate Colony Management Needs" Insects 16, no. 5: 517. https://doi.org/10.3390/insects16050517
APA StyleVillalobos, E. M., Medina Medina, L., Zhang, Z., Nikaido, S., Miranda, E., Wong, J., Santamaria, J., & Buteler, M. (2025). A Little Peek May Be Enough: How Small Hive Beetle Estimates Can Help Address Immediate Colony Management Needs. Insects, 16(5), 517. https://doi.org/10.3390/insects16050517








